A case of gastric ulceration from aberrant deposition of microspheres with significant consequences despite detailed pretreatment screening, investigation, and patient selection by experienced clinicians is reported. The mechanisms of gastrointestinal complications and recommendations for prevention are reviewed with reference to the current literature.
Keywords: Liver neoplasms, Radiopharmaceuticals, Peptic ulcer, Literature review
Abstract
The use of selective internal radiation therapy (SIRT) with SIR-Spheres® (Sirtex, Sydney, Australia) is increasingly recognized as a potential therapeutic modality of primary and secondary malignant liver tumors. A number of treatment-related complications have been described despite technical expertise and detailed pretreatment investigations to assess suitability. We describe a case of gastric ulceration from nontargeted deposition of SIR-spheres® in the gastric mucosa with life-threatening consequences. This case highlights the need for careful screening and appropriate patient selection, and the need to recognize ulceration from SIRT as a potential complication of treatment. The characteristic endoscopic, radiologic, and histopathologic findings are illustrated and recommendations are reviewed with regard to the current literature.
Case Report
Selective internal radiation therapy (SIRT) with yttrium-90 (90Y)-emitting microspheres is increasingly recognized as an effective therapy of both primary and secondary hepatic malignancies [1–5]. Increasing reports have shown this to be a useful treatment for unresectable hepatic metastases from colorectal carcinoma [6], neuroendocrine tumors [7], and primary hepatocellular carcinoma [3, 8, 9]. A number of treatment-related complications have been described despite technical expertise and detailed pretreatment investigations to assess suitability. We describe a case of gastric ulceration from aberrant deposition of SIR-Spheres® (Sirtex, Sydney, Australia) in the gastric mucosa with life-threatening consequences.
The patient, a 63-year-old male with metastatic colorectal cancer, received SIRT 17 weeks earlier at another institution for recurrent, inoperable hepatic metastatic disease. The original diagnosis of rectosigmoid adenocarinoma was made 3 years earlier following an episode of infective endocarditis with Streptococcus viridians.
Dual aortic and mitral valve replacements were undertaken with metallic prostheses and, following resolution of cardiac failure, a low anterior resection was performed for a T3N1 tumor without complications. Subsequent staging with positron emission tomography (PET), computed tomography (CT), and magnetic resonance imaging (MRI) scans of the liver revealed two hepatic metastases confined to the right lobe (segments 7 + 8) that were initially unresectable and no extrahepatic disease. The patient declined oxaliplatin because of the risks for neurotoxicity interfering with his occupation; however, the hepatic metastases were successfully resected following eight cycles of chemotherapy with irinotecan, 5-fluorouracil, leucovorin, and bevacizumab on the First-BEAT trial [10]. The patient declined further chemotherapy post hemi-hepatectomy and was well and disease free for 18 months.
Thirty months following his original diagnosis, he was found to have asymptomatic hepatic-isolated recurrence of disease that was deemed inoperable after investigation with PET and MRI. The patient declined further systemic therapy in favor of treatment with SIR-Spheres®.
Prior to receiving SIRT, pretreatment hepatic angiography, CT hepatic arteriography, and a macroaggregated albumin scan through the indwelling catheter was performed to evaluate for liver-to-lung shunts and remaining vascular connections between the liver and gastrointestinal tract. Liver function tests were conducted to establish adequate liver function prior to implantation. When the pretreatment angiography was performed, the gastroduodenal artery (GDA) was coil embolized to prevent nontargeted flow to the bowel because the GDA was situated in close approximation to the origin of the left hepatic artery. Ten days later, the hepatic angiogram was repeated and a whole liver implantation with 1.70 Gbq of 90Y SIR-Spheres® was performed. The patient took esomeprazole, 20 mg twice daily, for one month following the procedure.
Seventeen weeks after receiving SIRT, the patient presented to the emergency department with a 3-day history of melaena, increasing lethargy, and presyncope. He was taking warfarin for his metallic cardiac valve prostheses. On admission, his hemoglobin (Hb) was 44 g/l, international normalized ratio (INR) was 4.2, platelet count was 426 × 109/l (normal range, 150–450), albumin was low at 22 g/l (normal range, 33–48), and γ-glutamyl transferase was 97 U/l (normal range, 0–50), but other liver function tests, including bilirubin, were within normal limits. He was hemodynamically unstable and an urgent gastroscopy was performed, which revealed a 2-cm oozing linear ulcer at the pylorus. Hemostasis was achieved with adrenaline injection, and his INR was lowered with fresh frozen plasma and maintained in the range of 1.5–2.0 with i.v. unfractionated heparin. In total, eight units of packed RBCs were transfused during the admission. He was discharged on the fifth day after admission following resolution of bleeding and reinstitution of warfarin. On the day of discharge, his INR was 2.7 and Hb was 130 g/l. Serology for Helicobacter pylori was negative.
Thirteen days after discharge, the patient represented with melaena of 2 days duration. On admission, his INR was 1.8, Hb was 100 g/l and platelet count was 459 × 109/l (normal range, 150–450). Infusion of a proton pump inhibitor was commenced and a gastroscopy was performed within 24 hours. The area of anteropyloroduodenal ulceration with active bleeding was again visualized. This was treated with thermocoagulation and hemostasis was achieved. However, on the fourth postprocedure day the patient had further episodes of melaena, and over the next 2 weeks he continued to have active upper gastrointestinal bleeding with a labile INR of 1.8–3.6. A further gastroscopy was performed on day 17 of the admission. Three raised, pigmented vessels were visualized after an overlying fresh clot was washed away. Two vessels in the prepyloric portion and one in the pyloric channel were coagulated with a gold probe, and two endoclips were deployed in the prepylorus. The duodenum was normal.
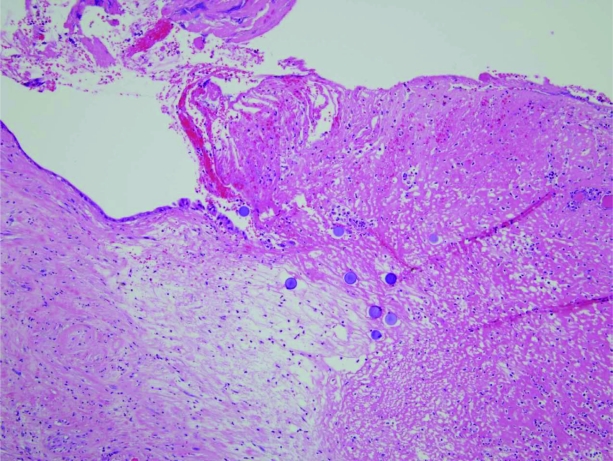
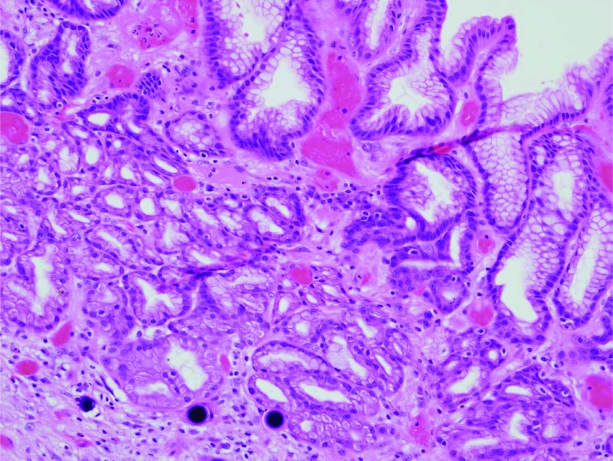
Despite maintaining an INR in the range of 1.8–2.5, the patient experienced further major bleeding, and 6 weeks after his initial presentation with melaena a palliative partial gastrectomy was performed. In a slide from the gastrectomy, SIR-Spheres® can clearly be seen within the gastric mucosa in close proximity to a clean-based ulcer at 10× magnification (Fig. 1). In the second slide (Fig. 2, hematoxylin and eosin stained, 20× magnification), multiple round, purple orbs are visualized within lamina propria vessels within the gastric mucosa.
Figure 1.
Gastrectomy specimen. SIR-Spheres® can clearly be seen within the gastric mucosa in close proximity to a clean-based ulcer. Magnification, 10×; hematoxylin and eosin stain.
Figure 2.
Gastrectomy specimen. SIR-Spheres® are visualized as round, purple orbs within lamina propria vessels in the gastric mucosa. Magnification, 20×; hematoxylin and eosin stain.
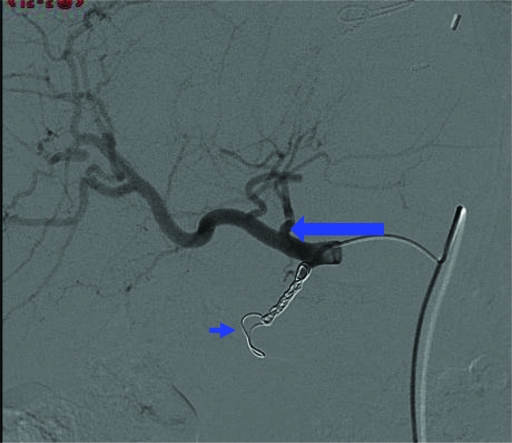
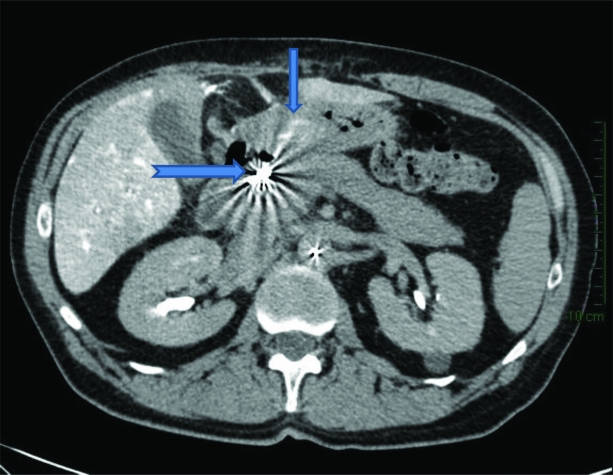
On review of the pretreatment hepatic arteriograms (Fig. 3) and CT hepatic angiogram (Fig. 4), an accessory right gastric artery branching off the base of the left hepatic artery was identified, allowing passage of 90Y 90 SIR-Spheres® into gastric mucosa. In Figure 3, the accessory right gastric artery (large arrow) is seen branching off the left hepatic artery, with coils seen in place in the GDA (short arrow). Retrospectively, enhancement of the gastric mucosa was appreciated on the CT hepatic angiogram (Fig. 4, small arrow), with coils seen in situ in the GDA (Fig. 4, large arrow).
Figure 3.
Pretreatment hepatic arteriogram illustrating the accessory right gastric artery (large arrow) branching off the left hepatic artery, with coils in place in the gastroduodenal artery (short arrow).
Figure 4.
Computed tomography hepatic angiogram revealing enhancement of the gastric mucosa (small arrow) with coils seen in situ in the gastroduodenal artery (large arrow).
Although no acute perioperative morbidity occurred, the subsequent 2-week postoperative period was complicated by ongoing fever, poor wound healing, and hospital-acquired pneumonia. A transesophageal echocardiogram excluded bacterial endocarditis as a cause for the fever, and multiple blood cultures were negative. Anticoagulation was achieved with i.v. unfractionated heparin with a target activated partial thromboplastin time of 40–60 seconds.
Throughout this period, his liver function tests gradually worsened in a mixed pattern. Four weeks after gastrectomy, an abdominal ultrasound was performed that confirmed intrahepatic disease progression. At that point, the patient had an Eastern Cooperative Oncology Group (ECOG) performance status score of 3 and was unfit for further systemic treatment. He was discharged home for palliation and died 32 weeks after treatment with SIRT.
Discussion
SIR-Spheres® consist of microspheres containing 90Y, a high-energy pure β-emitting isotope with a range of tissue penetrance of 2.5–11 mm [11]. The spheres are 20–30 μm in diameter and their size allows them to become preferentially lodged in the microvasculature of the tumor. Combined with selective angiography, this allows focused delivery of ionizing radiation to tumors, which derive their blood supply almost exclusively from the hepatic artery [12], although some irradiation of surrounding normal tissue does occur.
Used alone or in combination with systemic chemotherapy, SIR-Spheres® are approved for use in a number of countries, including Australia, for the treatment of inoperable primary or secondary neoplasms of the liver. Recent reports have described their use as monotherapy for inoperable hepatoma [3, 5], as well as in combination with chemotherapy for unresectable hepatic metastases from colorectal cancer [1]. Although potentially an effective therapy, a number of complications can occur, even in the hands of an experienced operator. Gastrointestinal side effects are common, ranging from nausea, epigastric discomfort, vomiting, and anorexia [13] to cholecystitis, hepatitis, and gastroduodenal ulceration [14–16]. However, the majority of side effects are mild and serious complications are uncommon in appropriately selected patients, with several large series documenting the feasibility of delivering this treatment to a wide variety of patients, including a significant number of elderly patients (>65 years) and those with an ECOG performance status score >1 [4, 17].
Prior to treatment, it is recommended that all patients undergo routine assessments to exclude those individuals at high risk for serious or potentially fatal complications, such as hepatic decompensation, radiation pneumonitis, or nontargeted flow to the gastrointestinal tract [12, 14, 16, 18]. A diagnostic hepatic angiogram is performed to define aberrant vascular anatomy and embolize any anomalous arterial branches of the hepatic arteries that may provide connections between the liver and gastrointestinal tract [19]. Nonhepatic arteries supplying the antrum of the stomach or duodenum frequently arise from the hepatic arteries or its branches, as in this patient. One reported angiographic series of 250 patients describes the prevalence of right gastric artery and accessory left gastric artery arising from hepatic arteries as 78.4% and 17.2% of patients, respectively [20].
Despite a detailed pretreatment angiography, cases of duodenal ulceration from inadvertent deposition of SIR-Spheres® have been reported in the literature [21, 22], with reported incidence rates of 0%–20% (median, 8%) [12, 13, 16]. The two largest series of patients with secondary liver tumors from colorectal or other malignancies reported rates of grade 3 gastrointestinal ulceration of 1%–2% [4, 17]. Cases have been reported both with and without the use of prophylactic proton pump inhibitors, with ulcers most commonly occurring in the pylorus, antrum, or duodenum [12, 13, 16]. Aside from aberrant vasculature, no series have identified other risk factors for ulceration, although cases with poor outcomes have described other comorbidities complicating treatment options, as with our case.
When gastric or duodenal ulceration is attributed to SIRT, it occurs as a consequence of indirect irradiation or direct deposition of 90Y microspheres. In patients with nonhealing ulceration after SIRT, biopsy can help differentiate between the two pathologies. The characteristic appearance of perfectly round, purple microspheres under light microscopy, as seen in Figures 1 and 2, is pathognomonic of this type of injury. The absence of lamellation differentiates the spheres from psammoma bodies [12, 23, 24].
In our patient, aberrant microsphere deposition occurred despite detailed pretreatment investigations. The combination of nonhealing pyloric ulceration together with an ongoing need for anticoagulation for prevention of thromboembolism with dual metallic cardiac valves resulted in significant, near fatal toxicity. Our case illustrates the need to consider the potential side effects of treatment in their differential diagnoses, even in well-screened and appropriately selected patients.
Acknowledgments
We thank Dr. A. Kasim Ismail, Registrar, Anatomical Pathology Department, Prince of Wales Hospital, Sydney, Australia, for the illustrations.
Author Contributions
Conception/Design: Katrin M. Sjoquist, David Goldstein
Provision of study material or patients: David Goldstein, Lourens Bester
Collection and/or assembly of data: Katrin M. Sjoquist, Lourens Bester
Manuscript writing: Katrin M. Sjoquist, David Goldstein, Lourens Bester
Final approval of manuscript: Katrin M. Sjoquist, David Goldstein, Lourens Bester
References
- 1.van Hazel GA, Pavlakis N, Goldstein D, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol. 2009;27:4089–4095. doi: 10.1200/JCO.2008.20.8116. [DOI] [PubMed] [Google Scholar]
- 2.Kuebler JP. Radioembolization of liver metastases in patients with colorectal cancer: A nonsurgical treatment with combined modality potential [editorial] J Clin Oncol. 2009;27:4041–4042. doi: 10.1200/JCO.2009.23.2785. [DOI] [PubMed] [Google Scholar]
- 3.Riaz A, Ryu RK, Kulik LM, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: Oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27:5734–5742. doi: 10.1200/JCO.2009.23.1282. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy AS, McNeillie P, Dezarn WA, et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74:1494–1500. doi: 10.1016/j.ijrobp.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Townsend A, Price T, Karapetis C. Selective internal radiation therapy for liver metastases from colorectal cancer. Cochrane Database Syst Rev. 2009;4:CD007045. doi: 10.1002/14651858.CD007045.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King J, Quinn R, Glenn DM, et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer. 2008;113:921–929. doi: 10.1002/cncr.23685. [DOI] [PubMed] [Google Scholar]
- 8.Reardon KA, McIntosh AF, Shilling AT, et al. Treatment of primary liver tumors with yttrium-90 microspheres (TheraSphere) in high risk patients: Analysis of survival and toxicities. Technol Cancer Res Treat. 2009;8:71–77. doi: 10.1177/153303460900800109. [DOI] [PubMed] [Google Scholar]
- 9.Van De Wiele C, Defreyne L, Peeters M, et al. Yttrium-90 labelled resin microspheres for treatment of primary and secondary malignant liver tumors. Q J Nucl Med Mol Imaging. 2009;53:317–324. [PubMed] [Google Scholar]
- 10.Michael M, Vancutsem E, Kretzschmar A, et al. Feasibility of metastasectomy in patients treated with bevacizumab in first-line mCRC - Preliminary results from the First Beat-study. J Clin Oncol. 2006;24(18 suppl):3523. [Google Scholar]
- 11.Sirtex Medical. SIR-Spheres® [package insert] [accessed July 27, 2010]. Available at http://www.sirtex.com/files/US20Package20Insert1.pdf.
- 12.Konda A, Savin MA, Cappell MS, et al. Radiation microsphere-induced GI ulcers after selective internal radiation therapy for hepatic tumors: An underrecognized clinical entity. Gastrointest Endosc. 2009;70:561–567. doi: 10.1016/j.gie.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Murthy R, Brown DB, Salem R, et al. Gastrointestinal complications associated with hepatic arterial yttrium-90 microsphere therapy. J Vasc Interv Radiol. 2007;18:553–562. doi: 10.1016/j.jvir.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Szyszko T, Al-Nahhas A, Tait P, et al. Management and prevention of adverse effects related to treatment of liver tumours with 90Y microspheres. Nucl Med Commun. 2007;28:21–24. doi: 10.1097/MNM.0b013e3280121a8f. [DOI] [PubMed] [Google Scholar]
- 15.Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25:1099–1106. doi: 10.1200/JCO.2006.08.7916. [DOI] [PubMed] [Google Scholar]
- 16.Riaz A, Lewandowski RJ, Kulik LM, et al. Complications following radioembolization with yttrium-90 microspheres: A comprehensive literature review. J Vasc Interv Radiol. 2009;20:1121–1130. doi: 10.1016/j.jvir.2009.05.030. quiz 1131. [DOI] [PubMed] [Google Scholar]
- 17.Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: Radioembolization with 90Y microspheres—safety, efficacy, and survival. Radiology. 2008;247:507–515. doi: 10.1148/radiol.2472062029. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: A consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13–23. doi: 10.1016/j.ijrobp.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 19.Salem R, Lewandowski RJ, Sato KT, et al. Technical aspects of radioembolization with 90Y microspheres. Tech Vasc Interv Radiol. 2007;10:12–29. doi: 10.1053/j.tvir.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Song S-Y, Chung JW, Lim HG, et al. Nonhepatic arteries originating from the hepatic arteries: Angiographic analysis in 250 patients. J Vasc Interv Radiol. 2006;17:461–469. doi: 10.1097/01.rvi.0000202718.16416.18. [DOI] [PubMed] [Google Scholar]
- 21.Silvanto A, Novelli M, Lovat L. SIRT—an uncommon cause of gastroduodenal ulceration. Histopathology. 2009;55:114–115. doi: 10.1111/j.1365-2559.2009.03333.x. [DOI] [PubMed] [Google Scholar]
- 22.South CD, Meyer MM, Meis G, et al. Yttrium-90 microsphere induced gastrointestinal tract ulceration. World J Surg Oncol. 2008;6:93–99. doi: 10.1186/1477-7819-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmermann L, Dudeck O, Schmitt J, et al. Duodenal ulcer due to yttrium microspheres used for selective internal radiation therapy of hepatocellular cancer. Gastrointest Endosc. 2009;69:977–978. doi: 10.1016/j.gie.2008.05.063. [DOI] [PubMed] [Google Scholar]
- 24.Newland LR, Walsh A, Gilbert DR, et al. Selective internal radiation therapy: A case of SIR-Sphere associated duodenal ulceration. Pathology. 2007;39:526–528. doi: 10.1080/00313020701444598. [DOI] [PubMed] [Google Scholar]