The study examines the possible linkage between fatigue and intestinal injury during pelvic radiotherapy in anal and uterine cancer patients.
Keywords: Radiotherapy, Cancer-related fatigue, Citrulline, Acute-phase proteins
Abstract
Background.
The association between cancer-related fatigue and pathological processes in the body is largely unknown. This study was designed to investigate a possible linkage between fatigue and intestinal injury during pelvic radiotherapy.
Methods.
Twenty-nine women undergoing pelvic radiotherapy for anal or uterine cancer were prospectively followed. Fatigue and diarrhea were assessed using patient self-reported questionnaires. Plasma citrulline concentration, as a sign of intestinal injury, and C-reactive protein, orosomucoid, albumin, α1-antitrypsin, and haptoglobin, as signs of systemic inflammation, were analyzed.
Results.
Fatigue increased significantly (p < .001) and citrulline decreased significantly (p < .001) during treatment. A significant negative correlation (r = −0.40; p < .05) was found between fatigue and epithelial atrophy in the intestine (as assessed by plasma citrulline) after 3 weeks of treatment and a significant positive correlation (r = 0.75; p < .001) was found between fatigue and diarrhea. Signs of systemic inflammation were evident, with significant increases in serum orosomucoid, serum haptoglobin (p < .05) and serum α1-antitrypsin (p < .001) and a significant decrease in serum albumin (p < .001).
Conclusion.
The present study indicates a link between fatigue and intestinal injury during pelvic radiotherapy. This observation should be considered as a preliminary finding because of the small sample size but may serve as a rationale for therapeutic interventions aimed at alleviating both fatigue and gastrointestinal symptoms during pelvic radiotherapy.
Introduction
Patients undergoing pelvic radiotherapy experience general symptoms, such as fatigue [1–4], as well as gastrointestinal symptoms directly related to irradiation of the intestine [3, 5, 6]. Despite extensive literature on cancer-related fatigue, there are still gaps in the knowledge concerning its development and alleviation. This study investigated whether there is a link between the signs of intestinal injury on one hand and fatigue on the other hand. Such a link could enable therapeutic interventions against intestinal injury that could alleviate both the gastrointestinal symptoms and the accompanying fatigue.
Gastrointestinal symptoms such as diarrhea, abdominal pain, and fecal incontinence, which affect daily life, are experienced by patients during pelvic radiotherapy [3, 5–7]. These symptoms can persist and affect quality of life several years after treatment [7]. The severity of gastrointestinal symptoms during pelvic radiation is related to the total radiation dose and the irradiated volume of the small intestine [8, 9].
Fatigue is a prevalent symptom in connection with cancer and/or its therapy and has emotional, cognitive, and physical corollaries [2, 10]. Patients report that cancer-related fatigue affects their physical and psychosocial well-being and ability to work [11, 12]. Moreover, several studies have found a significant correlation between global quality of life and cancer-related fatigue [12–14]. Cancer-related fatigue is often experienced in clusters of other symptoms, such as pain, sleeplessness, and anxiety [15, 16].
The pathophysiology of fatigue in patients with cancer has not been fully elucidated [17]. Many factors are thought to contribute and several studies have demonstrated high levels of fatigue in, apparently, different cancer populations. For example, anemia, circadian rhythm disruption, altered muscle endurance, and an inflammatory reaction caused by the release of inflammatory mediators such as cytokines have all been proposed to contribute to the development of fatigue [17]. One possible mechanism to the development of fatigue during pelvic radiotherapy could be that radiation-induced intestinal injury promotes translocation of bacteria and other proinflammatory luminal components into the systemic circulation, resulting in a cytokine-induced inflammatory reaction, which in turn may elicit fatigue. Supporting this hypothesis, intestinal permeability has been shown to be greater at the end of pelvic radiotherapy [18], which might enable such a translocation. In reviewing 18 studies (n = 1,037), Schubert et al. [19] found evidence for a correlation between fatigue and the release of the cytokines interleukin-6 and interleukin-1ra and the inflammatory marker neopterin. Wang et al. [20] showed a positive correlation between multiple symptoms, including fatigue, and high levels of interleukin-6 following allogeneic hematopoietic stem cell transplantation. Further linking intestinal injury to the development of fatigue, Ahlberg et al. [12] showed that intensity of fatigue correlated with severity of diarrhea. Wang et al. [1] showed that uncontrolled diarrhea predicted fatigue in 18% of the patients after 3 weeks of pelvic radiotherapy.
Citrulline is a biomarker of epithelial cell mass in the small bowel [21–23]. It is an amino acid that is produced from the degradation of glutamine (the main fuel for enterocytes) in the small intestine [22]. Enterocytes lack the enzymes to further degrade citrulline, which is released into the bloodstream and contributes to the production of arginine in the kidneys. Plasma citrulline concentration has been shown to decrease during pelvic radiotherapy [24, 25] and, given that glutamine is available, this observation has been interpreted as an illustration of a reduction in functional enterocytes. Other studies investigating citrulline concentrations in patients with HIV [26], Crohn's disease [27], and villous atrophy disease (celiac disease) [28] have all shown decreased citrulline levels to correlate with severity of disease.
To the best of our knowledge, no published studies have examined if cancer-related fatigue is associated with radiation-induced intestinal injury, as assessed by both plasma citrulline concentration and diarrhea. The aim of the present study was therefore to investigate whether the intensity of fatigue during pelvic radiotherapy is correlated with plasma citrulline concentration and the severity of diarrhea.
Methods
Patient Population
The patient population comprised two groups of patients scheduled to receive curative pelvic radiotherapy in 2-Gy fractions to 46 Gy: (a) patients with stage I–III anal cancer and (b) patients with stage I–III uterine cancer. In order to detect a large effect-size correlation coefficient of 0.50, a sample size of 26 patients was estimated to achieve a power of 80% with a significance level of 0.05 (Pitman's nonparametric permutations test). Thirty patients were projected for inclusion to allow for longitudinal attrition.
Study Variables and Instruments
The primary variables for this study were general fatigue, diarrhea, plasma citrulline, and plasma glutamine.
Cancer-Related Fatigue
The Multidimensional Fatigue Inventory (MFI)-20, Swedish version [2, 29, 30], was used to investigate cancer-related fatigue. The MFI-20 measures five dimensions of fatigue. For the purposes of this study, only the subscale of general fatigue was used. It consists of four statements (I feel fit, I feel tired, I am rested, I tire easily). The time frame is fatigue experienced in the preceding days and statements are rated against a five-point scale from “yes, that is true” to “no, that is not true.” The subscale score range is 4–20, whereby a higher score indicates greater fatigue. Scores were dichotomized into scores of 4 to <12 and scores of 12–20 based on patients' reports of fatigue in face-to-face interviews.
Gastrointestinal Symptoms
To investigate the intensity of diarrhea, the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30 [31] was used. The EORTC QLQ-C30 uses a four-point response scale (not at all, a little, quite a bit, very much) and a 1-week time frame. Scale scores are transformed to a 0–100 scale, whereby higher scores represent greater symptom burden.
Diarrhea was also assessed with the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0, because diarrhea was of most interest along with fatigue. According to the CTCAE grading system, grade 1 diarrhea refers to an increase of four or fewer stools per day, grade 2 refers to an increase of four to six stools per day, and grade 3 refers to an increase of seven or more stools per day. Grade 4 diarrhea indicates life-threatening consequences.
Biochemical Markers
Plasma citrulline concentration was analyzed as a sign of intestinal injury. Plasma glutamine, the source of citrulline, was analyzed to rule out malnutrition as a confounding cause of an expected reduction in citrulline concentration. Serum C-reactive protein (CRP), serum orosomucoid, serum haptoglobin, serum α1-antitrypsin, and serum albumin were measured to investigate systemic inflammatory responses. Serum hemoglobin was assessed to control for possible anemia.
Data Collection
Data were collected prospectively at one center. All variables were measured at baseline (before the start of radiotherapy), after 3 weeks of treatment (30 ± 2 Gy), and after 5 weeks of treatment (46 ± 2 Gy). The measurement at 30 Gy was chosen with the expectation that, at this point, an effect on both symptoms and signs would be apparent, based on the onset of symptoms from a previous study by Ahlberg et al. [12].
Most patients were interviewed face-to-face or, in some cases, by telephone. In the interviews, items and response alternatives from the instruments were read to the patient in the order they appeared in the scale. Patients were also asked to estimate their loperamide consumption (Dimor®; Merckle GmbH, Blaubeuren, Germany) in relation to diarrhea.
Clinical data (e.g., tumor stage, therapy) were collected from the patients' records.
The study adhered to the Declaration of Helsinki and was approved by the ethical board of the University of Gothenburg (dnr. 009–08). All included patients signed an informed consent form before data collection.
Statistical Analysis
Associations between general fatigue and plasma citrulline were analyzed at baseline, 3 weeks (30 Gy), and 5 weeks (46 Gy) with Spearman's rank order correlation. Partial correlations were computed between general fatigue and plasma citrulline at 3 weeks (30 Gy), controlling for potential fatigue covariates, including EORTC QLQ-C30 sleep disturbance, pain, and emotional functioning. Spearman's rank order correlation was also used to investigate associations between symptoms. Changes over time in intensity of symptoms were analyzed with the nonparametric Friedman's test followed by the Wilcoxon signed rank test, because of skewed distributions of the data. Changes in biochemical parameters were analyzed in patients with a complete set of markers over the three time points. To assess change over time for these variables, parametric one-way analysis of variance followed by paired-samples t-tests were used because the data were assumed to be normally distributed. All tests were two-tailed, and a significance level of p < .05 was applied. The MFI-20 general fatigue subscale was chosen as the correlating variable with plasma citrulline based on the general statements in that subscale and the correlation shown by Ahlberg et al. [12] between general fatigue and diarrhea. The strength of the relationship between two variables was interpreted according to Cohen's criteria for interpreting effect sizes [32]. All analyses were performed using SPSS, version 17.0, software (SPSS, Inc., Chicago, IL).
Results
Patient Characteristics
In total, 65 patients were screened consecutively for inclusion over the course of 1 year. Fifteen patients did not fulfill inclusion criteria, three patients were missed, and 17 patients declined to participate. Thirty patients agreed to participate, of whom 20 had uterine cancer and 10 had anal cancer. One patient with uterine cancer dropped out of the study after baseline, leaving 29 patients for the final analysis. Of the 29 patients, seven patients with anal cancer and two patients with uterine cancer underwent chemotherapy before the start of radiotherapy. All patients received a daily fraction of 1.8–2.0 Gy up to a cumulative dose of 46 Gy. Conventional radiotherapy was given to all patients except one who, after 14 Gy, received intensity-modulated radiotherapy. All patients were female, with a median age of 64 years (range, 49–85 years).
Of the patients who declined to join the study, 16 had uterine cancer and one had anal cancer. Reasons for declining were “not enough energy to cope with both radiotherapy and this study” (n = 13), “lack of interest” (n = 2), “felt too old” (n = 1), and “too sad” (n = 1). These patients were significantly older (median, 71 years; range, 61–90 years) than the included patients.
Cancer-Related Fatigue
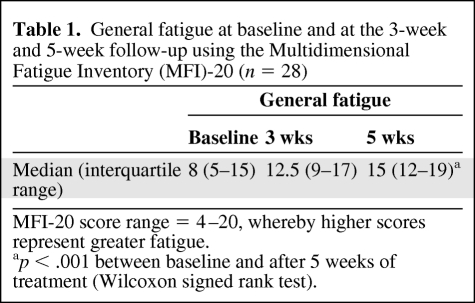
As shown in Table 1, there was a significant increase (p < .001) in the MFI-20 general fatigue subscale score during radiotherapy. From baseline to 5 weeks of treatment, the number of patients with scores ≥12 on the MFI-20 general fatigue subscale increased from seven to 22 patients. Two patients were not fatigued during treatment (scores of 4 and 6 in general fatigue).
Table 1.
General fatigue at baseline and at the 3-week and 5-week follow-up using the Multidimensional Fatigue Inventory (MFI)-20 (n = 28)
MFI-20 score range = 4–20, whereby higher scores represent greater fatigue.
ap < .001 between baseline and after 5 weeks of treatment (Wilcoxon signed rank test).
Diarrhea
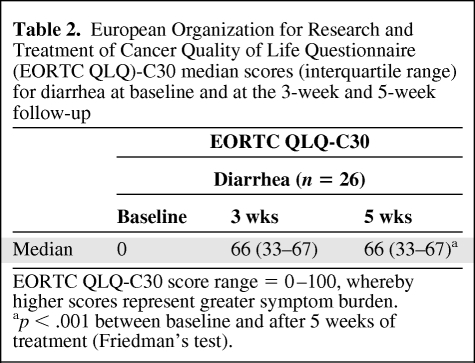
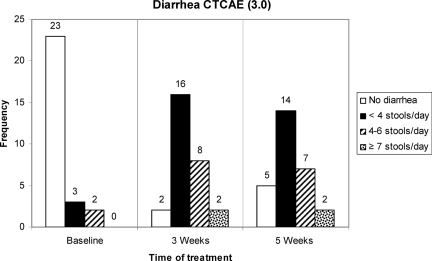
Scores on the EORTC QLQ-C30 diarrhea subscale increased significantly (p < .001) during treatment (Table 2). The greatest increase in diarrhea was seen between baseline and 3 weeks of treatment. No significant change occurred during the last 2 weeks of treatment. CTCAE grades of diarrhea are shown in Figure 1. The median numbers of loperamide tablets consumed during treatment were one tablet per day (range, 0–6 tablets) at 3 weeks and two tablets per day (range, 0–6 tablets) at 5 weeks.
Table 2.
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30 median scores (interquartile range) for diarrhea at baseline and at the 3-week and 5-week follow-up
EORTC QLQ-C30 score range = 0–100, whereby higher scores represent greater symptom burden.
ap < .001 between baseline and after 5 weeks of treatment (Friedman's test).
Figure 1.
Number of patients (n = 28) with each Common Terminology Criteria for Adverse Events (CTCAE), version 3.0, grade of diarrhea at baseline and after 3 weeks and 5 weeks of treatment.
Correlations Between Fatigue and Diarrhea
A strong positive correlation (r = 0.75; p < .001) was found between general fatigue (MFI-20) and intensity of diarrhea (EORTC QLQ-C30) at the 3-week follow-up. General fatigue also correlated strongly (r = 0.55; p < .01) with frequency of diarrhea measured by the CTCAE at this point. At 5 weeks, no significant correlations were found between fatigue and intensity or frequency of diarrhea.
Biochemical Markers
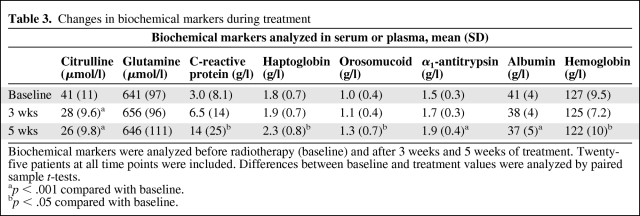
Citrulline concentrations decreased by 15 μmol/l (p < .001) from baseline (median, 41 μmol/l; standard deviation [SD], 11) to 5 weeks of treatment (median, 26 μmol/l; SD, 10) (Table 3). The major decrease occurred between baseline and 3 weeks of treatment (median, 13; p < .001). Plasma glutamine showed no significant changes during treatment (Table 3). There were significant increases in markers of systemic inflammation—serum orosomucoid and serum haptoglobin (p < .05) and serum α1-antitrypsin (p < .001). Serum albumin decreased significantly (p < .001).
Table 3.
Changes in biochemical markers during treatment
Biochemical markers were analyzed before radiotherapy (baseline) and after 3 weeks and 5 weeks of treatment. Twenty-five patients at all time points were included. Differences between baseline and treatment values were analyzed by paired sample t-tests.
ap < .001 compared with baseline.
bp < .05 compared with baseline.
There was a statistically significant decrease (p < .05) in the hemoglobin level from baseline (median, 127 g/l; SD, 9.5) to 5 weeks (median, 122 g/l; SD, 10) (Table 3). Three patients received one blood transfusion during treatment and one patient received two blood transfusions during treatment.
Serum CRP (Table 3) did not pass the normality check, with extreme outliers at all time points (e.g., range <5–84 after 5 weeks of treatment). In total, 11 patients had an elevated CRP level during treatment, of whom eight had an increase during treatment and three had consistently elevated levels from baseline.
Correlations Between Symptoms and Signs
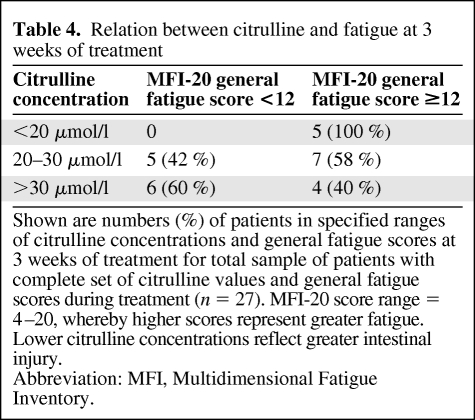
At the 3-week follow-up, a significant negative correlation (r = −0.40; p < .05) was found between MFI-20 general fatigue subscale score and epithelial atrophy in the intestine as assessed by plasma citrulline concentration after controlling for EORTC sleep disturbance, pain, and emotional functioning. This correlation was not seen at 5 weeks. To characterize the relation between citrulline and fatigue at 3 weeks of treatment, patients were categorized into groups representing high, medium, and low citrulline concentrations and high and low fatigue scores (Table 4).
Table 4.
Relation between citrulline and fatigue at 3 weeks of treatment
Shown are numbers (%) of patients in specified ranges of citrulline concentrations and general fatigue scores at 3 weeks of treatment for total sample of patients with complete set of citrulline values and general fatigue scores during treatment (n = 27). MFI-20 score range = 4–20, whereby higher scores represent greater fatigue. Lower citrulline concentrations reflect greater intestinal injury.
Abbreviation: MFI, Multidimensional Fatigue Inventory.
No correlation was found between the frequency of diarrhea (CTCAE) or the intensity of diarrhea (EORTC QLQ-C30) and plasma citrulline at 3 weeks or 5 weeks.
Because of the increases in haptoglobin, orosomucoid, and α1-antitrypsin, it was of interest to correlate these parameters with general fatigue. At both 3 weeks and 5 weeks, haptoglobin and general fatigue correlated significantly (r = 0.46; p < .05 versus r = 0.40; p < .05).
Discussion
A moderate inverse association between citrulline concentration and general fatigue was found at the 3-week follow-up. The association is illustrated in Table 4 by the fact that all patients who had low citrulline concentrations (<20 μmol/l) also had high general fatigue scores (≥12; maximum, 20) and that more patients with medium citrulline concentrations (20–30 μmol/l) had high general fatigue scores. No association was found between these variables after 5 weeks of treatment, which may be a result of the large deviation of scores in general fatigue and in citrulline values.
We could not show a correlation between the frequency of diarrhea and plasma citrulline level at any time point during treatment. Two previous studies with heterogeneous groups of patients receiving abdominal and pelvic radiotherapy (n = 23, n = 59) investigated the correlation between citrulline levels in plasma and gastrointestinal symptoms experienced [24, 25]. Lutgens et al. [24] found a correlation between grade 2 diarrhea and citrulline level at week 4 and week 6 of treatment. Wedlake et al. [25] could not show a correlation between gastrointestinal symptoms and citrulline.
Plasma citrulline decreased significantly during treatment, which replicated the findings of earlier studies [24, 25]. In line with Lutgens et al. [24], we demonstrated that most of the decrease in citrulline occurred during the first 3 weeks of treatment. The normal citrulline concentration in the blood is 30- 50 μmol/l, with a mean of 40 μmol/l [33]. Citrulline values <20 μmol/l have been shown to predict permanent intestinal failure in patients with short bowel syndrome [33], and a value <10 μmol/l has been shown to predict the need for parenteral nutrition in patients with celiac disease [28]. Therefore, we categorized patients according to their citrulline values (Table 4).
In relation to the elevated markers orosomucoid, haptoglobin, and α1-antitrypsin along with the decreased serum albumin levels, we can conclude signs of systemic inflammation [34, 35]. The increases in haptoglobin, orosomucoid, and α1-antitrypsin could be explained by different factors, apart from intestinal injury, for example, decay of tumor cells or damage to normal tissue [35]. To further investigate these elevated markers in relation to intestinal injury, intestinal permeability tests will be performed in future studies based on the assumption that an increased translocation of proinflammatory luminal components could contribute to systemic inflammation. The correlation between fatigue and haptoglobin supports a linkage between inflammatory markers and fatigue. CRP levels were very skewed in our study, and hence no conclusions can be drawn about this variable. Wedlake et al. [25] did not find increased CRP levels or a correlation between gastrointestinal symptoms and CRP after 4 or 5 weeks of treatment. The elevation in CRP, analyzed in plasma, found by Cengiz et al. [36] was more prominent in patients receiving pelvic-paraaortic irradiation and had no correlation with acute enteritis. Further, even though there was a statistically significant decrease in hemoglobin levels from baseline to 5 weeks of treatment in our study (Table 3), the numeric difference was small and we do not believe that this decrease is clinically relevant to the development of fatigue.
As in the studies of Fürst and Åhsberg [2] and Ahlberg et al. [4], our study showed an increase in general fatigue during treatment. Patients described an onset of fatigue at the end of week 2 or during week 3. Diarrhea measured by the EORTC QLQ-C30 and CTCAE correlated highly with fatigue after 3 weeks of treatment. The absence of a correlation between fatigue and diarrhea after 5 weeks can be explained by several factors. As Figure 1 shows, diarrhea stabilized between week 3 and week 5 of treatment and fatigue continued to increase (Table 1). This stabilization has been observed in other studies [5, 12] and can be explained by patients' use of antidiarrhea medicine (loperamide) or by patients' efforts to overcome gastrointestinal symptoms by modifying their food intake.
Patients were consecutively included and only three were missed. However, a limitation to this study is that 17 patients (26%) declined participation in the study. The main reason for declining was that patients felt too weak to take part in the study (n = 13). These patients were significantly older (median, 71 years; range, 61–90 years) than the included patients (median, 64 years; range, 49–85 years); thus, the oldest patients (mostly patients with uterine cancer) were not represented in the study. Another limitation is that the radiation fields over the pelvic region differed among patients because of the different primary diagnoses. Further, patients with uterine cancer had undergone a hysterectomy, which could increase the volume of small intestine descending into the irradiated pelvic area. The size of our sample precluded conceivably relevant subgroup analyses. Finally, our sample was comprised exclusively of women.
Conclusion
The preliminary finding from this study indicates a possible link between cancer-related fatigue and intestinal injury as assessed by plasma citrulline and intensity of diarrhea during pelvic radiotherapy. This observation definitely needs further validation but may serve as a rationale for therapeutic interventions aimed at protecting the intestine during pelvic radiotherapy, thereby hypothetically alleviating both the fatigue and the diarrhea, two important symptoms for patient well-being during pelvic radiotherapy.
Acknowledgments
The study was supported by a grant from King Gustav V Jubilee Clinic Cancer Research Foundation.
Author Contributions
Conception/Design: Sofie Jakobsson, Karin Ahlberg, Tor Ekman
Provision of study material or patients: Sofie Jakobsson, Tor Ekman
Collection and/or assembly of data: Sofie Jakobsson
Data analysis and interpretation: Sofie Jakobsson, Karin Ahlberg, Tor Ekman, Charles Taft
Manuscript writing: Sofie Jakobsson, Karin Ahlberg, Tor Ekman, Charles Taft
Final approval of manuscript: Sofie Jakobsson, Karin Ahlberg, Tor Ekman, Charles Taft
References
- 1.Wang XS, Janjan NA, Guo H, et al. Fatigue during preoperative chemoradiation for resectable rectal cancer. Cancer. 2001;92(6 suppl):1725–1732. doi: 10.1002/1097-0142(20010915)92:6+<1725::aid-cncr1504>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 2.Fürst CJ, Ahsberg E. Dimensions of fatigue during radiotherapy. An application of the Multidimensional Fatigue Inventory. Support Care Cancer. 2001;9:355–360. doi: 10.1007/s005200100242. [DOI] [PubMed] [Google Scholar]
- 3.Guren MG, Dueland S, Skovlund E, et al. Quality of life during radiotherapy for rectal cancer. Eur J Cancer. 2003;39:587–594. doi: 10.1016/s0959-8049(02)00741-4. [DOI] [PubMed] [Google Scholar]
- 4.Ahlberg K, Ekman T, Wallgren A, et al. Fatigue, psychological distress, coping and quality of life in patients with uterine cancer. J Adv Nurs. 2004;45:205–213. doi: 10.1046/j.1365-2648.2003.02882.x. [DOI] [PubMed] [Google Scholar]
- 5.Christman NJ, Oakley MG, Cronin SN. Developing and using preparatory information for women undergoing radiation therapy for cervical or uterine cancer. Oncol Nurs Forum. 2001;28:93–98. [PubMed] [Google Scholar]
- 6.Abayomi J, Kirwan J, Hackett A. The prevalence of chronic radiation enteritis following radiotherapy for cervical or endometrial cancer and its impact on quality of life. Eur J Oncol Nurs. 2009;13:262–267. doi: 10.1016/j.ejon.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Abayomi J, Kirwan J, Hackett A, et al. A study to investigate women's experiences of radiation enteritis following radiotherapy for cervical cancer. J Hum Nutr Diet. 2005;18:353–363. doi: 10.1111/j.1365-277X.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- 8.Baglan KL, Frazier RC, Yan D, et al. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:176–183. doi: 10.1016/s0360-3016(01)01820-x. [DOI] [PubMed] [Google Scholar]
- 9.Gunnlaugsson A, Kjellén E, Nilsson P, et al. Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncol. 2007;46:937–944. doi: 10.1080/02841860701317873. [DOI] [PubMed] [Google Scholar]
- 10.Ahlberg K, Ekman T, Gaston-Johansson F, et al. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362:640–650. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- 11.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. The Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 12.Ahlberg K, Ekman T, Gaston-Johansson F. The experience of fatigue, other symptoms and global quality of life during radiotherapy for uterine cancer. Int J Nurs Stud. 2005;42:377–386. doi: 10.1016/j.ijnurstu.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Smets EM, Visser MR, Willems-Groot AF, et al. Fatigue and radiotherapy: (A) experience in patients undergoing treatment. Br J Cancer. 1998;78:899–906. doi: 10.1038/bjc.1998.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holzner B, Kemmler G, Meraner V, et al. Fatigue in ovarian carcinoma patients: A neglected issue? Cancer. 2003;97:1564–1572. doi: 10.1002/cncr.11253. [DOI] [PubMed] [Google Scholar]
- 15.Gaston-Johansson F, Fall-Dickson JM, Bakos AB, et al. Fatigue, pain, and depression in pre-autotransplant breast cancer patients. Cancer Pract. 1999;7:240–247. doi: 10.1046/j.1523-5394.1999.75008.x. [DOI] [PubMed] [Google Scholar]
- 16.Beck SL, Dudley WN, Barsevick A. Pain, sleep disturbance, and fatigue in patients with cancer: Using a mediation model to test a symptom cluster. Oncol Nurs Forum. 2005;32:542–E55. doi: 10.1188/04.ONF.E48-E55. [DOI] [PubMed] [Google Scholar]
- 17.Ryan JL, Carroll JK, Ryan EP, et al. Mechanisms of cancer-related fatigue. The Oncologist. 2007;12(suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 18.Pía de la Maza M, Gotteland M, Ramírez C, et al. Acute nutritional and intestinal changes after pelvic radiation. J Am Coll Nutr. 2001;20:637–642. doi: 10.1080/07315724.2001.10719161. [DOI] [PubMed] [Google Scholar]
- 19.Schubert C, Hong S, Natarajan L, et al. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Wang XS, Shi Q, Williams LA, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer. 2008;113:2102–2109. doi: 10.1002/cncr.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutgens L, Lambin P. Biomarkers for radiation-induced small bowel epithelial damage: An emerging role for plasma Citrulline. World J Gastroenterol. 2007;13:3033–3042. doi: 10.3748/wjg.v13.i22.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curis E, Crenn P, Cynober L. Citrulline and the gut. Curr Opin Clin Nutr Metab Care. 2007;10:620–626. doi: 10.1097/MCO.0b013e32829fb38d. [DOI] [PubMed] [Google Scholar]
- 23.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Lutgens LC, Deutz N, Granzier-Peeters M, et al. Plasma citrulline concentration: A surrogate end point for radiation-induced mucosal atrophy of the small bowel. A feasibility study in 23 patients. Int J Radiat Oncol Biol Phys. 2004;60:275–285. doi: 10.1016/j.ijrobp.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 25.Wedlake L, McGough C, Hackett C, et al. Can biological markers act as non-invasive, sensitive indicators of radiation-induced effects in the gastrointestinal mucosa? Aliment Pharmacol Ther. 2008;27:980–987. doi: 10.1111/j.1365-2036.2008.03663.x. [DOI] [PubMed] [Google Scholar]
- 26.Crenn P, De Truchis P, Neveux N, et al. Plasma citrulline is a biomarker of enterocyte mass and an indicator of parenteral nutrition in HIV-infected patients. Am J Clin Nutr. 2009;90:587–594. doi: 10.3945/ajcn.2009.27448. [DOI] [PubMed] [Google Scholar]
- 27.Papadia C, Sherwood RA, Kalantzis C, et al. Plasma citrulline concentration: A reliable marker of small bowel absorptive capacity independent of intestinal inflammation. Am J Gastroenterol. 2007;102:1474–1482. doi: 10.1111/j.1572-0241.2007.01239.x. [DOI] [PubMed] [Google Scholar]
- 28.Crenn P, Vahedi K, Lavergne-Slove A, et al. Plasma citrulline: A marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology. 2003;124:1210–1219. doi: 10.1016/s0016-5085(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 29.Smets EM, Garssen B, Bonke B, et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 30.Hagelin CL, Wengstrom Y, Runesdotter S, et al. The psychometric properties of the Swedish Multidimensional Fatigue Inventory MFI-20 in four different populations. Acta Oncol. 2007;46:97–104. doi: 10.1080/02841860601009430. [DOI] [PubMed] [Google Scholar]
- 31.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. Statistical Power Analysis for the Behavioural Sciences. Second Edition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. pp. 1–558. [Google Scholar]
- 33.Crenn P, Coudray-Lucas C, Thuillier F, et al. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000;119:1496–1505. doi: 10.1053/gast.2000.20227. [DOI] [PubMed] [Google Scholar]
- 34.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 35.Mölne J, Wold A. Inflammation. Stockholm, Sweden: Liber AB; 2007. pp. 1–351. [Google Scholar]
- 36.Cengiz M, Akbulut S, Atahan IL, et al. Acute phase response during radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:1093–1096. doi: 10.1016/s0360-3016(00)01426-7. [DOI] [PubMed] [Google Scholar]