An update of the most recent data on the current therapy for pseudomyxoma peritonei and mucinous colorectal adenocarcinoma with metastatic disease confined to the peritoneum is provided.
Keywords: Pseudomyxoma peritonei, Mucinous colorectal cancer, Treatment, Peritoneal carcinomatosis
Abstract
Peritoneal carcinomatosis has been considered a terminal disease with a median survival time of 5.2–12.6 months. Systemic chemotherapy and cytoreductive surgery (CRS) have long been used to treat macroscopic disease, with limited success. However, a comprehensive treatment approach involving cytroreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) has evolved into a novel approach for peritoneal carcinomatosis. Surgery removes the primary cancer and any dissemination within the peritoneal cavity and adjuvant HIPEC eradicates macroscopic or microscopic tumor residue, thus reducing the risk for recurrence. This approach offers a new potential treatment option for patients with metastatic disease confined to the peritoneum. The present review provides an update of the most recent data on the current therapy for pseudomyxoma peritonei (PMP) and mucinous colorectal adenocarcinoma (MCA) with metastatic disease confined to the peritoneum.
Introduction
In the past, peritoneal carcinomatosis has been regarded as a fatal manifestation of gastrointestinal cancer with a median survival time of 5.2–12.6 months [1, 2]. Treatment options for patients with unresectable metastatic disease have improved significantly in the past decade. However, the management of disease limited to the peritoneal cavity is controversial. Systemic chemotherapy is palliative and generally provides limited improvement in survival. Currently, there are no published data that outline the impact of new therapeutic regimens when given to patients with mucinous gastrointestinal adenocarcinomas with metastatic disease confined to the peritoneum. During the past decade, there has been a new multimodal therapeutic approach involving cytroreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). In the 1990s, Sugarbaker et al. [3, 4] proposed CRS and perioperative intraperitoneal (i.p.) chemotherapy as treatment for peritoneal dissemination from appendiceal and colonic neoplasms. Over the last 5 years, international treatment centers have published their prospective results showing a survival benefit for patients treated with CRS and HIPEC in the management of peritoneal surface malignancies for pseudomyxoma peritonei (PMP) and for those of colorectal origin.
PMP and mucinous colorectal adenocarcinoma (MCA) have a high propensity for spread limited to the peritoneal surface, with similar patterns of distribution [5]. Therefore, CRS and i.p. chemotherapy might be a potential treatment option for the particular group of patients with PMP and MCA with metastatic disease confined to the peritoneum. However, combined treatment procedures are not standardized and many variations exist in exposure techniques, drugs, drug doses, duration, temperature, and flow rates [6]. In addition, there is ongoing controversy about the pathological classification and prognosis of PMP and mucinous colorectal cancer (CRC) [7–9].
The aim of this review is to provide an update of the most recent data on the current therapy for PMP and MCA with metastatic disease confined to the peritoneum.
Gastrointestinal Mucinous Adenocarcinoma
PMP
PMP is a rare clinical disorder with an estimated incidence of approximately one person per million per year. It is a locoregional disease within the abdomen, characterized by mucinous tumor on peritoneal surfaces producing excessive amounts of mucinous ascites [10]. The mucinous feature in PMP is mucinous ascites and not intracellular mucin accumulation in tumor cells. The primary tumor is predominantly a minimally invasive appendiceal mucinous epithelial neoplasm with a high propensity for spread to peritoneal surfaces, but almost no lymphatic or hematogenous metastases [11, 12]. The initial cancer dissemination is through the wall of the appendix into the peritoneal space [13]. Tumor cells from the ruptured appendiceal neoplasm are spread throughout the peritoneal cavity by the i.p. fluid current and gravity. The absence of adhesive characteristics on the cell surface might explain such passive movement. Accumulation and the reproduction of free and implanted tumor cells leads to progressive peritoneal mucinous tumor and ascites, but invasion of the peritoneal surface usually remains absent. The locoregional progression—also referred to as redistribution phenomenon [14]—of the cancer results in a starvation phase of the disease and death resulting from the failure of intestinal function because of intra-abdominal pressure, fistula formation, or infection [10]. In the past it was a uniformly lethal condition.
Previously, PMP was applied as a pathologic diagnostic term to both benign and malignant mucinous appendiceal neoplasms, resulting in a variable and poor prognosis. With the recognition that a broad spectrum of aggressiveness exists within PMP, Ronnett et al. [9] proposed three pathological subtypes of PMP with different pathological characteristics and different prognoses: (a) disseminated peritoneal adenomucinosis (DPAM), a low-grade lesion that is marked by its abundant extracellular mucin and lack of cytological atypia or mitotic activity, usually derived from mucinous neoplasms of the appendix, and with a good prognosis; (b) peritoneal mucinous adenocarcinoma (PMCA), which is histopathologically a high-grade metastatic adenocarcinoma, usually derived from the appendix and colon, and is characterized by abundant mucinous epithelium with architectural and cytologic features of carcinoma and a grim prognosis; and (c) intermediate type PMP (PMCA-I), which includes those in whom the peritoneal lesions demonstrate predominantly features of DPAM but also contained focal areas of PMCA and a prognosis between that of DPAM and PCMA. In contrast, Misdraji et al. [8] proposed classifying appendiceal mucinous tumors into low-grade appendiceal mucinous neoplasms (LAMNs) and high-grade mucinous adenocarcinomas (MACAs). LAMNs are papillary or flat mucinous tumors with low-grade cytologic atypia analogous to low-grade dysplasia in other parts of the gastrointestinal tract. MACAs are appendiceal tumors with destructive invasion of the appendiceal wall, high-grade cytologic atypia, or complex epithelial proliferation, and with a more aggressive clinical course than that of LAMNs. More recently, Bradley et al. [7] proposed that the only relevant categories are low- and high-grade mucinous carcinoma peritonei based on the clinically malignant behavior of all PMPs. The most widely used histopathologic classification is the one proposed by Ronnett et al. [9].
MCA
MCA is one of the histological subtypes of CRC and accounts for 10%–20% of all colorectal neoplasms [15]. MCA is diagnosed when ≥50% of the tumor comprises a mucinous pattern on histological examination and a large amount of extracellular mucin is produced by secreting acini [16]. The mucin is intraluminal in the case of well-differentiated or moderately differentiated CRC and forms interstitial pools surrounding the irregular trabeculae in poorly differentiated CRC [17]. This subtype of tumor is to be differentiated from signet-ring cell carcinoma, which is constituted by ≥50% single tumor cells with intracytoplasmatic mucin displacing their nuclide acid and is well known for its aggressiveness [18]. Compared with the more common nonmucinous variety, mucinous tumors metastasize to lymph nodes with greater frequency, are more prone to peritoneal carcinomatosis, and are typically diagnosed at an advanced stage [19]. This might be explained by the production of mucus under pressure, which allows the MCA to separate tissue planes in the bowel wall and more frequently gain access to the peritoneal cavity. Moreover, the fluid produced by MCAs is taken up by lymphatics, which might help to promote tumor spread into regional lymph nodes [20].
The prognostic significance of the MCA histological subtype is controversial. In some studies, the MCA histological subtype has been shown to be a negative prognostic factor [19, 21, 22], but not in others [15, 23, 24]. These differing results for the MCA subtype could be explained by the striking geographical variations in the epidemiology of CRC [25], advanced tumor stage at presentation [22], differences in diagnostic histopathological criteria used to define MCA [16, 22, 26, 27], and specific tumor localization (e.g., rectum) [28]. Therefore, some investigators have divided MCAs into two subgroups on the basis of clinicopathological characteristics, genetic pathways, and behavior, which may help to explain the above mentioned conflicting results [29]. Both the American Joint Committee on Cancer and the College of American Pathologists consider that the mucinous subtype has not been proven to be a statistically significant prognostic factor independent of histological grade [30, 31].
Two major molecular genetic pathways in colorectal carcinogenesis can be differentiated—the chromosomal instability pathway and the DNA mismatch repair pathway. The chromosomal instability pathway involves the mutational activation of oncogenes (KRAS) coupled with the loss of several genes that normally suppress tumorigenesis (APC, DCC, p53) [32]. The DNA mismatch repair pathway is associated with CRCs arising from hereditary nonpolyposis colon cancer. The key element of this pathway is dysfunction in DNA mismatch repair enzymes that results from germline mutations in one of several DNA mismatch repair genes, most commonly MLH1 or MSH2. The result is the development of microsatellite instability (MSI) in tumors derived through this genetic pathway [33].
Mucinous adenocarcinomas are characterized by a low occurrence of p53 alterations, a high frequency of MSI (MSI-H), a high frequency of KRAS mutations, and a higher apoptotic index than in corresponding nonmucinous tumors [34]. Based on these genetic differences and the more aggressive behavior, some authors have suggested that an alternate mucinous phenotype–related pathway of carcinogenesis might exist and that MCA should be categorized and treated as a biological entity distinct from other colorectal adenocarcinomas [34, 35]. Moreover, a recent study by Leopoldo et al. [29] identified molecular alterations (e.g., MSI, hMLH1, p27) in subsets of MCAs associated with different clinicopathological and molecular characteristics and different outcomes, suggesting the existence of different subtypes of MCA. The first subtype is characterized by MSI-H, often localized in the proximal colon, frequently associated with altered expression of hMLH1 and p27, and better outcome. The second subtype of MCA is microsatellite stable, more frequently localized in the distal colorectum, shows normal expression of hMLH1 and p27, and has a worse outcome [29]. Another study, by Liu et al. [36], distinguished MCAs according to aneuploid versus diploid status. Further clinicopathological and molecular analyses will help to clarify the role of the two subtypes of MCA.
Management of Gastrointestinal Mucinous Adenocarcinomas with Peritoneal Dissemination
PMP
Patients with PMP conventionally have been treated with repeated interval debulking procedures for relief of symptoms, but with limited expectation of long-term survival and no prospect of cure. In 1994, Gough et al. [37] reported 5- and 10-year survival rates of 53% and 32%, respectively, in 56 patients with PMP treated with serial debulking procedures and selectively treated with i.p. or systemic radiotherapy or chemotherapy. Miner et al. [38] reported a 10- year survival rate of 21% in 97 PMP patients treated with serial debulking, systemic chemotherapy, and/or delayed intermittent i.p. chemotherapy using a 5-fluorouracil (FU)-based agent. Although a subset of patients remain asymptomatic for many years, disease almost always recurs, and repeated debulking procedures become more ineffective. In addition, Yan et al. [39] showed that patients underwent transitions from a less aggressive to a more aggressive histology from one cytoreduction to the next.
Sugarbaker proposed a comprehensive treatment approach involving CRS and HIPEC. The aim of surgery is macroscopic complete cytoreduction and complete lysis of adhesions between the bowel loops in combination with visceral resection to leave tumor deposits <0.25 cm. This is followed by adjuvant HIPEC (commonly mitomycin C, cisplatin, 5-FU, or a combination of these for usually 40–120 minutes) to eradicate any macroscopic or microscopic tumor residue and thus reduce recurrence [40, 41]. Timing and delivery of chemotherapy are critical, because it is given before the formation of any adhesions and allows direct chemotherapy and tumor cell contact.
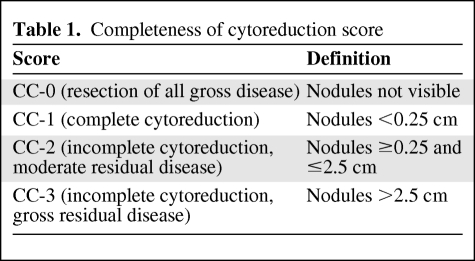
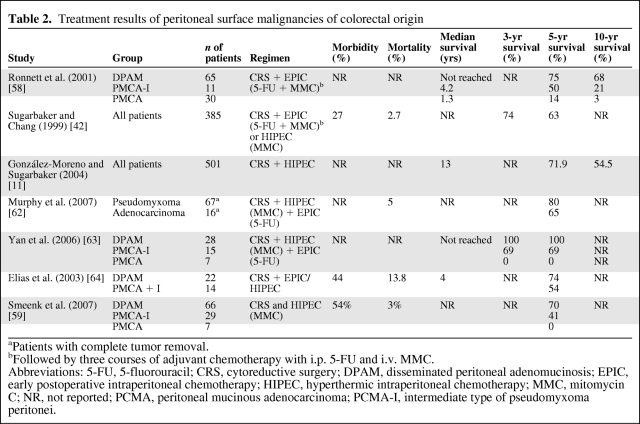
Favorable results with combined modality treatment have been achieved for patients with benign disease (DPAM) and complete cytoreduction, with 5- and 10-year survival rates of approximately 75%–100% and 68%, respectively [9, 10, 41]. However, patients with malignant disease (PMCA) or intermediate disease (PMCA-I) showed a significantly worse prognosis, with 5- and 10-year survival rates, respectively, of 50% and 21% for PMCA-I and 14% and 3% for PMCA [9]. Patients with DPAM seem to benefit most from this approach. However, it remains controversial whether patients with PMCA benefit from this aggressive treatment. Moreover, completeness of cytoreduction (CC score; Table 1) has additional prognostic value and is strongly associated with extent of disease. Patients with extensive PMP are prone to incomplete cytoreduction and a complicated recovery. Sugarbaker et al. [42] reported a significant survival difference (p < .0001) in favor of patients with complete cytoreduction (CC-0 and CC-1), versus incomplete cytoreduction (CC-2 and CC-3) (Table 2).
Table 1.
Completeness of cytoreduction score
Table 2.
Treatment results of peritoneal surface malignancies of colorectal origin
aPatients with complete tumor removal.
bFollowed by three courses of adjuvant chemotherapy with i.p. 5-FU and i.v. MMC.
Abbreviations: 5-FU, 5-fluorouracil; CRS, cytoreductive surgery; DPAM, disseminated peritoneal adenomucinosis; EPIC, early postoperative intraperitoneal chemotherapy; HIPEC, hyperthermic intraperitoneal chemotherapy; MMC, mitomycin C; NR, not reported; PCMA, peritoneal mucinous adenocarcinoma; PCMA-I, intermediate type of pseudomyxoma peritonei.
Although combined modality treatment has shown favorable results in a subset of patients with PMP, the aggressiveness of this treatment strategy and concomitant high morbidity and mortality rates are probably the main reasons for skepticism. In a systematic review by Yan et al. [41], the overall morbidity rate varied in the range of 33%–56% and the overall mortality was in the range of 0%–18%. Most frequent serious complications were small bowel perforation and suture leaks resulting in abscesses and fistula. However, the experience of the center has a strong prognostic impact [43].
The efficacy and possible benefit of systemic therapy in PMP might be diminished by the locoregional spread of well-differentiated tumor with a poor blood supply. However, two recently reported studies have suggested benefit to patients with PMP who were considered surgically unresectable. Farquharson et al. [44] found, in a prospective analysis including 39 PMP patients treated with a combination of capecitabine and mitomycin C, 15 patients (38%) with a response or stable disease. Furthermore, Shapiro and colleagues reported on a subset of 54 of 186 patients with appendiceal neoplasms considered to be suboptimal surgical candidates and therefore treated with modern systemic chemotherapy. That retrospective analysis indicated prolonged disease control of 7.6 months in patients who were deemed suboptimal candidates for CRS and/or HIPEC [45]. In summary, the primary modality of treatment for patients with PMP is CRS followed by HIPEC. Systemic chemotherapy may provide a benefit for patients who are not optimal surgical candidates (e.g., high tumor burden, comorbidities, grossly residual disease after prior CRS) but is still considered as a palliative treatment in patients with recurrent or progressive disease [10, 46].
MCA
Peritoneal carcinomatosis (PC) of colorectal origin is common, occurring in 10%–15% of patients at initial diagnosis, and the second most frequent cause of death after metastatic disease to the liver. Moreover, 25% of patients with recurrence have disease confined to the peritoneal cavity [47]. PC arising from CRC has long been considered as a generalized disease and has been treated with systemic chemotherapy. The most significant reports investigating the prognosis of isolated PC of colorectal origin with chemotherapy included 50–392 patients and showed a median survival time of 5.2–12.6 months [1, 2, 47, 48]. Modern systemic therapy has improved the median survival time for those patients with hematogenous dissemination, but the role of these newer combinations of cytotoxic chemotherapy and biological agents remains undefined in patients with stage IV MCA with metastatic disease confined to the peritoneum.
Sugarbaker has suggested PC of colorectal origin as transcoelomic invasion by the primary cancer or i.p. seeding during surgical or radiological interventions for diagnosis or treatment. Therefore, PC should be regarded as locoregional extension of disease rather than another manifestation of systemic metastasis [49]. MCAs and non-MCAs have similar patterns of hematogenous and lymphatic metastasis, but their peritoneal surface distribution is different. In contrast, MCAs present patterns of peritoneal surface distribution similar to those of PMP [5]. Therefore, CRS and i.p. chemotherapy might be a potential treatment option for the particular group of patients with stage IV MCAs with metastatic disease confined to the peritoneum.
However, no data are currently available for this particular group of patients. Nevertheless, an increasing number of international treatment centers have published their results using CRS and HIPEC in the management of peritoneal surface malignancies of colorectal origin.
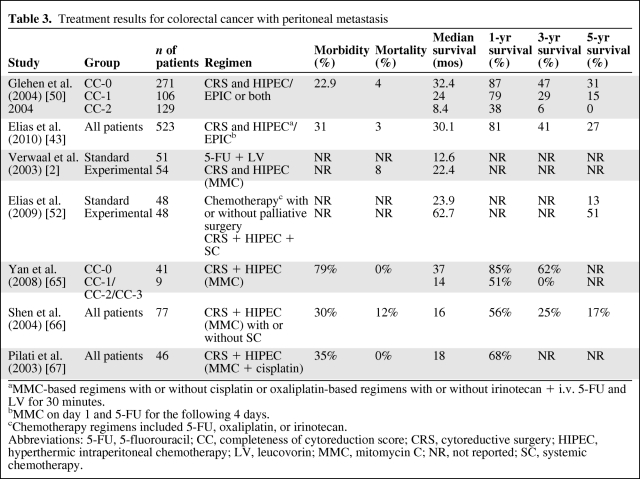
In a retrospective multicenter study by Glehen et al. [50] of CRS combined with perioperative i.p. chemotherapy for the management of PC of CRC, 506 patients were analyzed. Patients in whom CRS was complete (CC-0 and CC-1) had a median survival time of 32.4 months (5-year survival rate, 31%), compared with 8.4 months (3-year survival rate, 6%) for incomplete cytoreduction (CC-2 and CC-3; p < .001). The median overall survival time was 19.2 months and the overall 5-year survival rate was 19%. Recently, Elias et al. [43] performed a retrospective-cohort, multicenter French study of peritoneal colorectal carcinomatosis treated with surgery and perioperative i.p. chemotherapy including 523 patients. That study confirmed the results obtained by Glehen et al. [50], with a median overall survival time of 30.1 months and an overall 5-year survival rate of 27%. The mortality and morbidity rates were low in this study, at 3% and 23%, respectively. These are the two largest series of patients with PC from CRC treated with combined CRS and HIPEC, but they are likely to host large heterogeneity in patients and treatment (e.g., selection criteria, chemotherapy doses, different levels of experience, and different techniques).
To evaluate selection factors that have been used to limit patients treated with CRS and HIPEC, a phase III study was initiated. Verwaal and colleagues preoperatively randomized patients with known colorectal carcinomatosis to standard treatment with palliative surgery followed by systemic fluorouracil and leucovorin or treatment with maximal CRS with HIPEC [2]. The recently published 8-year follow-up data showed median survival times of 12.6 months in the standard arm and 22.2 months in the experimental arm (p = .028) and a 5-year overall survival rate of 45% for those patients for whom a complete cytoreduction (CC-0 and CC-1) could be achieved [51]. The trial was stopped early because of the large survival difference in favor of HIPEC.
Finally, Elias et al. [52] compared the long-term survival of CRC patients with isolated and resectable PC in comparable groups of patients treated with systemic chemotherapy including oxaliplatin or irinotecan (standard group) and with CRS plus HIPEC and systemic chemotherapy (SC). The median survival time was 23.9 months in the standard group, versus 62.7 months in the CRS + HIPEC + SC group (p < .05), and the 5-year overall survival rates were 13% versus 51%, respectively. Moreover, there was no statistically significant difference in survival rates between HIPEC and early postoperative intraperitoneal chemotherapy (EPIC). However, this was a nonrandomized cohort study, which might imply a potential selection bias, as mentioned by the authors (Table 3).
Table 3.
Treatment results for colorectal cancer with peritoneal metastasis
aMMC-based regimens with or without cisplatin or oxaliplatin-based regimens with or without irinotecan + i.v. 5-FU and LV for 30 minutes.
bMMC on day 1 and 5-FU for the following 4 days.
cChemotherapy regimens included 5-FU, oxaliplatin, or irinotecan.
Abbreviations: 5-FU, 5-fluorouracil; CC, completeness of cytoreduction score; CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; LV, leucovorin; MMC, mitomycin C; NR, not reported; SC, systemic chemotherapy.
The effect of mucinous histology on treatment response and survival was recently examined by Negri et al. [53]. In that study, patients with CRC had a lower response rate to 5-FU (22% versus 47%) and shorter survival time (11.8 months versus 17.9 months) than patients with nonmucinous CRC. Moreover, Catalano et al. [54] reported, in patients with mucinous CRC treated with irinotecan and/or oxaliplatin-based chemotherapy, a significantly lower response rate and overall survival time than in patients with nonmucinous CRC (18.4% versus 49% and 14 months versus 23.4 months, respectively).
Currently, no studies focusing on outcome for different molecular subtypes of MCA with metastatic disease confined to the peritoneum are available. However, molecular markers were examined for differences in response of mucinous and nonmucinous CRCs. Glasgow et al. [35] reported that the thymidylate synthase (TS) and GSTP1 genes were significantly overexpressed in mucinous tumors. Numerous studies have demonstrated that overexpression of TS correlates with poor response to 5-FU [55–57]. In addition, GSTP1 belongs to the glutathione S-transferase superfamily and is a major mechanism of detoxification of platinum agents. The information derived from these studies may help clinicians in making decisions that will improve the outcome of patients with mucinous CRC.
Discussion
Improved treatment modalities, insights into the mechanisms of i.p. spread, and differences in histopathologic types of mucinous adenocarcinomas have contributed to a better understanding and a different perception of mucinous CRC and PMP. Despite exciting results for combined treatment involving CRS and HIPEC, the results should be interpreted with caution.
For instance, there is ongoing controversy about the pathological features of PMP defined by Ronnett et al. [9]. A group of patients who had surgery for PMP were categorized into three histological subcategories and the histopathologic differences determined the prognosis of these patients. Most, but not all, centers use the criteria of Ronnett et al. [9]; however, there is a great degree of subjectivity, as pointed out by Yan et al. [39]. Because of common concurrent findings of PMCA within DPAM, the exact pathology and subsequent behavior of PMP are difficult to assess and make the classification more subjective. Moreover, confusion still exists because of different histopathologic nomenclature. In the past, designations such as malignant mucoceles, colloid carcinomas, or cystadenocarcinoma were used, and recently, Misdraji et al. [8] and Bradley et al. [7] proposed new histopathologic classifications. Many reports have included more indolent histologic findings, and this heterogeneity makes conclusions problematic. However, survival analysis of the different subtypes proposed by Ronnett et al. [9] has shown that DPAM patients are most likely to benefit from a combined treatment regimen, whereas PMCA patients should be classified and treated as having PC of nonmucinous colorectal origin because they do not seem to benefit from aggressive treatment [58, 59]. Despite the fact that much progress has been made, there is need for a prospective multi-institutional trial to standardize the histopathologic classification of PMP.
Although MCA is a well-recognized subtype of CRC by the World Health Organization classification, the prognostic impact and the genetic mechanism by which the mucinous phenotype arises are still matters of controversy. Some studies have reported that variations in survival could be related to differences in tumor location or stage at presentation rather than to histologic type [30]. Others have suggested that the MCA histologic subtype is an independent predictor of poor outcome, because patients with these tumors are more likely to have peritoneal metastasis and lymph node involvement than those with nonmucinous adenocarcinomas [19]. Both the American Joint Committee on Cancer and the College of American Pathologists consider that the mucinous subtype has not been proven to be a statistically significant prognostic factor independent of histological grade [30, 31]. Recent studies have proposed that MCA include two molecular subtypes, reflecting distinct clinicopathological and phenotypic characteristics [29, 60]. These differences could have potential influences on therapeutic choices, considering different responses with distinct genetic profiles. Moreover, we lack data investigating the outcome of patients with mucinous CRC treated with novel agents (anti–vascular endothelial growth factor receptor or anti–epidermal growth factor receptor monoclonal antibodies) in combination with traditional drugs (5-FU, oxaliplatin, or irinotecan).
The main criticism against combined treatment is the lack of standardized treatment techniques. Most centers are currently using HIPEC, whereas only a few are using EPIC. Both procedures are not standardized and many variations exist in exposure techniques, drugs, drug doses, duration, temperature, and flow rates, which may contribute to the differences in the results [6]. Moreover, surgical treatments are even less standardized than drug treatments and are dependent on the skill and level of experience of the surgeon [61]. In addition, the overall morbidity rate has varied in the range of 33%–56% and the overall mortality rate has been in the range of 0%–18%, and thus also dependent on the experience of the center's surgeon [41]. Therefore, results achieved by international experts may not be replicated in routine clinical practice. Nevertheless, the use of perioperative i.p. chemotherapy and surgical treatment needs to be standardized and validated by randomized studies.
Finally, proper patient selection is essential to benefit from combined CRS and i.p. chemotherapy. The outcome of patients with advanced disease or incomplete cytoreduction is poor and they are not likely to benefit from this major treatment. However, patients with certain histological subtypes who have minimal residual disease isolated to peritoneal surfaces accessible to chemotherapy and an absence of systemic metastasis may benefit.
Future Directions
The novel therapeutic approach of combining CRS and HIPEC may be the most promising new treatment for patients with mucinous gastrointestinal adenocarcinomas with metastatic disease confined to the peritoneum. This treatment strategy offers hope for cure in a disease that has, in the past, been regarded as a terminal event. However, careful patient selection is critical and perioperative i.p. chemotherapy and surgical treatment need to be standardized and validated by randomized studies. The exact role of chemotherapy remains to be elucidated. In addition, future studies should include predictive and prognostic molecular markers trying to identify subsets of patients who are likely to derive the most benefit from a particular treatment.
Acknowledgments
Thomas Winder is supported in part by a research grant of the Austrian Society of Hematology and Oncology and the Kurt und Senta-Herrmann Stiftung.
Author Contributions
Conception/Design: Thomas Winder, Heinz-Josef Lenz
Collection and/or assembly of data: Thomas Winder
Manuscript writing: Thomas Winder, Heinz-Josef Lenz
Final approval of manuscript: Thomas Winder, Heinz-Josef Lenz
References
- 1.Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker PH, Graves T, DeBruijn EA, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: Pharmacological studies. Cancer Res. 1990;50:5790–5794. [PubMed] [Google Scholar]
- 4.Sugarbaker PH, Landy D, Pascal R. Intraperitoneal chemotherapy for peritoneal carcinomatosis from colonic or appendiceal cystadenocarcinoma: Rationale and results of treatment. Prog Clin Biol Res. 1990;354B:141–170. [PubMed] [Google Scholar]
- 5.Carmignani CP, Sugarbaker TA, Bromley CM, et al. Intraperitoneal cancer dissemination: Mechanisms of the patterns of spread. Cancer Metastasis Rev. 2003;22:465–472. doi: 10.1023/a:1023791229361. [DOI] [PubMed] [Google Scholar]
- 6.Elias DM, Ouellet JF. Intraperitoneal chemohyperthermia: Rationale, technique, indications, and results. Surg Oncol Clin N Am. 2001;10:915–933. xi. [PubMed] [Google Scholar]
- 7.Bradley RF, Stewart JH, 4th, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: A clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30:551–559. doi: 10.1097/01.pas.0000202039.74837.7d. [DOI] [PubMed] [Google Scholar]
- 8.Misdraji J, Yantiss RK, Graeme-Cook FM, et al. Appendiceal mucinous neoplasms: A clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27:1089–1103. doi: 10.1097/00000478-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Ronnett BM, Zahn CM, Kurman RJ, et al. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei.”. Am J Surg Pathol. 1995;19:1390–1408. doi: 10.1097/00000478-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Smeenk RM, Verwaal VJ, Zoetmulder FA. Pseudomyxoma peritonei. Cancer Treat Rev. 2007;33:138–145. doi: 10.1016/j.ctrv.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 11.González-Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;91:304–311. doi: 10.1002/bjs.4393. [DOI] [PubMed] [Google Scholar]
- 12.Sugarbaker PH. Pseudomyxoma peritonei. Cancer Treat Res. 1996;81:105–119. doi: 10.1007/978-1-4613-1245-1_10. [DOI] [PubMed] [Google Scholar]
- 13.Sugarbaker PH. Epithelial appendiceal neoplasms. Cancer J. 2009;15:225–235. doi: 10.1097/PPO.0b013e3181a9c781. [DOI] [PubMed] [Google Scholar]
- 14.Sugarbaker PH. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg. 1994;219:109–111. doi: 10.1097/00000658-199402000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green JB, Timmcke AE, Mitchell WT, et al. Mucinous carcinoma—just another colon cancer? Dis Colon Rectum. 1993;36:49–54. doi: 10.1007/BF02050301. [DOI] [PubMed] [Google Scholar]
- 16.Symonds DA, Vickery AL. Mucinous carcinoma of the colon and rectum. Cancer. 1976;37:1891–1900. doi: 10.1002/1097-0142(197604)37:4<1891::aid-cncr2820370439>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 18.Nissan A, Guillem JG, Paty PB, et al. Signet-ring cell carcinoma of the colon and rectum: A matched control study. Dis Colon Rectum. 1999;42:1176–1180. doi: 10.1007/BF02238570. [DOI] [PubMed] [Google Scholar]
- 19.Nozoe T, Anai H, Nasu S, et al. Clinicopathological characteristics of mucinous carcinoma of the colon and rectum. J Surg Oncol. 2000;75:103–107. doi: 10.1002/1096-9098(200010)75:2<103::aid-jso6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Sugarbaker PH. Mucinous colorectal carcinoma. J Surg Oncol. 2001;77:282–283. doi: 10.1002/jso.1111. [DOI] [PubMed] [Google Scholar]
- 21.Secco GB, Fardelli R, Campora E, et al. Primary mucinous adenocarcinomas and signet-ring cell carcinomas of colon and rectum. Oncology. 1994;51:30–34. doi: 10.1159/000227306. [DOI] [PubMed] [Google Scholar]
- 22.Umpleby HC, Ranson DL, Williamson RC. Peculiarities of mucinous colorectal carcinoma. Br J Surg. 1985;72:715–718. doi: 10.1002/bjs.1800720915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Consorti F, Lorenzotti A, Midiri G, et al. Prognostic significance of mucinous carcinoma of colon and rectum: A prospective case-control study. J Surg Oncol. 2000;73:70–74. doi: 10.1002/(sici)1096-9098(200002)73:2<70::aid-jso3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Enriquez JM, Díez M, Tobaruela E, et al. Clinical, histopathological, cytogenetic and prognostic differences between mucinous and nonmucinous colorectal adenocarcinomas. Rev Esp Enferm Dig. 1998;90:563–572. [PubMed] [Google Scholar]
- 25.Levin KE, Dozois RR. Epidemiology of large bowel cancer. World J Surg. 1991;15:562–567. doi: 10.1007/BF01789199. [DOI] [PubMed] [Google Scholar]
- 26.Kanemitsu Y, Kato T, Hirai T, et al. Survival after curative resection for mucinous adenocarcinoma of the colorectum. Dis Colon Rectum. 2003;46:160–167. doi: 10.1007/s10350-004-6518-0. [DOI] [PubMed] [Google Scholar]
- 27.Wu CS, Tung SY, Chen PC, et al. Clinicopathological study of colorectal mucinous carcinoma in Taiwan: A multivariate analysis. J Gastroenterol Hepatol. 1996;11:77–81. doi: 10.1111/j.1440-1746.1996.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki O, Atkin WS, Jass JR. Mucinous carcinoma of the rectum. Histopathology. 1987;11:259–272. doi: 10.1111/j.1365-2559.1987.tb02631.x. [DOI] [PubMed] [Google Scholar]
- 29.Leopoldo S, Lorena B, Cinzia A, et al. Two subtypes of mucinous adenocarcinoma of the colorectum: Clinicopathological and genetic features. Ann Surg Oncol. 2008;15:1429–1439. doi: 10.1245/s10434-007-9757-1. [DOI] [PubMed] [Google Scholar]
- 30.Compton C, Fenoglio-Preiser CM, Pettigrew N, et al. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739–1757. doi: 10.1002/(sici)1097-0142(20000401)88:7<1739::aid-cncr30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 31.Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–994. doi: 10.5858/2000-124-0979-PFICC. [DOI] [PubMed] [Google Scholar]
- 32.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 33.Noffsinger AE. Serrated polyps and colorectal cancer: New pathway to malignancy. Annu Rev Pathol. 2009;4:343–364. doi: 10.1146/annurev.pathol.4.110807.092317. [DOI] [PubMed] [Google Scholar]
- 34.Hanski C. Is mucinous carcinoma of the colorectum a distinct genetic entity? Br J Cancer. 1995;72:1350–1356. doi: 10.1038/bjc.1995.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glasgow SC, Yu J, Carvalho LP, et al. Unfavourable expression of pharmacologic markers in mucinous colorectal cancer. Br J Cancer. 2005;92:259–264. doi: 10.1038/sj.bjc.6602330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu XP, Sato T, Oga A, et al. Two subtypes of mucinous colorectal carcinoma characterized by laser scanning cytometry and comparative genomic hybridization. Int J Oncol. 2004;25:615–621. [PubMed] [Google Scholar]
- 37.Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219:112–119. doi: 10.1097/00000658-199402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miner TJ, Shia J, Jaques DP, et al. Long-term survival following treatment of pseudomyxoma peritonei: An analysis of surgical therapy. Ann Surg. 2005;241:300–308. doi: 10.1097/01.sla.0000152015.76731.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan H, Pestieau SR, Shmookler BM, et al. Histopathologic analysis in 46 patients with pseudomyxoma peritonei syndrome: Failure versus success with a second-look operation. Mod Pathol. 2001;14:164–171. doi: 10.1038/modpathol.3880276. [DOI] [PubMed] [Google Scholar]
- 40.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan TD, Black D, Savady R, et al. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2007;14:484–492. doi: 10.1245/s10434-006-9182-x. [DOI] [PubMed] [Google Scholar]
- 42.Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727–731. doi: 10.1007/s10434-999-0727-7. [DOI] [PubMed] [Google Scholar]
- 43.Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 44.Farquharson AL, Pranesh N, Witham G, et al. A phase II study evaluating the use of concurrent mitomycin C and capecitabine in patients with advanced unresectable pseudomyxoma peritonei. Br J Cancer. 2008;99:591–596. doi: 10.1038/sj.bjc.6604522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro JF, Chase JL, Wolff RA, et al. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: A single-institution experience. Cancer. 2010;116:316–322. doi: 10.1002/cncr.24715. [DOI] [PubMed] [Google Scholar]
- 46.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76. doi: 10.1016/S1470-2045(05)70539-8. [DOI] [PubMed] [Google Scholar]
- 47.Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 48.Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364–367. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 49.Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol. 1998;14:254–261. doi: 10.1002/(sici)1098-2388(199804/05)14:3<254::aid-ssu10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 50.Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J Clin Oncol. 2004;22:3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 52.Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 53.Negri FV, Wotherspoon A, Cunningham D, et al. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol. 2005;16:1305–1310. doi: 10.1093/annonc/mdi244. [DOI] [PubMed] [Google Scholar]
- 54.Catalano V, Loupakis F, Graziano F, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer. 2009;100:881–887. doi: 10.1038/sj.bjc.6604955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnston PG, Lenz HJ, Leichman CG, et al. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995;55:1407–1412. [PubMed] [Google Scholar]
- 56.Leichman CG, Lenz HJ, Leichman L, et al. Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted-infusion fluorouracil and weekly leucovorin. J Clin Oncol. 1997;15:3223–3229. doi: 10.1200/JCO.1997.15.10.3223. [DOI] [PubMed] [Google Scholar]
- 57.Lenz HJ, Leichman CG, Danenberg KD, et al. Thymidylate synthase mRNA level in adenocarcinoma of the stomach: A predictor for primary tumor response and overall survival. J Clin Oncol. 1996;14:176–182. doi: 10.1200/JCO.1996.14.1.176. [DOI] [PubMed] [Google Scholar]
- 58.Ronnett BM, Yan H, Kurman RJ, et al. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer. 2001;92:85–91. doi: 10.1002/1097-0142(20010701)92:1<85::aid-cncr1295>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 59.Smeenk RM, Verwaal VJ, Antonini N, et al. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245:104–109. doi: 10.1097/01.sla.0000231705.40081.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li D, Semba S, Wu M, et al. Molecular pathological subclassification of mucinous adenocarcinoma of the colorectum. Pathol Int. 2005;55:766–774. doi: 10.1111/j.1440-1827.2005.01903.x. [DOI] [PubMed] [Google Scholar]
- 61.Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg. 2007;94:1408–1414. doi: 10.1002/bjs.5863. [DOI] [PubMed] [Google Scholar]
- 62.Murphy EM, Sexton R, Moran BJ. Early results of surgery in 123 patients with pseudomyxoma peritonei from a perforated appendiceal neoplasm. Dis Colon Rectum. 2007;50:37–42. doi: 10.1007/s10350-006-0741-9. [DOI] [PubMed] [Google Scholar]
- 63.Yan TD, Links M, Xu ZY, et al. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal mucinous neoplasms. Br J Surg. 2006;93:1270–1276. doi: 10.1002/bjs.5427. [DOI] [PubMed] [Google Scholar]
- 64.Elias D, Laurent S, Antoun S, et al. [Pseudomyxoma peritonei treated with complete resection and immediate intraperitoneal chemotherapy] Gastroenterol Clin Biol. 2003;27:407–412. In French. [PubMed] [Google Scholar]
- 65.Yan TD, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for isolated colorectal peritoneal carcinomatosis: Experimental therapy or standard of care? Ann Surg. 2008;248:829–835. doi: 10.1097/SLA.0b013e31818a15b5. [DOI] [PubMed] [Google Scholar]
- 66.Shen P, Hawksworth J, Lovato J, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11:178–186. doi: 10.1245/aso.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Pilati P, Mocellin S, Rossi CR, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol. 2003;10:508–513. doi: 10.1245/aso.2003.08.004. [DOI] [PubMed] [Google Scholar]