The article examines the potential reasons for the delayed clinical benefits seen with therapeutic cancer vaccines and the broader implications for evolving treatment paradigms.
Keywords: Prostate cancer, Therapeutic cancer vaccines, Overall survival, Progression-free survival, Clinical endpoints
Abstract
Therapeutic cancer vaccines represent a new class of agents in the treatment of cancer. Sipuleucel-T is an antigen-presenting cell–based vaccine that recently demonstrated a significant 4.8-month improvement in overall survival in advanced prostate cancer patients and was well tolerated. The findings of that study have been met with skepticism, primarily because the agent did not change initial disease progression and yet led to longer survival. Although the commonly accepted treatment paradigm suggests that treatments should initially decrease tumor volume, perhaps vaccines work differently. Vaccines may induce delayed responses not seen in the first few months of therapy or they may initiate a dynamic immune response that ultimately slows the tumor growth rate, resulting in longer survival. Subsequent therapies may also combine with the induced immune response, resulting in a combination that is more effective than conventional treatments alone. Also, other treatments may alter tumor-associated antigen expression, enhancing the immune response. Future trials are currently planned to investigate these hypotheses; however, the results of the sipuleucel-T vaccine in prostate cancer should not be dismissed. Results with another vaccine in prostate cancer are similar, perhaps suggesting a class effect. In a broader context, clinicians may need to reconsider how they measure success. Several agents have been approved that produce superior disease progression results, but do not affect overall survival. Given the toxicity and costs of cancer therapies, perhaps studies should put more weight on long-term survival endpoints than on short-term endpoints that may be less consequential.
Current Approaches to Treating Prostate Cancer
Six years after the U.S. Food and Drug Administration (FDA) approved the use of docetaxel for the treatment of prostate cancer, a therapeutic cancer vaccine has demonstrated improved survival times in a large randomized, phase III trial in metastatic castration-resistant prostate cancer (CRPC) patients. Although this was, in fact, the second trial of this agent to show superior survival results in CRPC patients, data from the phase III trial of the sipuleucel-T vaccine (Provenge®; Dendreon Corp., Seattle, WA) have raised many questions [1].
Of the most prevalent solid tumor malignancies (lung, colon, breast, and prostate), prostate cancer has the fewest therapeutic options for patients with advanced disease. In fact, until earlier this year, only one chemotherapeutic regimen had been shown to confer a survival benefit for the nearly 200,000 men who will be diagnosed with prostate cancer in 2010 and the >27,000 who will succumb to the disease [2]. The regimen of docetaxel and prednisone was sanctioned by the FDA in 2004 for the treatment of metastatic CRPC based on longer survival time, by approximately 3 months [3, 4]. In spite of the obvious need for additional treatments and promising preliminary studies, most subsequent clinical trials in metastatic CRPC patients have fallen short of clinical expectations [5–9].
Sipuleucel-T is a patient-specific therapeutic cancer vaccine generated through the in vitro stimulation of the patient's own peripheral blood mononuclear cells obtained by leukapheresis. Antigen-presenting cells (APCs), including dendritic cells, are activated by exposure to a prostatic acid phosphatase (PAP)–GM-CSF fusion protein in vitro. The activated cellular product is then reinfused into the same patient in three biweekly doses, with the goal of initiating a dynamic immune response targeting PAP, within the context of class I major histocompatibility complex, on the surface of prostate cancer cells [10, 11]. It is important to note that the therapeutic cancer vaccines discussed in this review differ from traditional preventive vaccines (e.g., for infectious diseases) in that the primary goal of therapeutic cancer vaccines is not to prevent disease but rather to generate an active immune response against an existing malignancy.
After promising preliminary clinical trials, an initial placebo-controlled phase III trial failed to meet its primary endpoint of progression-free survival (PFS). Sipuleucel-T did, however, provide evidence of a longer overall survival (OS) time (25.9 months versus 21.4 months; p = .01) [12–16]. Recently, a larger phase III trial that enrolled >500 patients demonstrated a similar survival benefit (25.8 months versus 21.7 months; p = .032), even though there was no difference in terms of the PFS interval between the vaccine and placebo [17]. The results of this latest study ultimately led to FDA approval of this first-in-class agent in April of 2010, despite the fact that many clinicians and researchers in the oncology community are still wrestling with the concept of a survival benefit without any difference in the PFS time [18]. The formal indication for this treatment is for metastatic CRPC patients who have minimal or no symptoms from their disease [19].
How Did We Get Here? Origins of Standard Benchmarks
As new agents are evaluated, standard criteria of clinical benefit are required to determine efficacy. The World Health Organization first addressed efficacy criteria in 1979, but the advent of new imaging techniques in the following two decades necessitated a more modern approach [20, 21]. The Response Evaluation Criteria in Solid Tumors (RECIST) were developed in 2000 through a collaboration of the European Organization for Research and Treatment of Cancer, the National Cancer Institute of Canada, and the National Cancer Institute (NCI) of the U.S. [22, 23]. RECIST measure the success of a treatment by a reduction in the size of metastatic lesions after therapy commences. Progressive disease is defined as an increase in the cumulative size of target lesions by 20%, or the development of any new lesions. It is important to note, however, that although RECIST were developed to be used as a tool in determining antitumor activity, improving survival should be the ultimate goal of cancer therapeutics [22].
The true value of RECIST depends on the type and chemosensitivity of the tumor being treated and particular treatment goals. For acute leukemias, RECIST are not relevant because persistent marrow involvement (even with normal x-rays) heralds rapid clinical deterioration. Likewise, in patients with newly diagnosed high-grade lymphomas or testicular cancer, a minimal RECIST response merits an immediate change in treatment [24]. The true clinical benefit of short-lived RECIST responses for patients with widely metastatic lung, breast, or prostate cancer remains enigmatic, especially with the use of some newer targeted therapies.
The natural history of metastatic CRPC presents additional confounding variables when evaluating the benefits of treatment. The majority of disease is found in bone lesions, as demonstrated in phase III trials of docetaxel in which 50%–60% of patients had metastasis only to bone [3, 4]. Significant changes in whole-body scintigraphy are rare and remain unaddressed by even the most recent version of RECIST [25]. For the remainder of the metastatic CRPC patients with soft tissue lesions, a meager 12%–17% had radiographic “responses” [3, 4]. The fact that most soft tissue disease in CRPC patients consists of lymph node metastasis also presents problems, because nonspecific inflammation or inflammation from an immune response generated by a therapeutic cancer vaccine would certainly be misinterpreted as progressive disease by the RECIST [26–28].
Somewhat unique to prostate cancer is the serum tumor marker prostate-specific antigen (PSA), which plays a vital role in managing and assessing response to hormonal therapies, but whose value in metastatic CRPC is still evolving [29]. Preclinical studies involving the multikinase inhibitor sorafenib (Nexevar®; Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ) demonstrated that the drug actually increases PSA secretion by tumor cells, resulting in patient serum PSA elevations regardless of therapeutic benefit, including improvement on imaging studies [30]. These findings highlight the likelihood that, depending on the agent used, changes in PSA may be difficult to interpret in patients with metastatic CRPC. Regardless of the state of disease, anxiety over rising PSA levels can cause a patient or practitioner to prematurely discontinue a potentially effective therapy, either in standard practice or in a clinical trial [31]. To forestall this result, the Prostate Cancer Clinical Trials Working Group recently updated their recommendations, encouraging practitioners not to use rising PSA alone as a reason to discontinue therapy in advanced disease [32].
How Can Cancer Vaccines Improve OS Without Changing PFS?
Sipuleucel-T is not the only therapeutic cancer vaccine to demonstrate a benefit in terms of OS without any difference in the PFS interval in metastatic CRPC patients. PSA-TRICOM (Prostvac™, developed by the NCI and licensed to BN Immunotherapeutics, Mountain View, CA) is a vector-based vaccine that demonstrated an 8.5-month OS benefit relative to placebo (p = .015) in a 43-center randomized phase II trial (n = 125), in spite of no difference in PFS, compared with placebo [33–35]. A smaller trial at the NCI demonstrated that PSA-TRICOM can generate a PSA-specific T-cell response within 3 months, and that these immune responses are associated with favorable survival outcomes in spite of a short PFS interval [36].
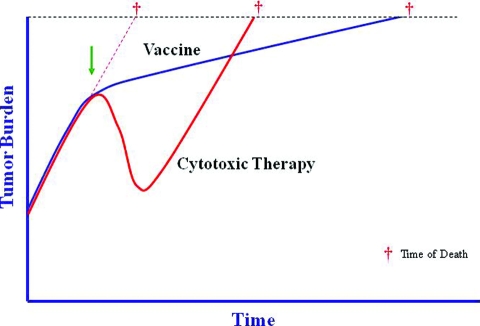
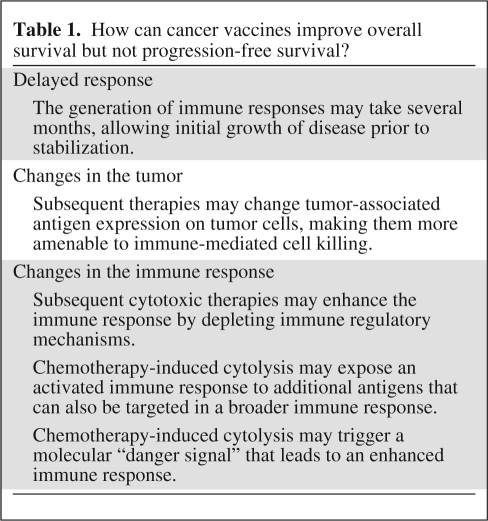
The findings of these vaccine trials may suggest an important characteristic of therapeutic vaccines. A recent analysis of metastatic CRPC patients demonstrated that patients with a short PFS interval had a very poor prognosis. It should be noted, however, that that analysis did not include patients treated with sipuleucel-T or PSA-TRICOM [37]. As opposed to cytoreductive agents, which have their greatest effects soon after the initiation of therapy, vaccines require time to generate an immune response, and evidence of activity may therefore be delayed [38, 39]. This point is demonstrated by mathematical models that use PSA kinetics to predict time of death for patients with metastatic CRPC [40]. A review of prostate cancer clinical trials done at the NCI in the last decade revealed an interesting pattern. For patients who received chemotherapy, there was a very close relationship between time on treatment and survival. After treatment was discontinued, pretreatment PSA kinetics resumed; time to death was thus predictable based on similar pre- and post-treatment PSA trajectories. For patients treated with the PSA-TRICOM vaccine, PSA kinetics did not immediately change while on treatment, but time of death was well beyond what was predicted by the models (Fig. 1) [41]. This analysis of patients treated with a vaccine indicates that something, possibly an immune response, is inducing a more gradual disease progression post-treatment, and thus altering OS without short-term changes in disease progression (Table 1). Although immune responses can be initiated within 3 months of receiving a vaccine, it appears that these responses are not sufficient at that point to significantly reduce tumor size, but may eventually alter the rate of tumor growth. Moreover, it is possible that the immune response most relevant to antitumor therapy may not be the one targeted by the vaccine, but a new immune response to other tumor antigens in a phenomenon known as antigen cascade or epitope spreading [42, 43]. For instance, the initial immune response to a vaccine can lead to T cell–mediated killing, causing APCs to take up dying tumor cells and presenting other, more relevant, antigens to the immune system. This combined, broader antitumor immune response may be more clinically relevant and may lead to slower growth rates. Furthermore, this immune response can be maintained or even augmented following subsequent therapies [39, 44].
Figure 1.
Tumor growth is a dynamic biologic process that is the combined result of cells dividing and other cells dying. Intrinsic tumor biology, as well as extrinsic factors such as therapies, affect the tumor's growth rate. However, chemotherapy (red line) only affects the tumor growth rate while it is being administered, which may result in a dramatic but transient response. Following discontinuation of chemotherapy, the growth rate returns to its pretreatment slope, driven by the underlying biology of the tumor. Immunotherapy (blue line), on the other hand, can alter the biology of the host by inducing an active antitumor immune response including a memory response. This may not cause an immediate or dramatic change in tumor burden, but continued cumulative slowing pressure on tumor growth rate, especially if started early in the disease course, may lead to substantially longer overall survival. The arrow indicates the initiation of treatment; cross indicates time of death from cancer.
Table 1.
How can cancer vaccines improve overall survival but not progression-free survival?
There is a common perception that, because many patients treated with a vaccine receive chemotherapy after progression, the chemotherapy alone is actually improving survival, not the vaccine [1]. There are strong preclinical and emerging clinical data suggesting that this perception is not accurate. Murine studies indicate that the combination of docetaxel and a vaccine creates a more effective antitumor response than either treatment alone, and that a vaccine followed by taxanes maximizes the vaccine-induced immune response [45, 46]. Several follow-up studies in vaccine trials have also indicated that patients treated with a vaccine do better than expected on subsequent chemotherapy [47, 48]. In patients with metastatic CRPC in particular, there has been an intriguing analysis of response to chemotherapy after a vaccine. Patients treated in an earlier study with either sipuleucel-T vaccine or placebo were evaluated for OS after treatment with docetaxel. For the 51 patients treated with the vaccine followed by chemotherapy, the OS time was 34.5 months, compared with 25.4 months for the 31 patients who were randomized to placebo and then went on to receive chemotherapy (p = .023) [49]. Taken together, these data suggest that docetaxel administered after a vaccine may take advantage of a smoldering immune response, resulting in better outcomes. Chemotherapy may also alter the phenotype of tumor cells, induce an antigen cascade, or deplete regulatory cells, thereby enhancing or boosting the immune response in addition to its cytotoxic effects, resulting in a better outcome than with chemotherapy alone. Emerging data also suggest that cytotoxic chemotherapy may trigger a molecular “danger signal” that ultimately leads to an enhanced immune response that could capitalize on an ongoing immune response [50, 51].
It is important to note that therapeutic cancer vaccines are not the only agents evaluated in patients with metastatic CRPC that have led to a longer OS time without altering the PFS interval. ZD4054 (zibotentan; AstraZeneca, London, U.K.) is an endothelin-A receptor antagonist that was tested in 312 metastatic CRPC patients at two doses, compared with placebo. Although neither dose of zibotentan led to a longer PFS interval, a subsequent analysis demonstrated that both doses produced a longer survival time, by >6 months (p = .052 for the 15-mg dose; p = .008 for the 10-mg dose) [5]. A larger phase III study is planned to confirm these findings [52].
Another immune-based therapy, ipilimumab (MDX-1010; Bristol-Myers Squibb, New York), demonstrated an OS advantage in metastatic melanoma patients without a change in disease progression. This agent blocks the cytotoxic T lymphocyte antigen 4 molecule on T cells, which can result in an augmented immune response [53–55]. In that three-arm study, patients were treated with either ipilimumab alone, ipilimumab in combination with an older peptide-based vaccine (GP100), or GP100 alone used as an active control. The PFS times for all three arms were 2.8–2.9 months, but the OS time significantly favored patients receiving ipilimumab (10.1 months and 10.0 months, respectively), compared with patients receiving GP100 alone (6.4 months; p = .0026, p = .0004) [56].
Delayed Progression Versus Longer Survival
While the OS benefit in vaccine trials without a longer PFS interval has been met with great skepticism, a longer PFS time without the benefit of a longer OS time has led to FDA approval of bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA) for metastatic breast cancer. This antibody, which targets the human vascular endothelial growth factor ligand, demonstrated a longer PFS time when used as a first-line treatment in metastatic breast cancer patients. Patients treated with paclitaxel and bevacizumab had a median PFS interval of 11.8 months, compared with a median PFS interval of 5.9 months for those treated with paclitaxel alone (p < .001). In spite of the impressive doubling of the PFS time, the median OS times were 26.7 months and 25.2 months (p = 0.16) [57]. Nonetheless, these findings were sufficient for the FDA to give fast-track approval for bevacizumab in this setting [58]. Two other, more recent, trials also combined chemotherapy with bevacizumab in the same population, again with a longer PFS interval without a significant difference in the OS time at the current follow-up [59, 60].
Further evidence of the unreliability of PFS as an endpoint in clinical trials is seen in the fleeting effects of bevacizumab in the adjuvant treatment of colon cancer patients. Over 2,500 men with stage II and stage III colon cancer were treated with adjuvant chemotherapy with or without bevacizumab. Patients treated with bevacizumab received the drug for either 6 months or 12 months. There was a suggestion that patients on bevacizumab for 12 months had an initially higher PFS rate after 1 year; however, the superior PFS result was no longer significant at a later follow-up [61]. These and other studies cast doubt on the sustainability of PFS benefits seen in some clinical trials, and this, in turn, has implications for patient care, given the potential side effects of some treatments as well as related financial considerations [62, 63].
Although multiple therapies are available for patients with metastatic colon and breast cancer, and these therapies may obscure the ultimate OS benefit in spite of an initial improvement in PFS, this is not the case with prostate cancer. Prior to 2010, in metastatic CRPC patients, there was only one regimen demonstrated to affect survival and approved by the FDA. If the vaccines are truly inert and the lack of a difference in PFS accurately demonstrates a lack of activity, one would expect all patients to move on to the one standard regimen and derive a similar clinical outcome, instead of a 4-month survival benefit in patients treated with sipuleucel-T [17].
Where Do We Go from Here?
With the approval of this first-in-class agent, sipuleucel-T has become the second FDA-approved treatment regimen for patients with metastatic CRPC, based on a survival benefit. Moving forward, clinicians ultimately have to determine how to integrate therapeutic cancer vaccines into clinical practice. They will then ask, “How do I assess the success or failure of a vaccine if there is no immediate change in pain, PSA, or PFS?” The question is appropriate, especially in the absence of standardized immunologic biomarkers of response. The answer may involve fixed dosing of vaccines and sequential therapies with standard agents such as docetaxel. Emerging immunologic response data suggest that patients who derive benefit from therapeutic vaccines do so after only a few months of treatment [64, 65]. To further simplify matters, the sipuleucel-T vaccine is only administered three times in 2-week intervals. However, because it is unlikely that all patients treated with sipuleucel-T will see a survival benefit, better tests are needed to identify which patients are most likely to live longer following treatment with the vaccine.
Although fixed dosing addresses the above question with regard to sipuleucel-T, the broader question about the benefits of sequential treatment with a vaccine followed by chemotherapy remains. The Eastern Cooperative Oncology Group will investigate this issue in an upcoming study. Metastatic CRPC patients will be randomized to initial treatment with either standard docetaxel and prednisone or 3 months of PSA-TRICOM vaccine followed by docetaxel and prednisone. The endpoint of the study will be OS. The results of that study will determine whether chemotherapy can indeed capitalize on a vaccine-induced immune response, or whether survival is unaffected by previous treatment with a vaccine.
The studies with the sipuleucel-T and PSA-TRICOM vaccines outlined above not only provide important data on potential clinical benefits but also force us to re-evaluate current approaches to therapy and clinical research. Appropriate endpoints in clinical studies are vital to understanding the benefits of emerging agents and combinations. Given growing public concern about the costs of health care, it may become harder to justify the use of agents based on fleeting changes in PFS that do not affect survival outcomes. Perhaps the questions surrounding prostate cancer vaccines will broaden our understanding of this new class of agents and lead to a re-evaluation of our current standards. This will help to ensure that we are not confusing potentially transient intermediate endpoints such as PFS with successful long-term benefit for our patients.
Acknowledgment
The authors acknowledge Ms. Bonnie L. Casey, National Cancer Institute, for her editorial assistance in the preparation of this manuscript.
Author Contributions
Conception/Design: Ravi A. Madan, James Gulley, Tito Fojo, William L. Dahut
Financial support: Ravi A. Madan
Manuscript writing: Ravi A. Madan, James Gulley, Tito Fojo, William L. Dahut
Final approval of manuscript: Ravi A. Madan, James Gulley, Tito Fojo, William L. Dahut
References
- 1.Eisenberger M. Discussion: Immunotherapy for Prostate Cancer? Mario A Eisenberger, MD, Discussant. [accessed June 25, 2010]. Available at http://www.asco.org/ASCOv2/MultiMedia/Virtual+Meeting?&vmview=vm_session_presentations_view&confID=65&trackID=3&sessionID=362.
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.James ND, Caty A, Borre M, et al. Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: A double-blind, placebo-controlled, randomised, phase 2 trial. Eur Urol. 2009;55:1112–1123. doi: 10.1016/j.eururo.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Higano C, Saad F, Somer B, et al. A phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC) [abstract 150]. Presented at the Genitourinary Cancers Symposium; February 26–28, 2009; Orlando, FL. [Google Scholar]
- 7.Small E, Demkow T, Gerritsen W, et al. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC) [abstract 7]. Presented at the Genitourinary Cancers Symposium; February 26–28, 2009; Orlando, FL. [Google Scholar]
- 8.ClinicalTrials.gov. DN-101 in Combination With Docetaxel in Androgen-Independent Prostate Cancer (AIPC) (AIPC Study of Calcitriol Enhancing Taxotere [ASCENT-2]) [accessed August 3, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00273338?term=prostate+cancer+DN101&rank=1.
- 9.Sartor A, Petrylak D, Witjes J, et al. Satraplatin in patients with advanced hormone-refractory prostate cancer (HRPC): Overall survival (OS) results from the phase III satraplatin and prednisone against refractory cancer (SPARC) trial [abstract 5003] J Clin Oncol. 2008;26(suppl 15):250s. [Google Scholar]
- 10.Rini BI. Technology evaluation: APC-8015, Dendreon. Curr Opin Mol Ther. 2002;4:76–79. [PubMed] [Google Scholar]
- 11.Patel PH, Kockler DR. Sipuleucel-T: A vaccine for metastatic, asymptomatic, androgen-independent prostate cancer. Ann Pharmacother. 2008;42:91–98. doi: 10.1345/aph.1K429. [DOI] [PubMed] [Google Scholar]
- 12.Burch PA, Breen JK, Buckner JC, et al. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6:2175–2182. [PubMed] [Google Scholar]
- 13.Burch PA, Croghan GA, Gastineau DA, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: A phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 14.Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 15.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration. Cellular, Tissue and Gene Therapies Advisory Committee: Sipuleucel-T Briefing Document. Dendreon Corporation. [accessed August 12, 2009]. Available at http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007–4291B1_01.pdf.
- 17.American Urological Association. AUA Late-Breaking Science Forum: Review of the Provenge Trial. [accessed August 3, 2010]. Available at http://www.aua2009.org/program/lbsciforum.asp.
- 18.U.S. Food and Drug Administration. U.S. FDA Approves First Cancer Vaccine. [accessed August 3, 2010]. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm210174.htm.
- 19.U.S. Food and Drug Administration. Highlights of Prescribing Information: PROVENGE. [accessed June 25, 2010]. Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM210031.pdf+provenge+product+insert&hl=en&gl=us&pid=bl&srcid=ADGEESi40y_otXMFZNPKTHxc9UQU-wnOck_Gfxu45V_36uMLWQ4p0zGsF_ul4-wE4mIXMUryrhc2pBoA_QEPJlKqN20zh6KMVywCRoqPaobqDMALHfum-Op8SMIz9aK5b6uK4WZ5XnpJ&sig=AHIEtbRhiPUpSetO1jIy1QjOlrvtq53Idw.
- 20.World Health Organization. Geneva, Switzerland: WHO; 1979. Handbook for Reporting Results of Cancer Treatment; pp. 1–45. [Google Scholar]
- 21.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: A review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–1039. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Ilizalitturri FJ, Czuczman MS. Therapeutic options in relapsed or refractory diffuse large B-cell lymphoma. Part 1. Current treatment approaches. Oncology (Williston Park) 2009;23:546–553. [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Gulley JL, Arlen PM, Tsang KY, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones RL, Cunningham D, Cook G, et al. Tumour vaccine associated lymphadenopathy and false positive positron emission tomography scan changes. Br J Radiol. 2004;77:74–75. doi: 10.1259/bjr/19323466. [DOI] [PubMed] [Google Scholar]
- 28.Loveland BE, Zhao A, White S, et al. Mannan-MUC1-pulsed dendritic cell immunotherapy: A phase I trial in patients with adenocarcinoma. Clin Cancer Res. 2006;12:869–877. doi: 10.1158/1078-0432.CCR-05-1574. [DOI] [PubMed] [Google Scholar]
- 29.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 30.Aragon-Ching JB, Jain L, Gulley JL, et al. Final analysis of a phase II trial using sorafenib for metastatic castration-resistant prostate cancer. BJU Int. 2009;103:1636–1640. doi: 10.1111/j.1464-410X.2008.08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson JB, Love W, Chin JL, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478–2487. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantoff P, Glode L, Tannenbaum S, et al. Randomized, double-blind, vector-controlled study of targeted immunotherapy in patients (pts) with hormone-refractory prostate cancer (HRPC) [abstract 2501] J Clin Oncol. 2006;24(suppl 18):100s. [Google Scholar]
- 34.PROSTVAC. Promising Phase II results lead to Phase III in 2010. [accessed June 25, 2010]. Available at http://www.bavarian-nordic.com/pipeline/prostvac.aspx.
- 35.Madan R, Arlen PM, Mohebtash M, et al. Prostvac-VF: A vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18:1001–1011. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halabi S, Vogelzang NJ, Ou SS, et al. Progression-free survival as a predictor of overall survival in men with castrate-resistant prostate cancer. J Clin Oncol. 2009;27:2766–2771. doi: 10.1200/JCO.2008.18.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: Moving beyond current paradigms. Clin Cancer Res. 2007;13:3776–3782. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein WD, Figg WD, Dahut W, et al. Tumor growth rates derived from data for patients in a clinical trial correlate strongly with patient survival: A novel strategy for evaluation of clinical trial data. The Oncologist. 2008;13:1046–1054. doi: 10.1634/theoncologist.2008-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulley J, Stein W, Schlom J, et al. A retrospective analysis of intramural NCI prostate cancer trials: Progress made and insights gleaned. J Clin Oncol. 2010;28(suppl 15):4657. [Google Scholar]
- 42.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 43.Kudo-Saito C, Schlom J, Hodge JW. Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res. 2005;11:2416–2426. doi: 10.1158/1078-0432.CCR-04-1380. [DOI] [PubMed] [Google Scholar]
- 44.Gulley JL, Madan RA, Arlen PM. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine. 2007;25(suppl 2):B89–B96. doi: 10.1016/j.vaccine.2007.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: Effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 47.Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11:4430–4436. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 48.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 49.Petrylak D. Defining the optimal role of immunotherapy and chemotherapy: Advanced prostate cancer patients who receive sipuleucel-T (Provenge) followed by docetaxel derive greatest survival benefit. Presented at the Chemotherapy Symposium 14th Annual Meeting; November 8–11, 2006; New York, NY. [Google Scholar]
- 50.Ma Y, Kepp O, Ghiringhelli F, et al. Chemotherapy and radiotherapy: Cryptic anticancer vaccines. Semin Immunol. 2010;22:113–124. doi: 10.1016/j.smim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Zitvogel L, Apetoh L, Ghiringhelli F, et al. The anticancer immune response: Indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.A Phase III Trial of ZD4054 (Endothelin A Antagonist) in Hormone Resistant Prostate Cancer With Bone Metastases (ENTHUSE M1) [accessed August 3, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00554229?term=zd4054+prostate+cancer&rank=1.
- 53.Egen JG, Kuhns MS, Allison JP. CTLA-4: New insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 54.Allison JP, Chambers C, Hurwitz A, et al. A role for CTLA-4-mediated inhibitory signals in peripheral T cell tolerance? Novartis Found Symp. 1998;215:92–98. doi: 10.1002/9780470515525.ch7. discussion 98–102, 186–190. [DOI] [PubMed] [Google Scholar]
- 55.Gulley JL, Dahut WL. Future directions in tumor immunotherapy: CTLA4 blockade. Nat Clin Pract Oncol. 2007;4:136–137. doi: 10.1038/ncponc0749. [DOI] [PubMed] [Google Scholar]
- 56.O'Day S, Hodi F, McDermott D, et al. A phase III, randomized, double-blind, multicenter study comparing monotherapy with ipilimumab or gp100 peptide vaccine and the combination in patients with previously treated, unresectable stage III or IV melanoma. J Clin Oncol. 2010;28(suppl 18):4. [Google Scholar]
- 57.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 58.National Cancer Institute. FDA Approval for Bevacizumab. [accessed August 3, 2010]. Available at http://www.cancer.gov/cancertopics/druginfo/fda-bevacizumab#Anchor-Metastati-43353.
- 59.Robert N, Dieras V, Glaspy J, et al. Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer [abstract 1005] J Clin Oncol. 2009;27(suppl 15):42s. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 60.Miles D, Chan A, Romieu G, et al. Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO [abstract LBA1011] J Clin Oncol. 2008;26(suppl 15):43s. [Google Scholar]
- 61.Wolmark N, Yothers G, O'Connell M, et al. phase III trial comparing mFOLFOX6 to mFOLFOX6 plus bevacizumab in stage II or III carcinoma of the colon: Results of NSABP Protocol C-08 [abstract LBA4] J Clin Oncol. 2009;27(suppl 15):6s. [Google Scholar]
- 62.Fojo T, Grady C. How much is life worth: Cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst. 2009;101:1044–1048. doi: 10.1093/jnci/djp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madan R, Gulley J, Dahut W, et al. Overall survival (OS) analysis of a phase II study using a pox viral-based vaccine, PSA-TRICOM, in the treatment of metastatic, castrate-resistant prostate cancer (mCRPC): Implications for clinical trial design [abstract 3005] J Clin Oncol. 2008;26(suppl 15):133s. [Google Scholar]
- 65.Palucka A, Fay J, Banchereau J, et al. Melanoma antigen specific Tregs and immune response to vaccination with dendritic cells. Presented at the American Association of Cancer Research Annual Meeting; April 18–22, 2009; Denver, CO. [Google Scholar]