This study evaluated the effects of epoetin alfa on patient-reported outcomes in patients with breast cancer receiving myelotoxic chemotherapy. Early intervention with epoetin alfa was well tolerated and improved anemia-related patient-reported outcomes.
Keywords: Breast Cancer, Anemia, Erythropoietin, Hemoglobin, Fatigue, Survival
Abstract
Purpose.
To evaluate the effects of epoetin alfa on patient-reported outcomes (PROs) in patients with breast cancer receiving myelotoxic chemotherapy.
Materials and Methods.
Women with hemoglobin concentrations ≤12.0 g/dl and an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–3 were randomized 1:1 to receive epoetin alfa (10,000 IU 3 times weekly) or best standard care (BSC) during chemotherapy. The primary endpoint was the change from baseline in the total anemia subscale assessed by the Functional Assessment of Cancer Therapy–Anemia (FACT-An) questionnaire after 12 weeks of treatment. The fatigue and nonfatigue subscales from the FACT-An, the Cancer Linear Analog Scale (CLAS), hemoglobin changes, ECOG PS score, tumor response, overall survival, and safety also were evaluated.
Results.
Of 223 patients randomized, 216 constituted the modified intent-to-treat population. Percentage changes in the total anemia subscale of the FACT-An were significantly different between epoetin alfa treatment (14.2%) and BSC (−0.5%; p = .002), favoring epoetin alfa; so were changes in the FACT-An fatigue subscale (epoetin alfa, 17.5%; BSC, −0.9%; p = .003) and nonfatigue subscale (epoetin alfa, 8.8%; BSC, 0.2%; p = .008). Similar results were observed with the CLAS. Hemoglobin concentrations >12 g/dl were more common with epoetin alfa (62.0%) than with BSC (27.6%). Tumor response, ECOG PS score, 12-month survival rate, and the incidence of serious treatment-emergent adverse events were similar between groups.
Conclusion.
Early intervention with epoetin alfa was well tolerated and improved anemia-related PROs in patients with breast cancer receiving myelotoxic chemotherapy.
Introduction
Breast cancer is one of the most common life-threatening malignancies in women. Survival rates generally have improved as a consequence of increased detection of early-stage disease and the introduction of more effective therapeutic regimens [1]. Maintenance of general health and well-being during and after treatment also is essential, and supportive care has become an important component of overall disease management. Anemia is a frequent complication of cancer that results from the disease and/or cytotoxic treatment regimens. If untreated/not managed properly, anemia can lead to physical and functional impairment, with fatigue and exhaustion being the most common symptoms. Results of the 2004 European Cancer Anemia Survey showed that most cancer patients with anemia (hemoglobin concentration <12.0 g/dl), including those with breast cancer, did not receive treatment for their anemia [2]. A more recent epidemiologic study (the Anaemia Cancer Treatment study) reviewed data from 2,807 patients with cancer-related anemia (hemoglobin concentration <11 g/dl) in 16 European countries [3]. Results showed that treatment patterns followed accepted guidelines, and that judicious use of erythropoiesis-stimulating agents (ESAs) generally was safe and effective.
The therapeutic benefit of correcting chemotherapy-induced anemia through the use of epoetin alfa in patients with cancer has been well documented in double-blind, placebo-controlled, randomized studies [4–6] and in nonrandomized, community-based, open-label studies [7–9]. Results from those studies consistently demonstrate that epoetin alfa significantly increases hemoglobin concentrations or hematocrit and decreases the incidence of blood transfusions when treatment is initiated at hemoglobin concentrations up to 11.5 g/dl, and that successful treatment of anemia is correlated with measurable and clinically beneficial increases in energy level and functional status. The current study was undertaken in women with breast cancer receiving myelotoxic chemotherapy who had hemoglobin concentrations ≤12.0 g/dl for the purpose of evaluating the effects of treatment with epoetin alfa on anemia-related patient-reported outcomes (PROs).
Materials and Methods
Study Population and Design
This was a phase IIIb, randomized, open-label, multicenter study conducted in six European countries. It consisted of a pretreatment visit, four visits during treatment at week 4–6, week ∼9, week 12, and up to week 28, depending on duration of treatment, and two follow-up visits at 6 and 12 months. The protocol was approved by the independent ethics committee at each center, and the study was conducted in accordance with the International Conference on Harmonisation Harmonised Tripartite Guidelines for Good Clinical Practice and with the Declaration of Helsinki, South Africa amendment of 1996. All patients gave written informed consent prior to study entry.
Women aged >18 years with a confirmed diagnosis of breast cancer were included in the study. Patients were mildly anemic (hemoglobin concentration ≤12.0 g/dl) and were receiving myelotoxic chemotherapy for a planned minimum of 12 weeks. The most common chemotherapy agents used were epirubicin, fluorouracil, and cyclophosphamide. Patients also had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–3, a life expectancy ≥6 months, and adequate renal, hepatic, and hematologic function (not the result of transfusion).
Exclusion criteria included the following: uncontrolled hypertension; dysfunction of any organ system not attributable to the malignancy or chemotherapy; active second primary malignancy within the last 3 years (other than basal cell carcinoma or cervical cancer in situ); symptomatic or untreated brain metastases; pregnancy or lactation; hypersensitivity to epoetin alfa; anemia caused by factors other than cancer or its therapy; untreated iron, folic acid, or vitamin B12 deficiency; acute major illness within 7 days of study entry; blood transfusion within 14 days of study entry; major infection within 1 month of study entry; treatment with ESAs within 4 weeks of study entry; and participation in any other anemia-related investigational drug trial within 30 days of randomization.
Eligible patients were randomized 1:1 to receive epoetin alfa (Eprex®; Ortho Biotech Europe, a division of Janssen-Cilag, Ltd, High Wycombe, U.K.) or best standard care (BSC). Epoetin alfa was initiated at 10,000 IU (5,000 IU if the patient was <45 kg) s.c. three times weekly for 4 weeks. Hemoglobin levels were monitored regularly (up to once weekly) until the increase in hemoglobin was stable, after which hemoglobin was monitored at least monthly until study completion (up to week 28). A gradual increase in hemoglobin concentration of up to 2 g/dl per month was recommended. Dose adjustments were made if the hemoglobin concentration had not increased by >1.0 g/dl above baseline after the first cycle of a 4-week cycle of chemotherapy or the first two cycles of a 3-week cycle of chemotherapy, with the intent to maintain hemoglobin concentrations in the range of 12.5–14 g/dl. Epoetin alfa was stopped in cases of a rise in hemoglobin 2 g/dl per month or a hemoglobin concentration >14 g/dl. Patients continued on epoetin alfa until 4 weeks after the end of their last chemotherapy cycle.
Evaluation of Efficacy and Safety
The primary efficacy endpoint was the change from baseline in the total anemia subscale from the Functional Assessment of Cancer Therapy–Anemia (FACT-An) after 12 weeks of treatment. The FACT-An questionnaire includes 20 items, of which 13 are fatigue-associated items and seven are non–fatigue-related items. The Cancer Linear Analog Scale (CLAS) was used subsequently to further assess PROs. The CLAS consists of three linear analog scales that measure energy, ability to do daily activities, and overall quality of life. Each patient was to complete the questionnaires at the pretreatment visit, as well as after 4–6 weeks, after ∼9 weeks, after 12 weeks, and at study completion (up to week 28). The protocol instructed that subjects should be unaware of any hematology results prior to completing the questionnaires. After completion of the FACT-An questionnaire, patients were asked to indicate which item of the questionnaire was of greatest importance to them and therefore that which would be most desirable for the treatment to improve.
Secondary endpoints included hematologic response; scores on the fatigue and nonfatigue subscales of the FACT-An; scores for CLAS energy, ability to do daily activities, and overall quality of life; ECOG PS score; tumor response to chemotherapy; and 6-month and 12-month overall survival rates. The number of patients transfused since the previous visit was assessed for each group at each visit, and the proportion of patients transfused at least once during the treatment period also was determined. Tumor response was assessed by the investigator at the study completion visit (up to week 28). Survival analyses were performed using all long-term follow-up data.
Safety was evaluated by monitoring adverse events (AEs), which were recorded regardless of their relationship to study drug and were rated as mild, moderate, or severe. Hematologic and iron parameters and vital signs also were evaluated.
Statistical Analysis
The target enrollment was 400 women. The study was powered (with 80% power) to detect a 3.65 difference between epoetin alfa and BSC in terms of the FACT-An total anemia subscale score change from baseline to week 12, assuming a common standard deviation (SD) of 12. However, slow and declining enrollment caused the study to be stopped after 223 patients were enrolled. Assuming two-sided tests of statistical significance and a type I error rate of 5%, the sample size was estimated retrospectively to yield 97% power to detect the specified clinically relevant difference of 7.4 [10] in the FACT-An total anemia subscale score, from baseline to week 12, between the epoetin alfa and BSC treatment groups, assuming an SD of 14.1.
The safety population was defined as all randomized patients who received chemotherapy, the modified intention-to-treat (mITT) population was defined as all randomized patients who received chemotherapy and were treated with at least a single dose of epoetin alfa (for the epoetin alfa group) and had at least a single postbaseline assessment of the primary variable, and the per-protocol (PP) population was defined as all patients from the mITT population with no major protocol violations. The mITT analysis was regarded as the primary analysis. Survival analyses were based on the all-patients-randomized (APR) population.
Statistical analyses were performed with SAS (SAS Institute, Cary, NC), version 8.1. FACT-An scores and laboratory parameters, including hemoglobin concentration, hematocrit, reticulocyte count, and transferrin measurement, were compared by one-way analysis of variance (ANOVA) or the Wilcoxon rank sum test. Changes in hemoglobin concentrations were analyzed using analysis of covariance. Tumor stage was compared using the Wilcoxon rank sum test; Pearson's exact test was used to compare metastatic disease, previous surgery, type of chemotherapy, further treatment at follow-up, and blood transfusions during the treatment period. Survival distributions were estimated by Kaplan–Meier curves and were compared by log-rank tests. Because the FACT-An data were skewed at the baseline, the nonparametric Wilcoxon rank sum test was used in parallel with a one-way ANOVA. Changes in PROs between baseline and each monthly visit were analyzed.
Results
Demographic and Baseline Characteristics
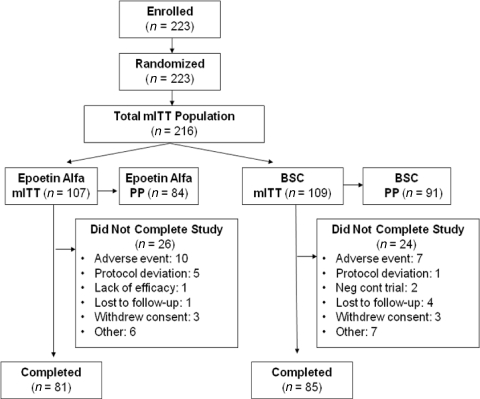
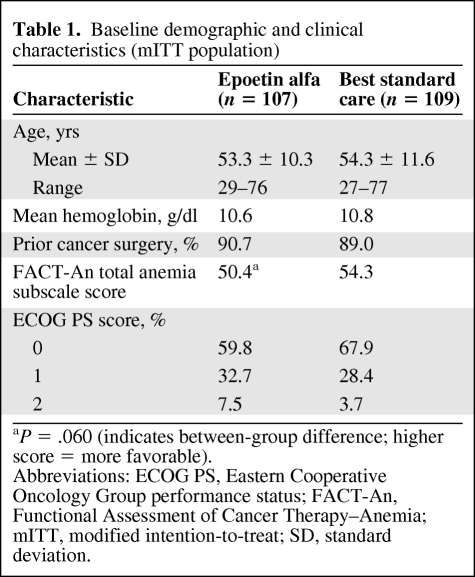
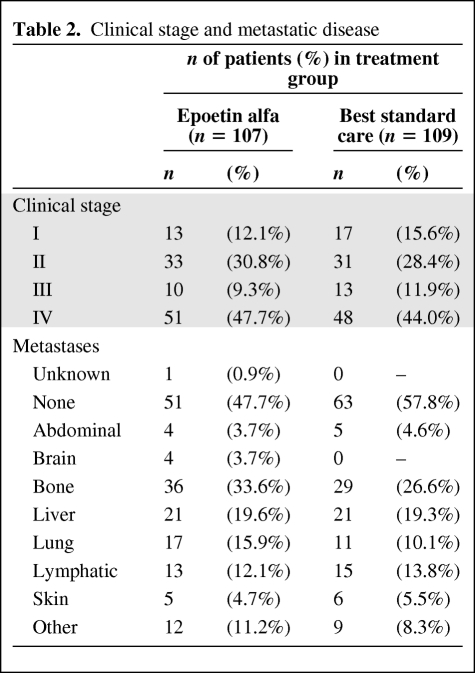
From January 2000 to July 2002, 223 patients were enrolled in this study (Fig. 1). Demographic and baseline characteristics of the mITT population (Table 1) were well balanced between the two groups. The most common staging was stage IV (47.7% of patients) disease (Table 2). The mean (± SD) time on study was 17.2 (± 6.7) weeks overall and was similar between groups.
Figure 1.
Patient disposition (mITT population).
Abbreviations: BSC, best standard care; mITT, modified intention-to-treat; PP, per protocol.
Table 1.
Baseline demographic and clinical characteristics (mITT population)
aP = .060 (indicates between-group difference; higher score = more favorable).
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; FACT-An, Functional Assessment of Cancer Therapy–Anemia; mITT, modified intention-to-treat; SD, standard deviation.
Table 2.
Clinical stage and metastatic disease
FACT-An and CLAS Changes
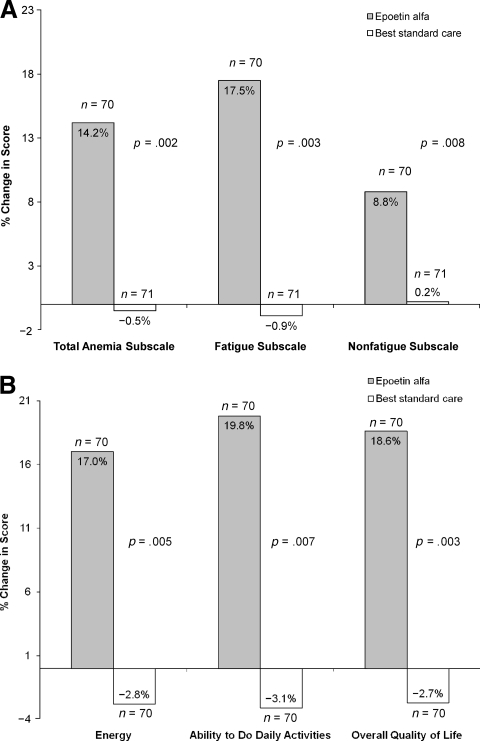
The FACT-An questionnaire was completed by 98.1% (212 of 216) of patients at baseline and by 98.0% (144 of 147) of patients at week 12. As is shown in Figure 2A, the percentage change from baseline in the mean score on the FACT-An total anemia subscale and on the fatigue subscale after 12 weeks of epoetin alfa treatment were significantly different (p = .002 and p = .003, respectively) from those for the BSC group. Even on the FACT-An nonfatigue subscale, which mainly evaluates subjective signs and symptoms of anemia, the change from baseline was significantly better in the epoetin alfa group (p = .008). Differences in the two groups were marked more by improvement in the epoetin alfa group than by deterioration in the BSC group. Changes from baseline to study end (up to week 28) also were significantly better for the epoetin alfa group for the FACT-An total anemia subscale (p < .001, data not shown).
Figure 2.
Comparing patient-reported outcomes between epoetin alfa treatment and best standard care. (A): Percent change in mean FACT-An subscale scores between baseline and week 12 (mITT population). (B): Percent change in mean CLAS score between baseline and week 12 (mITT population).
Abbreviations: CLAS, Cancer Linear Analog Scale; FACT-An, Functional Assessment of Cancer Therapy–Anemia; mITT, modified intention-to-treat.
Figure 2B shows results from the CLAS assessment. All three variables (energy, ability to do daily activities, and overall quality of life) were significantly (p ≤ .007) better than at baseline in the epoetin alfa group compared with the BSC group after 12 weeks of treatment. In addition, between-group differences for all three CLAS change scores from baseline to study end also were statistically significant (p ≤ .001), favoring epoetin alfa (data not shown).
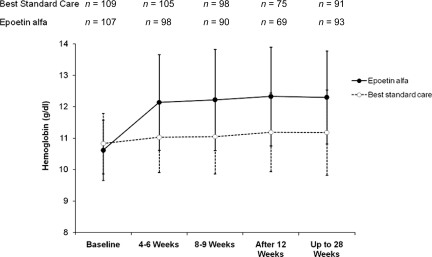
Hemoglobin Response
Figure 3 illustrates the change in hemoglobin over time for the mITT population (similar results were seen in the PP population). In the epoetin alfa group, the mean hemoglobin concentration increased from a baseline of 10.6 g/dl to 12.3 g/dl at the week 12 and week 28 assessments, whereas in the BSC group, it changed from 10.8 g/dl to 11.2 g/dl (p < .001, between-group difference). Hemoglobin and hematocrit levels were the highest within 4–6 weeks after initiation of epoetin alfa therapy and remained stable during the rest of the study. In the mITT population, a higher percentage of patients in the epoetin alfa group achieved a hemoglobin level >12 g/dl during the study period, compared with the BSC group at all evaluation times. At week 12, 62% of patients who received epoetin alfa therapy had achieved a hemoglobin level ≥12 g/dl, versus 28% in the BSC group. No correlation between stage of disease and hematologic response was noted (data not shown).
Figure 3.
Hemoglobin change (mean) over time during the treatment phase (mITT population).
Abbreviation: mITT, modified intention-to-treat.
ECOG PS
In the mITT population, the distributions of patients by ECOG PS score at baseline were similar in the two treatment groups (Table 1). Although no statistically significant differences between the groups were noted at the 12-week visit or at the up-to-28-week visit, in the follow-up period, the number of subjects with an ECOG PS score ≥2 was higher in the epoetin alfa group.
Assessment of Tumor Response
Tumor response was assessed at the end of the study (up to week 28). Among patients in whom a tumor response was recorded (65 patients in the epoetin alfa group and 56 patients in the BSC group), no differences were found between the treatment groups.
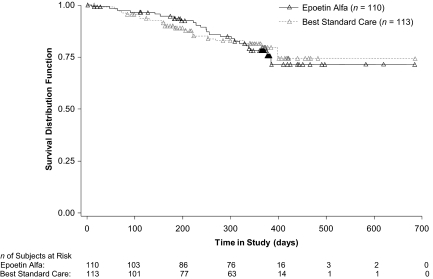
Overall Survival
Patients were followed for 1 year after their last study assessment. In the APR population, 43 patients died during the treatment and follow-up periods: 23 (20.9%) in the epoetin alfa group and 20 (17.7%) in the BSC group, with no statistically significant difference in survival noted between the groups (Fig. 4). The hazard ratio and 95% confidence interval (CI) were 1.054 and 0.578–1.919, respectively, with a p-value of .86. Similar results were seen in the mITT population.
Figure 4.
Overall survival of patients with breast cancer receiving myelotoxic chemotherapy (APR population; n = 223).
Abbreviation: APR, all-patients-randomized.
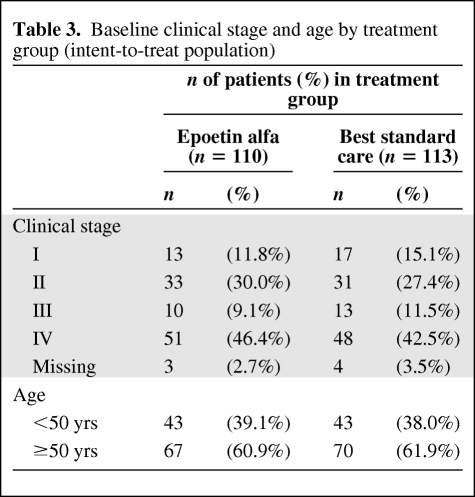
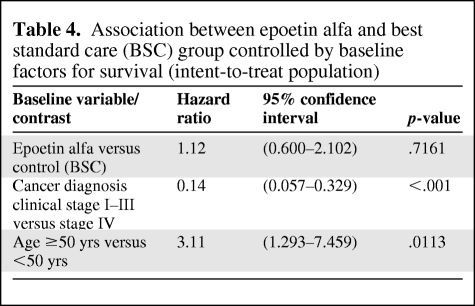
Among all baseline prognostic factors for survival, two factors—cancer diagnostic clinical stage and age (≥50 years versus <50 years)—appear to be significant predictors for survival, irrespective of treatment allocation (Table 3). More specifically, stage IV patients had a statistically significant higher mortality risk than stage I–III patients, and elderly patients (defined as age ≥50 years) had a statistically significant higher mortality risk than younger patients (Table 4). However, these two factors were well balanced between the two treatment groups.
Table 3.
Baseline clinical stage and age by treatment group (intent-to-treat population)
Table 4.
Association between epoetin alfa and best standard care (BSC) group controlled by baseline factors for survival (intent-to-treat population)
Transfusion Requirements
Overall, 16.5% (18 of 109) of patients in the BSC group received at least one transfusion during the treatment period, compared with 7.5% (8 of 107) of patients in the epoetin alfa group (p = .059). A statistically significant difference between the treatment groups in the number of transfusions was observed at the end of the study; significantly fewer patients in the epoetin alfa group than in the BSC group had required transfusions (3.0% versus 10.1%; p = .048).
Safety
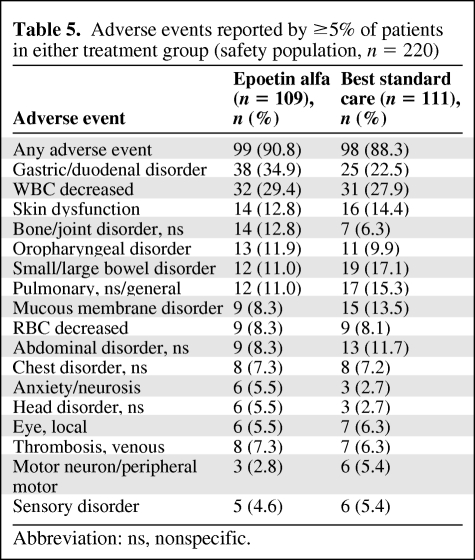
Epoetin alfa was well tolerated. The mean area under the concentration–time curve for epoetin alfa was 26,634 IU (range, 4,534.9–70,000 IU). The two treatment groups were similar in the nature of treatment-emergent AEs reported (Table 5), except for more reports in the epoetin alfa group of gastric/duodenal and bone/joint disorders, and more reports in the BSC group of small/large bowel and mucous membrane disorders. Rates of treatment-emergent venous thrombosis were similar in the two groups.
Table 5.
Adverse events reported by ≥5% of patients in either treatment group (safety population, n = 220)
Abbreviation: ns, nonspecific.
Eighteen patients (16.5%) in the epoetin alfa group reported serious AEs, compared with 16 patients (14.4%) in the BSC group. Serious thrombovascular events occurred in four patients in the epoetin alfa group and in one patient in the BSC group.
Discussion
In this prospective, randomized trial, we evaluated the impact of earlier intervention with epoetin alfa compared with BSC in patients with breast cancer receiving myelotoxic chemotherapy. The primary endpoint was change from baseline in the total anemia subscale of the FACT-An. Patients in the epoetin alfa group showed a significant improvement over baseline values during the course of chemotherapy, whereas patients in the BSC group did not. Changes in FACT-An total anemia, fatigue, and nonfatigue subscales were highly significantly different between the two groups and favored epoetin alfa treatment. These observations were supported by the CLAS results. In addition, >60% of the patients in the epoetin alfa group achieved a hemoglobin concentration >12 g/dl after 12 weeks of treatment, compared with 28% of patients in the BSC group.
Our results are consistent with findings of another recent trial of early intervention with epoetin alfa therapy in patients with breast cancer. In that study, treatment with epoetin alfa (40,000 IU once weekly for at least 12 weeks) maintained or improved hemoglobin concentrations and PROs, whereas decreases in hemoglobin concentrations and declines in PROs were observed in patients who received placebo [11].
Epoetin alfa was well tolerated in the current study, and almost all the AEs reported appeared to be related to chemotherapy. Although the rates of treatment-emergent venous thrombosis were found to be similar in the two treatment arms, serious thrombovascular events were more frequent in the epoetin alfa group. A higher incidence of thrombovascular events is considered a known and labeled event in cancer patients receiving ESAs. Although our study was neither designed nor powered to detect differences in survival, it is important to note that the survival curves were overlapping at 12 months.
However, some investigational clinical studies have suggested that ESA therapy might be harmful in certain groups of patients with cancer [12–14]. One of these studies was the Breast Cancer Erythropoietin Survival Trial. That large clinical trial enrolled 939 patients with metastatic breast cancer receiving first-line chemotherapy. Those with hemoglobin concentrations ≤13 g/dl were treated with either epoetin alfa or placebo for 12 months [13]. The study was stopped early because of poorer overall survival in the epoetin alfa arm than in the placebo arm over the 12 months (70% versus 76%; p = .01). However, most of the survival difference occurred during the first 4 months (epoetin alfa, 41 deaths; placebo, 16 deaths); the numbers of deaths over the subsequent period were similar (epoetin alfa, 97; placebo, 95). Furthermore, other studies evaluating the effect of ESAs in patients with breast cancer have reported comparable findings on survival and/or tumor progression in ESA-treated patients versus standard of care. In a multicenter phase III trial evaluating the effect of epoetin alfa on anemia and survival, 658 patients with breast cancer receiving a dose-dense epirubicin–cyclophosphamide–paclitaxel regimen (ETC) [15] were randomized to receive epoetin alfa (n = 333) or no epoetin alfa. Anemia was significantly less prevalent in the ETC–epoetin alfa arm (p < .0001). At a median follow-up of 62 months, no difference between the ETC-only arm and the ETC–epoetin alfa arm was observed in the 5-year disease-free survival rate (71% versus 72%, respectively; p = .86) and in the overall survival rate (83% versus 81%, respectively; p = .89) [15]. Similar results were observed in two other studies of ESAs in patients with breast cancer [16, 17]. A randomized, double-blind, multicenter study (Preoperative Epirubicin-Paclitaxel-Aranesp, PREPARE) evaluated the effects of darbepoetin in combination with chemotherapy in patients with breast cancer (n = 773); no difference in tumor response or progression was observed in those who received darbepoetin (n = 356) versus those who did not (n = 377), although the survival duration was shorter and there were more relapses in the group of patients treated with darbepoetin [17]. A randomized, open-label study evaluated the effects of epoetin beta on overall survival in patients with metastatic breast cancer receiving chemotherapy (Breast Cancer Anemia and the Value of Erythropoietin, BRAVE, n = 463) [16]. At 18 months of follow-up, no significant difference was detected in overall survival (hazard ratio, 1.07; 95% CI, 0.87–1.33) or progression-free survival (hazard ratio, 1.07; 95% CI, 0.89–1.30) between the epoetin beta group (n = 231) and the control group (n = 232). However, the authors cautioned that, because of the design of the study, clinically important differences in survival could not be excluded with absolute certainty.
Conclusion
In conclusion, the results of this study demonstrate that epoetin alfa is generally well tolerated and can protect against anemia and attenuate deteriorations in anemia-related PROs when administered early during the course of chemotherapy to patients with mild to moderate anemia. In particular, this early intervention allows patients to maintain a positive sense of well-being and ability to function, to experience less fatigue during chemotherapy, potentially avoiding the deterioration associated with more severe anemia, and to maintain productivity [18, 19].
Acknowledgments
Financial support for this study was provided by Johnson & Johnson Pharmaceutical Research & Development, L.L.C., Raritan, NJ.
Preliminary data from our study were presented previously at the 27th Congress of the European Society for Medical Oncology (ESMO), October 18–22, 2002, Nice, France: Pronzato P, Cortesi E, van der Rijt C et al. Early intervention with epoetin alfa in breast cancer (BC) patients (pts) undergoing chemotherapy (CT): Results of a randomized, multicenter, phase IIIb study (EPO-INT-47 Study Group) [abstract 620O]. Ann Oncol 2002;13(suppl 5):168.
Author Contributions
Conception/Design: Paolo Pronzato, Ricardo Rosso, Enrico Cortesi, Carin C. van der Rijt
Provision of study material or patients: Paolo Pronzato, Peter J. Ostler, Ricardo Rosso, Enrico Cortesi, Carin C. van der Rijt, Alain Bols, José A. Moreno-Nogueira, Carlos Freire de Oliveira, Peter Barrett-Lee
Collection and/or assembly of data: Paolo Pronzato, Peter J. Ostler, Ricardo Rosso, Enrico Cortesi, Carin C. van der Rijt, Alain Bols, José A. Moreno-Nogueira, Carlos Freire de Oliveira, Peter Barrett-Lee
Data analysis and interpretation: Paolo Pronzato, Ricardo Rosso, Enrico Cortesi, Carin C. van der Rijt
Manuscript writing: Paolo Pronzato, Ricardo Rosso, Enrico Cortesi, Carin C. van der Rijt
Final approval of manuscript: Paolo Pronzato, Peter J. Ostler, Ricardo Rosso, Enrico Cortesi, Carin C. van der Rijt, Alain Bols, José A. Moreno-Nogueira, Carlos Freire de Oliveira, Peter Barrett-Lee
The authors take full responsibility for the content of the paper but gratefully acknowledge the assistance of Els Vercammen, M.D., (Johnson & Johnson Pharmaceutical Research & Development, L.L.C.) for contributing to the concept and design, acquisition of data, analysis and interpretation, and editorial assistance; Steven Sun, Ph.D., (Johnson & Johnson Pharmaceutical Research & Development, L.L.C.) for contributing to the concept and design, acquisition of data, analysis and interpretation, and editorial assistance; Dennis D. Gagnon, M.S., (Johnson & Johnson Pharmaceutical Research & Development, L.L.C.) for contributing to the concept and design, acquisition of data, analysis and interpretation, and editorial assistance; and Namit Ghildyal, Ph.D., (Johnson & Johnson Pharmaceutical Research & Development, L.L.C.) for assistance with drafting/revising the article and editorial assistance. The authors also thank Roseanne Degnan, Pharm.D., Jinling Wu, Ph.D., and Candace Lundin, D.V.M., M.S., of Scientific Connexions Newtown, PA, for providing medical writing and editing services including assembling tables, figures, preparing references, and grammatical assistance. Financial support provided by Johnson & Johnson Pharmaceutical Research & Development, L.L.C.
References
- 1.American Cancer Society. Overview: Breast Cancer. [accessed November 9, 2009]. Available at http://www.cancer.org.
- 2.Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40:2293–2306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig H, Aapro M, Bokemeyer C, et al. Treatment patterns and outcomes in the management of anaemia in cancer patients in Europe: Findings from the Anaemia Cancer Treatment (ACT) study. Eur J Cancer. 2009;45:1603–1615. doi: 10.1016/j.ejca.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Abels R. Erythropoietin for anaemia in cancer patients. Eur J Cancer. 1993;29A(suppl 2):S2–S8. doi: 10.1016/s0959-8049(05)80281-3. [DOI] [PubMed] [Google Scholar]
- 5.Littlewood TJ, Bajetta E, Nortier JW, et al. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: Results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19:2865–2874. doi: 10.1200/JCO.2001.19.11.2865. [DOI] [PubMed] [Google Scholar]
- 6.Witzig TE, Silberstein PT, Loprinzi CL, et al. Phase III, randomized, double-blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. J Clin Oncol. 2005;23:2606–2617. doi: 10.1200/JCO.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Glaspy J, Bukowski R, Steinberg D, et al. Impact of therapy with epoetin alfa on clinical outcomes in patients with nonmyeloid malignancies during cancer chemotherapy in community oncology practice. Procrit Study Group. J Clin Oncol. 1997;15:1218–1234. doi: 10.1200/JCO.1997.15.3.1218. [DOI] [PubMed] [Google Scholar]
- 8.Demetri GD, Kris M, Wade J, et al. Quality-of-life benefit in chemotherapy patients treated with epoetin alfa is independent of disease response or tumor type: Results from a prospective community oncology study. Procrit Study Group. J Clin Oncol. 1998;16:3412–3425. doi: 10.1200/JCO.1998.16.10.3412. [DOI] [PubMed] [Google Scholar]
- 9.Gabrilove JL, Cleeland CS, Livingston RB, et al. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: Improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol. 2001;19:2875–2882. doi: 10.1200/JCO.2001.19.11.2875. [DOI] [PubMed] [Google Scholar]
- 10.Cella D, Eton DT, Lai JS, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 11.Chang J, Couture F, Young S, et al. Weekly epoetin alfa maintains hemoglobin, improves quality of life, and reduces transfusion in breast cancer patients receiving chemotherapy. J Clin Oncol. 2005;23:2597–2605. doi: 10.1200/JCO.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 13.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: A survival study. J Clin Oncol. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 14.Overgaard J, Hoff C, Sand Hansen H, et al. Randomized study of the importance of novel erythropoiesis stimulating protein (Aranesp®) for the effect of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC): The Danish Head and Neck Cancer Group (DAHANCA 10) randomized trial. Eur J Cancer. 2007;5(suppl):7. [Google Scholar]
- 15.Moebus V, Lueck H, Thomssen C, et al. The impact of epoetin-alpha on anemia, red blood cell (RBC) transfusions, and survival in breast cancer patients (pts) treated with dose-dense sequential chemotherapy: Mature results of an AGO phase III study (ETC trial) [abstract 569] J Clin Oncol. 2007;25(20 suppl):20s. [Google Scholar]
- 16.Aapro M, Leonard RC, Barnadas A, et al. Effect of once-weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and/or taxane-based chemotherapy: Results of the Breast Cancer-Anemia and the Value of Erythropoietin (BRAVE) study. J Clin Oncol. 2008;26:592–598. doi: 10.1200/JCO.2007.11.5378. [DOI] [PubMed] [Google Scholar]
- 17.Untch M, Fasching PA, Bauerfeind I, et al. PREPARE trial. A randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel and CMF with a standard dosed epirubicin/cyclophosphamide followed by paclitaxel {+/-} darbepoetin alfa in primary breast cancer: A preplanned interim analysis of efficacy at surgery [abstract 517] J Clin Oncol. 2008;26(10 suppl):10s. [Google Scholar]
- 18.Cella D. The effects of anemia and anemia treatment on the quality of life of people with cancer. Oncology (Williston Park) 2002;16(suppl 10):125–132. [PubMed] [Google Scholar]
- 19.Cella D, Kallich J, McDermott A, et al. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: Results from five randomized clinical trials. Ann Oncol. 2004;15:979–986. doi: 10.1093/annonc/mdh235. [DOI] [PubMed] [Google Scholar]