The incidence of melanoma is increasing worldwide, and the prognosis for patients with high-risk or advanced metastatic melanoma remains poor despite advances in the field. A systematic literature review of treatments for advanced, metastatic disease was conducted to present the success of current treatments and the promise of those still in clinical development that may yield incremental improvements in the treatment of advanced, metastatic melanoma. Advances in the understanding of the mechanism of chemotherapy resistance offer the hope for improved results with chemotherapy, and the triumvirate of more effective chemotherapy, immunotherapy, and targeted therapy are likely to be combined with one another for significant advances in melanoma over the coming few years.
Keywords: Melanoma, Adjuvant treatment, Interferon-alpha, Vaccines, Chemotherapy, CTLA-4, BRAF-inhibitors
Abstract
The incidence of melanoma is increasing worldwide, and the prognosis for patients with high-risk or advanced metastatic melanoma remains poor despite advances in the field. Standard treatment for patients with thick (≥2.0 mm) primary melanoma with or without regional metastases to lymph nodes is surgery followed by adjuvant therapy or clinical trial enrollment. Adjuvant therapy with interferon-α and cancer vaccines is discussed in detail. Patients who progress to stage IV metastatic melanoma have a median survival of ≤1 year. Standard treatment with chemotherapy yields low response rates, of which few are durable. Cytokine therapy with IL-2 achieves durable benefits in a greater fraction, but it is accompanied by severe toxicities that require the patient to be hospitalized for support during treatment. A systematic literature review of treatments for advanced, metastatic disease was conducted to present the success of current treatments and the promise of those still in clinical development that may yield incremental improvements in the treatment of advanced, metastatic melanoma.
Introduction
The incidence of melanoma is increasing worldwide, with a growing fraction of patients with advanced disease for which prognosis remains poor [1]. Treatment options are limited despite advances in immunotherapy and targeted therapy. For patients with surgically resected, thick (≥2 mm) primary melanoma with or without regional lymph node metastases, the only effective adjuvant therapy is interferon-α (IFN-α) [2]. However, because of the limited benefit upon disease-free survival and the smaller potential improvement of overall survival [3, 4], the indication for IFN-α treatment remains controversial. Standard recommended therapy for patients with stage IV metastasis according to the American Joint Committee on Cancer (AJCC) is single-agent dacarbazine (Bedford Laboratories, Bedford, Ohio), but responses to this agent and its oral analogue, temozolomide (Merck & Co. Inc., Whitehouse Station, New Jersey), are <15% and generally transient [5]. Among other treatment options, immunotherapy with high-dose interleukin (IL)-2 achieves long-term, durable, complete responses in a small percentage of patients but has never been established in a formal, phase III, randomized comparative study [6]. Biochemotherapy increases objective response rates but has not been shown to significantly improve survival compared with chemotherapy alone and is associated with additive toxicity [7–10]. Clearly, new therapies are needed.
Adjuvant therapies for high-risk melanoma and therapies for advanced, metastatic melanoma will be discussed. This systematic literature review was performed to update a previous review of 41 randomized trials published through 2001 [11] and to identify new randomized trials that may serve to change the paradigm of melanoma treatment in the future. Thus, this review augments and provides a current analysis of randomized trials in metastatic melanoma. Additionally, clinical trial databases have been reviewed to identify and overview ongoing trials in melanoma worldwide.
Methods
Search Strategy and Selection Criteria
A systematic search strategy was applied as used previously [11]; this review updates previous analyses. The Medline database was searched for articles published between January 1, 2002, and June 5, 2010. A combination of MeSH headings was used: “melanoma, advanced”; “melanoma, metastatic”; or “melanoma, disseminated” with the term “randomized clinical trial” for trials in advanced disease and “melanoma, adjuvant” or “melanoma, interferon” for adjuvant IFN-α trials for the trials conducted in the adjuvant setting. Searches were limited to clinical trials and publications in English, French, Italian, or German. The “related articles” feature of PubMed was used for all reports that met the requested criteria as an additional means of identifying potentially relevant investigations. The abstract databases of the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology annual congresses were searched for further up-to-date clinical trials. Textbook chapters and review articles were also consulted. Additionally, in all reports, the list of references was reviewed to find further relevant publications.
Statistical Analysis
Data derived from adjuvant trials with IFN-α were analyzed using RevMan (The Cochrane Collaboration, http://www.cc-ims.net/RevMan) software version 5.0. The standardized mean differences and 95% confidence interval (CI) were computed and displayed graphically.
Adjuvant Therapy of Melanoma
Patients with thick (>2.0 mm according to 2009 AJCC/International Union Against Cancer [UICC] classification) primary melanoma and regional lymph node metastases are at increased risk of recurrence and death [12]. Surgery is the standard treatment [13, 14], and surgical excision margins should be based on Breslow's tumor thickness [15, 16]. Complete lymphadenectomy is recommended for patients with involved regional nodes [13, 14]. Current recommendations for patients with stage II (>2.0 mm according to AJCC/UICC classification, but negative nodes) melanoma are for adjuvant therapy with IFN or enrollment in a clinical trial [13, 14]. Patients with stage III melanoma typically undergo complete lymphadenectomy followed by adjuvant therapy with IFN or enrollment in a clinical trial of adjuvant therapy [13, 14]. Over the past 25 years, adjuvant therapy for immediate-risk (stage II and IIIA) and high-risk (stage IIIB as well as resectable stage IV M1a, M1b) patients have shifted from regional radiotherapy, systemic immunostimulants such as Bacillus Calmette-Guerin (BCG) and Corynebacterium parvum, or pharmacologic immunomodulators such as levamisole, to recombinant DNA-produced biologic agents such as IFN-α, granulocyte-macrophage colony-stimulating factor, and antibodies that have immunoregulatory function such as those that block cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) [17, 18].
IFN-α
IFN-α2b (Intron A, Merck & Co. Inc., Whitehouse Station, New Jersey) was the first agent to show a significant survival benefit in patients with high-risk melanoma in a randomized, controlled trial [19]. In the Eastern Cooperative Oncology Group (ECOG) trial E1684, patients (N = 287) with high-risk resected cutaneous melanoma and regional lymph node metastases were randomized to standard observation or to receive IFN-α2b (20 million units [MIU]/m2 per day) intravenously for 1 month and 10 MIU/m2 subcutaneously 3 times per week for 48 weeks. Overall survival was significantly prolonged with IFN-α2b after a median follow-up time of 6.9 years (median overall survival 3.82 years [95% CI = 2.34–7.08] with IFN-α2b vs. 2.78 years [95% CI = 1.83–4.03] with observation only; p-value = .0237) [19]. Subsequent to this first positive trial, a number of studies have attempted to identify the optimal dose, schedule, and duration of IFN-α for adjuvant therapy (Figs. 1 and 2) [20–29]. In a follow-up trial comparing patients (N = 642) receiving high-dose IFN-α2b (HDI) for 1 year, low-dose IFN-α2b (LDI) for 2 years, or observation, relapse-free survival (RFS) was significantly enhanced with HDI versus observation (p = .03), but overall survival was not improved [21]. Although LDI was associated with a greatly reduced incidence of grade 3/4 adverse events (AEs) compared with HDI (1 [0.5%] vs. 17 [8.0%] grade 4 AEs, respectively) and the early RFS benefit was equivalent to HDI after 3–4 years, LDI failed to achieve statistically significant improvement in RFS or durable impact on relapse in this trial. It is notable that this trial was conducted in part before and in part after the U.S. FDA approval of HDI, and follow-up evaluation of patients assigned to observation in the trial demonstrated that 37 patients had been treated at subsequent nodal relapse with HDI, offering an explanation for the absence of an effect upon survival in this experience. In a controlled trial of two lower doses of IFN conducted in patients (N = 1388) randomized to observation or to treatment with an intermediate dose of IFN-α2b (4 weeks with 10 MIU administered 5 times per week, followed by 10 MIU 3 times per week for 1 year or 5 MIU 3 times per week for 2 years) for 13 or 25 months, intermediate-dose IFNα-2b did not significantly improve distant metastasis-free interval or overall survival outcomes [22]. Low-dose IFN-α2b also failed to improve survival outcomes versus observation alone when patients were randomized to treatment with 3 MIU 2 times weekly for 6 months (N = 95) or 3 MIU 3 times weekly for 2 years (N = 674) or 3 years (N = 444) [23, 25, 29]. However, LDI did improve disease-free survival compared versus observation alone when patients (N = 311) received 3 MIU daily for 3 weeks and then 3 times weekly for 1 year (p = .02) and when patients (N = 499) were treated with 3 MIU 3 times weekly for 18 months (p = .038) [24, 27].
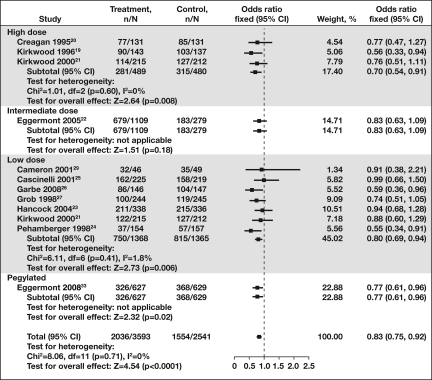
Figure 1.
Forest plot of disease-free survival of patients with high-risk melanoma treated with various doses of IFN-α adjuvant therapy. Disease-free survival among patients with high-risk melanoma was improved with IFN-α adjuvant therapy compared to control (p < .0001; odds ratio = 0.83; 95% CI = 0.75–0.92). Treatment improved disease-free survival compared with control regardless of dose or pegylation of the adjuvant IFN. Data analysis was performed using the program RevMan (The Cochrane Collaboration).
Abbreviations: CI, confidence interval; IFN-α, interferon-α; N, total number of patients per group; n, number of patients with disease progression.
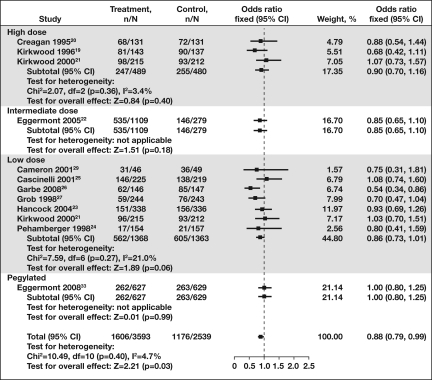
Figure 2.
Forest plot of overall survival in high risk patients treated with adjuvant interferon-α (IFN-α). Overall survival among patients with high-risk melanoma was improved with IFN-α adjuvant therapy compared to control (p < 0.03; odds ratio = 0.88; 95% CI = 0.79–0.99). Treatment improved overall survival compared with control regardless of dose or pegylation of the adjuvant IFN.
Abbreviations: CI, confidence interval; N, total number of patients per group; n, number of patients with disease progression.
The large number of clinical trials testing variations in dosage, schedule, and duration of IFN-α administration, and exploring these effects in different patient populations, has prompted a number of attempts to consolidate and review the available outcome data [3, 4, 30–32]. A meta-analysis of the available published data from randomized clinical trials was reported in 2007, summarizing event-free survival and overall survival in patients with high-risk melanoma treated with IFN-α adjuvant therapy [32]. Clinical data were sorted by IFN dose: high (20 MIU/m2), intermediate (5–10 MIU/m2), low (3 MIU/m2), and very low (1 MIU/m2) doses [32]. Groups were also stratified by duration of treatment (<6 months, 12–18 months, or >24 months). Although there was a statistically significant overall survival benefit for treatment of patients with IFN-α (p = .008), this assimilation did not find evidence of a clear difference in overall survival with different dose levels (p = .8) or durations of treatment (p = .9) [32]. Thus, adjuvant IFN improves overall survival of patients with stage II/III melanoma, although the absolute benefit in terms of survival rate at 5 years was small (∼3%, 95% CI = 1%–5%). In this review, disease-free and overall survival of patients treated with high-dose, intermediate-dose, low-dose, and pegylated IFN (PEG–IFN) were compared (Figs. 1 and 2) [19–27, 29, 33]. Similar to the meta-analysis by Wheatley et al. [32], there was a clear benefit for disease-free survival (odds ratio [OR] = 0.83; 95% CI = 0.75–0.92) and overall survival (OR = 0.88; 95% CI = 0.79–0.99) with adjuvant therapy using IFN-α, regardless of dose, schedule, duration, or formulation (pegylation).
Current trials are assessing the tolerability and efficacy of more intense but shorter regimens of dosing with IFN-α2b [34] or PEG–IFN-α2a and PEG–IFN-α2b [35, 36]. A fundamental question has been posed regarding whether the benefit of IFNs occurs through durable immunologic, antiangiogenic, or other antitumor mechanisms that would require prolonged, and perhaps indefinite, exposure to IFN. A recent phase III European Organization for Research and Treatment of Cancer (EORTC) study (N = 1256) showed that prolonged treatment with PEG–IFN-α2b (for up to 5 years) significantly improved recurrence-free survival compared with observation in patients with resected stage III melanoma (328 events compared with 368 events; hazard ratio (HR) = 0.82, 95% CI = 0.71–0.96; p = .01) [33]. This effect is remarkably consistent with the aggregate assessment of IFN effects on relapse in melanoma [3]. In the EORTC trial, both gross nodal disease and microscopic nodal disease were included, but the benefit of therapy appeared to be confined to the subset of patients with microscopic metastatic disease [33]. The magnitude of this effect appears to be less than the magnitude of the HDI effect, and there is no hint of a survival benefit with this regimen (HR = 0.99). Many patients in the PEG–IFN-α2b arm (191 of 627; 31%) discontinued therapy because of toxicities [33]. The median duration of dosage, despite the initial goals of this trial, was 12 months. A trial of the European Association of Dermatologic Oncology investigated adjuvant treatment efficacy of low-dose PEG–IFN-α2b (100 μg/wk) for 36 months in comparison to classic LDI for 18 months and did not find differences in disease-free or overall survival [37].
Other studies have investigated the benefit of derivative components of the approved regimen of HDI, and specifically the benefit of intravenous high-dose induction therapy alone. A randomized phase III trial by the Hellenic Cooperative Oncology Group tested a modified HDI regimen versus a modified HDI induction regimen and showed that 1 month of modified intravenous IFN-α2b induction was not inferior to 1 year of modified HDI therapy [38]. The current U.S. intergroup trial tests the IV HDI induction regimen versus observation. An early report on a study of DeCOG has found that 1 month of HDI induction therapy adds no benefit to LDI given for 2 years [39].
To date, the mechanism of the therapeutic effects of IFN-α is not completely known. A neoadjuvant trial with HDI applied for 4 weeks before complete lymphadenectomy in patients with macrometastatic lymph node metastases and histologic comparison of pre- and post-treatment specimens implicated an indirect immunomodulatory mechanism [40]. In this trial, 20 patients who had bulky nodal disease at presentation or recurrence were enrolled. Remarkably, 55% of patients demonstrated objective clinical response after only 20 doses of HDI, including 3 with a complete response demonstrated pathologically. The examination of the nodal tumor tissue taken before and after 20 doses of IFN revealed increased infiltration of the tumor tissue by dendritic cells (DCs) marked by CD11a; T cells positive for CD3, CD4, and CD8; and striking ablation of the STAT3 expression that is typically constitutively active in melanoma. These findings argue that HDI has an immunologic mechanism of action. Additional data from Yurkovetsky et al. [41] revealed significant decreases of serum levels of immunosuppressive and tumor angiogenic/growth stimulatory factors and increased levels of antiangiogenic IFN-γ inducible protein 10 under IFN-α treatment. This study also demonstrates a profile of pro-inflammatory cytokines that may serve to predict response to therapy.
Treatment benefits with IFN-α, however, remain suboptimal, and the balance of this benefit weighed against the toxicity and cost of therapy has led to differences in the therapy pursued in different countries. In all western nations, HDI has received regulatory approval for stage III melanoma. In the United States, where three randomized controlled trials have shown this regimen to be superior to observation, as well as superior to LDI and a ganglioside vaccine, most physicians offer this treatment to patients. In Europe, LDI has also been approved for stage II melanoma, and this treatment is offered to patients. However, despite regulatory approval, many countries (e.g., United Kingdom, Scandinavian countries, Australia, etc.) do not have financial support for treatment with HDI and do not routinely offer HDI to patients.
Cancer Vaccines and Immunotherapies
Cancer vaccines have been pursued in hopes of enhancing immune recognition and effector antitumor immune responses through improved antigen presentation and the ability to elicit effector memory T-cell responses that are durable [42]. Increased knowledge of the relevant antigenic epitopes capable of eliciting antitumor immunity has prompted a variety of vaccine approaches. Although vaccines are well tolerated, they rarely have been monitored with methods that are now recognized to be critical to detect whether or not the vaccine induced an immune response. Not surprisingly, the large phase III trials of older crude tumor cell vaccines have not demonstrated robust clinical evidence of antitumor activity either in advanced disease or in the adjuvant setting [43]. A randomized, phase III study (ECOG 1694) compared HDI with the ganglioside vaccine GMK (ganglioside conjugate [GM2] coupled to keyhole limpet hemocyanin and formulated with adjuvant QS-21 [Progenics Pharmaceuticals, Tarrytown, New York]) as adjuvant therapies for patients with high-risk melanoma [44]. The GMK vaccination induces antibodies against GM2 capable of specifically binding GM2 and killing melanoma cells in vitro through complement or antibody-dependent cell-mediated cytotoxicity. Patients were documented to have generated robust antibody immune responses to GM2 in this trial, and those who had immunity to GM2 with these antibodies had a trend to an improved survival. However, the study was terminated early when interim analyses demonstrated a markedly inferior relapse-free and overall survival among patients who received GMK vaccine compared with patients who received HDI [44]. Subsequent analyses comparing pre- and post-treatment patient sera found that GMK had induced persistent (for at least 1 year) antibodies in ∼80% of vaccinated patients [45], so these results were not explained by a lack of immune activation. In two other randomized, phase III trials, postoperative adjuvant therapy with BCG alone or in combination with an allogeneic melanoma vaccine (Canvaxin; Micromet, Munich, Germany) tested as adjuvant treatment for patients with resected stage III (n = 1160) or stage IV (n = 496) melanoma was terminated because of the statistically inferior relapse-free and overall survival of patients (stage III) who received Canvaxin plus BCG, as well as the futility of the original hypothesis that Canvaxin would show an improvement in relapse-free and overall survival [46]. The fact that relapse-free and overall survival was shorter among patients who received BCG plus vaccine suggests that the vaccine may have induced tolerance to tumor antigens rather than effector immunity. This question also arises in the context of another large adjuvant vaccine trial in patients with high-risk melanoma. The EORTC 18961 trial (N = 1314) in patients with stage II melanoma compared vaccination with a synthetic GM2 vaccine and observation. This trial was prematurely halted after the second interim analysis (267 recurrences, median follow-up of 1.8 years) because, for the primary endpoint (RFS), the criteria for stopping for futility were met. For distant metastasis-free and overall survival, the results suggested no advantageous effects of the vaccine cohort (143 vs. 152 events, p = .36; 112 vs. 124 events, p = .25) [47].
Currently, a large phase III vaccine trial is ongoing in patients with stage IIIB/C melanoma whose tumors express MAGE-A3 antigen in lymph node metastases [48]. This vaccine is conceptually quite different from the previously described vaccines because it targets a cancer germline family antigen that has been considerably better defined than the crude Canvaxin preparation, and it induces effector T-cell responses rather than antibody responses.
Peptide vaccines are another category of vaccine that has been studied in large multicenter ECOG trials in the United States. These studies in patients with advanced metastatic melanoma revealed the induction of immune responses; more than one-third of vaccinated patients had increased T-cell production of IFN gamma as detected by ELISPOT. Of interest, those patients who demonstrated immune response to any of the three peptides studied (gp100, MART-1/Melan-A, and tyrosinase HLA-A2 epitopes) had significantly improved survival that was nearly double that of patients who did not develop immunity to 1 or more of the peptide vaccine epitopes. This triple vaccine has now also been evaluated in the placebo-controlled E4697 intergroup adjuvant trial (N = 815), for which results are pending final evaluation [49].
In contrast to the various vaccines discussed above, antibodies to immunoregulatory checkpoint molecules such as CTLA-4 (for details, see below) have been shown to elicit a broader stimulation of the immune system, with autoimmune toxicities that are reminiscent of IL-2 [6] (details below), and the clinical and serologic evidence of autoimmunity associated with IFN-α2b [50]. An adjuvant therapy with CTLA-4–blocking antibodies compared against placebo is presently being conducted by the EORTC, whereasthe ECOG and U.S. Intergroup are conducting studies of these antibodies compared with HDI [51].
Treatment of Metastatic Melanoma
Among patients with AJCC stage IV metastatic melanoma, median survival time is estimated to be ∼8 months (±2 months), and only ∼10% patients survive >5 years from diagnosis of metastatic melanoma [12]. In the United States, there are only two agents approved for treatment of patients with metastatic melanoma: dacarbazine and high-dose IL-2. Current consensus is that there is no single standard therapy for metastatic melanoma [13, 14], and single agents are not likely to prove to be effective. Neither of the FDA-approved systemic therapies has been ever been shown to significantly prolong survival in phase III trials in patients with advanced stage IV melanoma [14].
Chemotherapy
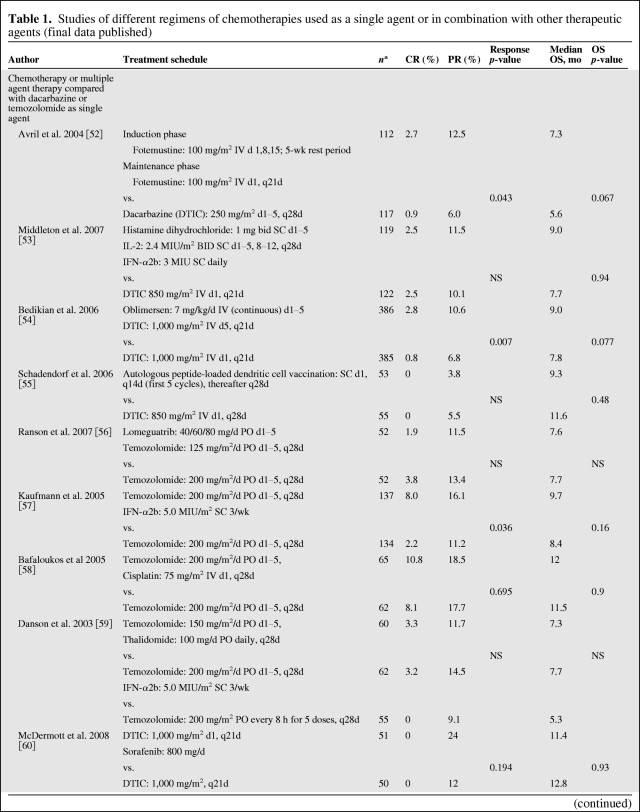
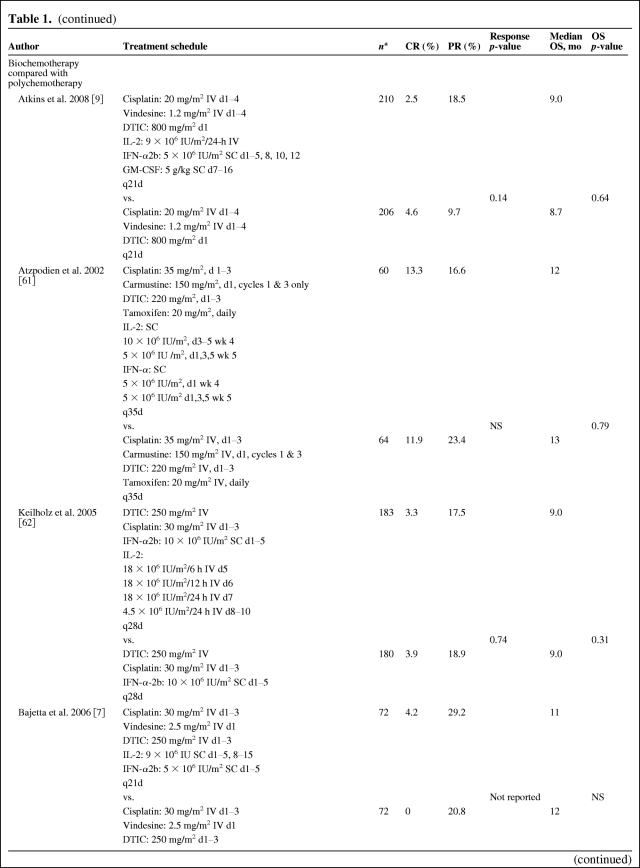
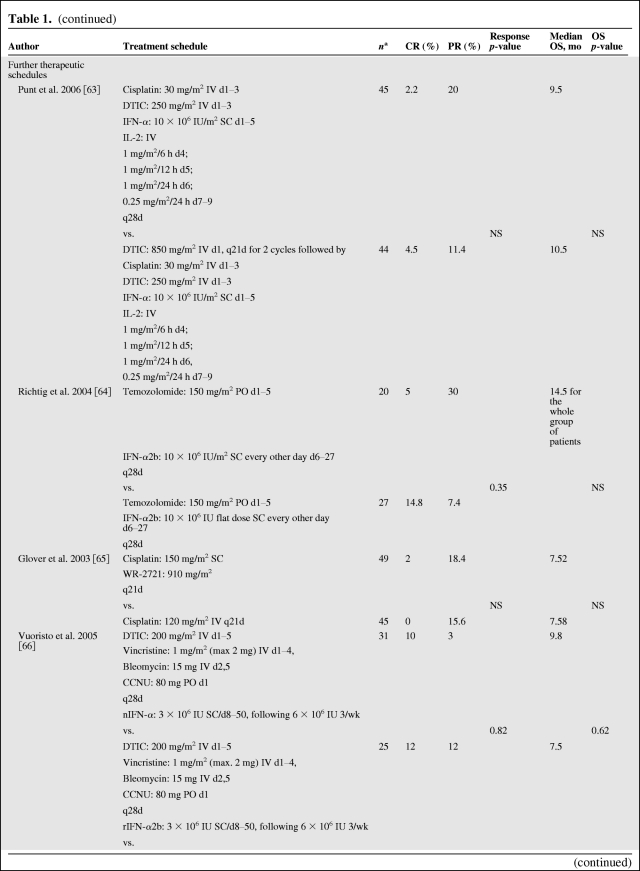
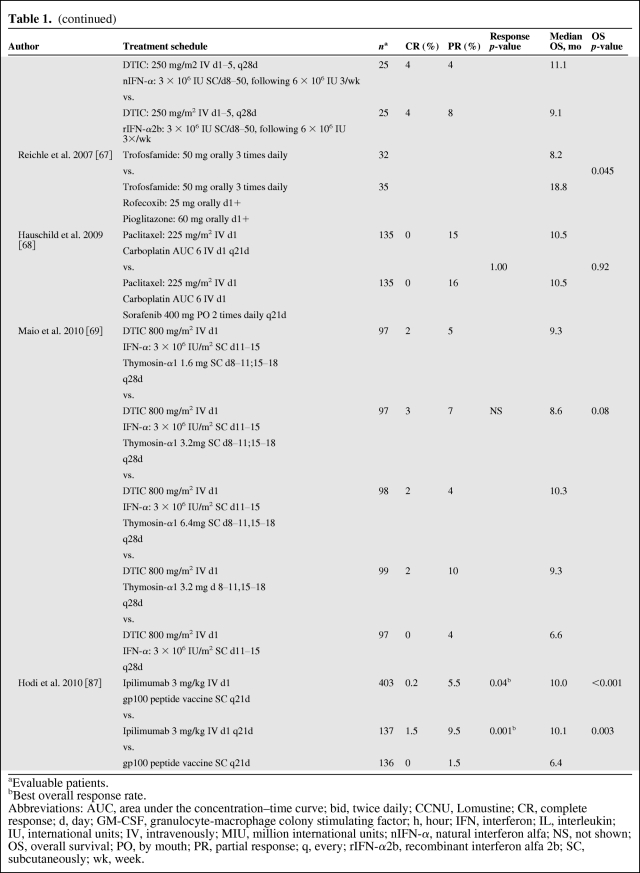
Chemotherapy is an accepted palliative therapy for stage IV metastatic disease (Table 1) [7, 9, 52–69], and dacarbazine is the most widely used single chemotherapeutic agent for the treatment of metastatic melanoma [70]. Dacarbazine was originally reported to yield objective responses in up to 25% of patients in older phase II trials, but current trials in more rigorous, large-scale, cooperative group settings have shown response rates of 5%–12% [52, 54, 55]. Unfortunately, most responses to this agent and its oral analogue temozolomide are transient; only 1%–2% of patients achieve a durable long-term response to chemotherapy [11]. Temozolomide, an oral prodrug that yields the same active intermediate (3-methyl-[triazen-1-yl]imidazole-4-carboxamide) as dacarbazine, has been demonstrated to be as effective as dacarbazine in phase III studies and is an oral, although more expensive, alternative to dacarbazine [71]. For symptomatic patients, or patients who are not eligible for current investigational trials, chemotherapy with one of these agents remains a reasonable palliative option; for novel agents being tested in clinical trials, chemotherapy is an accepted comparator (Table 1) [9, 52–69]. Other chemotherapies that have been explored include fotemustine (Servier, Gidy, France), a chloroethyl nitrosourea that has significantly improved the objective response rate (15.2% vs. 6.8% in the intent-to-treat populations; p = .043) and prolonged median overall survival, although not significantly (7.3 vs. 5.6 months; p = .067), when compared with dacarbazine in a phase III trial [52].
Table 1.
Studies of different regimens of chemotherapies used as a single agent or in combination with other therapeutic agents (final data published)
Table 1.
(continued)
Table 1.
(continued)
Table 1.
(continued)
aEvaluable patients.
bBest overall response rate.
Abbreviations: AUC, area under the concentration–time curve; bid, twice daily; CCNU, Lomustine; CR, complete response; d, day; GM-CSF, granulocyte-macrophage colony stimulating factor; h, hour; IFN, interferon; IL, interleukin; IU, international units; IV, intravenously; MIU, million international units; nIFN-α, natural interferon alfa; NS, not shown; OS, overall survival; PO, by mouth; PR, partial response; q, every; rIFN-α2b, recombinant interferon alfa 2b; SC, subcutaneously; wk, week.
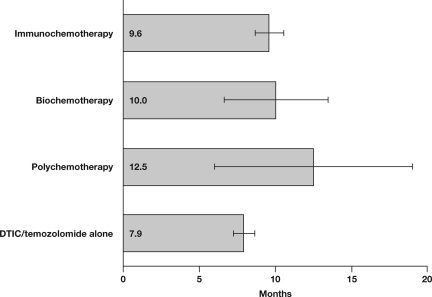
The antitumor activity of combinations of chemotherapeutic agents has been evaluated as a consequence of the increasingly frequently held belief that single agents are unlikely to improve the outcome of patients with advanced metastatic melanoma (Table 1 and Fig. 3) [7, 9, 52–69]. Other polychemotherapies tested in phase III trials (e.g., Dartmouth regimen: cisplatin/vinblastine/dacarbazine/tamoxifen) have failed to demonstrate a survival benefit compared with dacarbazine alone [9].
Figure 3.
Overall survival of patients treated with different therapies for melanoma. The data analyzed are listed in Table 1. On this figure, the error bars represent the 95% confidence interval. Abbreviation: DTIC, dacarbazine.
IL-2 and Other Immunotherapies
High-dose recombinant IL-2 (aldesleukin, Proleukin; Prometheus Laboratories Inc., San Diego, California) received U.S. FDA approval for the treatment of patients with metastatic melanoma in 1998. An objective response rate of ∼16% was observed in the collected set of phase II trials in patients (N = 47) with metastatic melanoma presented for regulatory review. Single-agent therapy was administered using the inpatient high-dose regimen of 600,000 U/kg IL-2 every 8 hours for up to 14 doses [72]. A small percentage of patients (∼5%) [73] experienced long-term, durable complete responses with IL-2, which has been interpreted as a potential cure. However, this therapy has not been shown to improve overall survival in the patient population and has never been evaluated in a phase III setting [74, 75]. In addition, IL-2 treatment-related toxicity is severe [72] and requires inpatient intensive care [76, 77]. Common dose-limiting toxicities include hemodynamic toxicity (e.g., hypotension, edema, weight gain, decreased renal function), respiratory insufficiency, and neurotoxicity [76, 77]. High-dose recombinant IL-2 treatment is the first immunotherapy of metastatic melanoma that induces a low percentage of long-term cancer remissions. Such long-term cancer remissions have not been convincingly demonstrated after chemotherapy. High-dose recombinant IL-2 has not been approved in Europe and is offered only in specialized cancer centers in the United States.
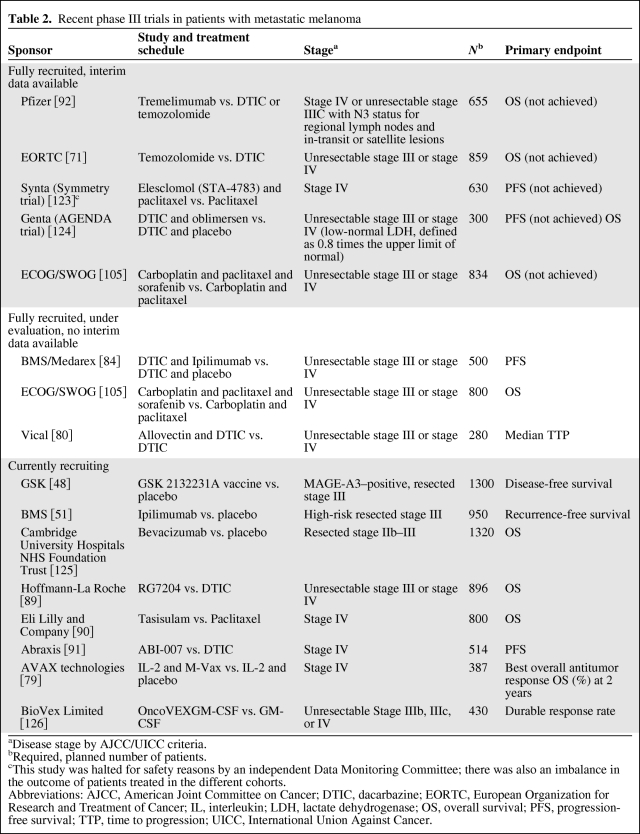
Furthermore, a recent multicenter study (N = 185) conducted by Schwartzentruber and colleagues [78] has built upon high-dose IL-2, evaluating the adding benefit of a peptide vaccine (gp100: 209–217[210M]). The remarkable finding that emerged from this trial was that peptide vaccination added significantly to the benefit of high-dose IL-2, improving response rates (22.1% vs. 9.7%, p = .02) and prolonging progression-free survival (1.6 vs. 2.9 months, p = .0101) significantly. Overall survival was also prolonged in the combination arm (17.6 vs. 12.8 months, p = .09) but did not reach significance. One ongoing study (N = 387) will determine whether the combination of IL-2 and vaccination with autologous melanoma cells can improve antitumor responses and overall survival compared with IL-2 alone (Table 2) [79]. Another vaccine currently under investigation in phase III is a DNA/lipid complex (allovectin) that enhances the expression of the major histocompatibility complex protein HLA-B7 and induces a fraction of antitumor responses (Table 2) [80].
Table 2.
Recent phase III trials in patients with metastatic melanoma
aDisease stage by AJCC/UICC criteria.
bRequired, planned number of patients.
cThis study was halted for safety reasons by an independent Data Monitoring Committee; there was also an imbalance in the outcome of patients treated in the different cohorts.
Abbreviations: AJCC, American Joint Committee on Cancer; DTIC, dacarbazine; EORTC, European Organization for Research and Treatment of Cancer; IL, interleukin; LDH, lactate dehydrogenase; OS, overall survival; PFS, progression-free survival; TTP, time to progression; UICC, International Union Against Cancer.
An area of great research interest focuses on the role of DCs that are recognized as the natural source of antigen presentation. The only randomized trial of a vaccine using DCs and melanoma peptides did not show any clinical benefit compared with chemotherapy (dacarbazine) [55], but newer, more highly polarized and immunogenic forms of DCs are now available and will doubtless be evaluated in the future (Table 1).
Biochemotherapy
Biochemotherapy (e.g., cisplatin, vinblastine, and dacarbazine combined with IFN-α ± IL-2) increases response rates but has not been shown to significantly improve survival compared with chemotherapy alone in randomized phase II and III trials [7, 8, 63]. In a systematic review of 41 randomized clinical trials of patients receiving various treatment schedules, including biochemotherapy regimens, none of them improved progression-free survival (PFS) or overall survival (Fig. 3) [11]. Similarly, objective response to polychemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen was also improved with the addition of subcutaneous IL-2 and IFN-α in a randomized study, but this did not result in significantly improved PFS or overall survival (Table 1) [61]. Furthermore, the addition of IL-2 does not significantly enhance the efficacy of dacarbazine/IFN [81] or dacarbazine/cisplatin/IFN-α2b (Table 1) [62]. Combinations with temozolomide have not been more successful than those with dacarbazine. In a phase III, randomized study, the addition of IFN-α to temozolomide improved the objective response rate but not overall survival compared with temozolomide alone (Table 1) [57]. A phase II study compared combinations of temozolomide with either thalidomide or IFN-α to treatment with temozolomide alone, but neither combination significantly improved objective response rate or median overall survival (Table 1) [59]. Another randomized phase II study analyzed the efficacy and tolerability of bleomycin, vincristine, lomustine, and dacarbazine (BOLD) combined with natural IFN-α or recombinant IFN-α2b (nIFN-α or rIFN-α2b, respectively) [66]. Treatment groups included dacarbazine plus nIFN-α, BOLD plus nIFN-α, dacarbazine plus rIFN-α2b, and BOLD plus rIFN-α2b. There were no significant differences in objective response rate or overall survival among these four treatments.
Although a recent meta-analysis of 18 trials and nearly 2,500 patients with metastatic melanoma suggested a benefit of biochemotherapy in terms of objective response, no benefit in terms of overall survival was found (p = .9) [82]. A similar pattern was observed in a phase III ECOG-led intergroup U.S. study comparing polychemotherapy treatment with cisplatin, vinblastine, and dacarbazine (CVD, n = 195) alone or given in combination with IL-2 and IFN-α2b (BCT, n = 200) administered as first-line treatment of patients with metastatic melanoma [9]. The objective response rate was slightly higher in the BCT arm than in the CVD arm (19.5% vs. 13.8%, respectively; p = .140); median PFS was significantly longer in the BCT arm than in the CVD arm (4.8 vs. 2.9 months; p = .015). However, the addition of IL-2 and IFN-α2b to chemotherapy was associated with greater toxicity and no improvements in overall survival or durable responses; the BCT regimen cannot be recommended for patients with metastatic melanoma [9]. An interesting observation has been reported by O'Day and colleagues from a study with 133 metastasized melanoma patients treated first line with a biochemotherapy induction regimen including CVD, IL-2, IFN-α, and granulocyte macrophage colony-stimulating factor (GM-CSF) [83]. Patients not experiencing disease progression received maintenance biotherapy with low-dose IL-2 and GM-CSF followed by intermittent pulses of decrescendo IL-2 over 12 months. The median survival time in this trial was 13.5 months, and the 12- and 24-month survival rates were 57% and 23%, respectively. It has been suggested that this promising regimen should be studied in a randomized clinical trial.
Until today, biochemotherapy has not demonstrated significant clinical benefit in adjuvant trials nor in randomized prospective studies in the metastatic setting, but it is associated with additive toxicity. The addition of maintenance biotherapy may be suitable to prolong overall survival. Presently, biochemotherapy regimens cannot be regarded as standard clinical practice and should be further evaluated in clinical trials.
Emerging Therapies for Patients with Metastatic Melanoma
An increased understanding of tumor biology and the complexity of immune antitumor response and immune regulation has led to the development of novel agents. Several approaches to overcoming tolerance that appear promising in clinical trials include blockade of inhibitory immune receptors, inhibition of oncogenic kinase pathways, downregulation of antiapoptotic proteins, and adoptive cell therapy after nonmyeloablative lymphodepletion (Table 2) [79, 80, 84–92].
Antibody Blockade of Cytotoxic T-Lymphocyte–Associated Antigen 4
Full T-cell activation requires stimulation through the T-cell receptor as well as a costimulatory signal provided by the binding of B7 on the antigen-presenting cell (e.g., dendritic cell) to CD28 on the T cell. Cytotoxic T-lymphocyte–associated antigen 4 is a homologue of CD28 and is an inhibitory T-cell receptor that is upregulated following T-cell activation. The normal function of CTLA4 is to compete with CD28 to bind B7 to downregulate T-cell activation, acting as a natural “brake” by removing the costimulatory signal. The CTLA4–B7 interaction can be blocked with an anti-CTLA4 monoclonal antibody (mAb), which has a higher affinity for CTLA4 than B7. Thus, the inhibitory signal is prevented and the “brake” on T-cell activation is released. Two fully human anti-CTLA4 mAb's are currently in clinical development: tremelimumab (CP-675,206; Pfizer Inc., New York) and ipilimumab (MDX-010; Medarex, Inc./Bristol-Myers Squibb, New York). Objective response rates of patients with metastatic melanoma treated with either of the two anti-CLTA4 mAb's as single agents are similar (∼7%–10%) [92–100] and resemble the response rate found in patients treated with high-dose IL-2. Responses to anti-CTLA4 mAb's are durable (as much as 70%) [87, 92] but may take as long as 12 weeks or even longer to develop [98, 101], and late-onset objective responses are sometimes preceded by months of stable disease or even transient disease progression [101]. Side effects with CTLA4 blockade are autoimmune-related but less acute than those observed with exogenous cytokine therapy and are manageable. Commonly reported AEs include diarrhea and rash [87, 92]. Several randomized studies are ongoing to assess whether these early observations of durable responses will translate into an overall survival benefit (Table 2) [84, 87, 92]. In a phase III randomized study of tremelimumab (15 mg/kg administered once every 3 months, n = 328) and chemotherapy (n = 327) with dacarbazine or temozolomide in treatment-naive patients, median survival was longer (11.76 months) in patients treated with tremelimumab compared with chemotherapy (10.71 months) [92]. However, the difference was not statistically significant (HR chemotherapy/tremelimumab 1.04; p = .729), and the trial was halted at the second interim analysis. However, patients with clinical benefit from tremelimumab treatment are continuing on study, and more mature survival and response data are anticipated. Ipilimumab is also currently being investigated in a large phase III trial in patients with metastatic melanoma (Table 2) [84]. The results of a randomized phase III trial for single ipilimumab treatment versus gp100 vaccination and versus the combination of ipilimumab and gp100 vaccination have been recently published, showing improved overall survival of a median duration of 10.1 and 10.0 months in the ipilimumab arm and the combined arm, respectively, in comparison to 6.4 months in the vaccination alone arm. Although objective response rates were rather low with 10.9% in the ipilimumab alone arm and 5.7% in the combined ipilimumab and vaccination arm versus 1.5% in the gp100 vaccination alone arm, highly significant differences in hazard rates for overall survival resulted were detected between ipilimumab alone versus vaccination alone (0.66; 95% CI = 0.51–0.87; p = .003) and between the combined arm versus vaccination alone (0.68; 95% CI = 0.55–0.85; p < .001) [87]. This observation may result in approval of ipilimumab by health authorities for the treatment of advanced melanoma.
Inhibitors of the Mitogen-Activated Protein Kinase Pathway
The most frequently (60%–70%) mutated oncogene identified to date in melanoma is BRAF, an upstream mediator of the mitogen-activated protein kinase (MAPK) pathway [102]. Increased activation of the MAPK pathway is implicated in melanoma tumorigenesis and is enhanced in advanced-stage melanoma [103]. Sorafenib (Nexavar, BAY 43–9006; Bayer HealthCare Pharmaceuticals Inc., Wayne, New Jersey, and Onyx Pharmaceuticals, Inc., Emeryville, California) is an oral multikinase BRAF inhibitor that has been widely investigated. Unfortunately, the majority of published clinical studies have failed to show any benefit associated with the addition of sorafenib to standard chemotherapy [60, 68, 104–106].
In contrast to nonselective multikinase inhibitors, RG7204, formerly PLX4032 (Hoffmann-La Roche, Basel, Switzerland/Plexxikon, Inc., Berkeley, California), is a novel selective inhibitor of the oncogenic V600E mutant BRAF kinase. A phase I dose-escalation study in patients with solid tumors carrying the V600E mutation was reported at ASCO 2009 and showed objective responses in ∼70% of patients treated with RG7204 [107]. At ASCO 2010, data from an international multicenter phase I study were reported showing an objective fluorodeoxyglucose positron emission tomography response in all 22 treated patients. The best overall response was determined by conventional assessment using Response Evaluation Criteria in Solid Tumors (RECIST) criteria, and an objective response rate of 86% has been described. No relationship was found between reduction in target lesions, maximum standardized uptake value (SUVmax) and response by RECIST, PFS, and time to achieve RECIST partial response [108]. A consecutive phase III study comparing RG7204 versus dacarbazine is currently recruiting patients worldwide (Table 2) [89]. Other BRAF inhibitors such as GSK2118436 (GlaxoSmithKline PLC, Brentford, U.K.) and RAF265 (Novartis, Basel, Switzerland) are likewise in first clinical trials [109, 110]. For GSK2118436 a clinical objective response rate of 66% has recently been reported in V600 mutant melanoma patients treated with >150 mg 2 times daily (b.i.d.) [111].
AZD6244 (ARRY-142886; AstraZeneca, Wilmington, Delaware) is a potent, selective inhibitor of MEK 1/2 kinase [112]. Its therapeutic target is downstream but in the same signaling pathway as the kinase targeted by the BRAF inhibitors. First results of AZD6244 antitumor activity were presented at the ASCO 2008 annual meeting [113], indicating partial responses in mainly BRAF-mutated patients. AZD6244 is currently being tested in a phase II, multicenter, open-label, randomized study comparing its antitumor activity in combination with dacarbazine versus dacarbazine alone for patients with stage III or IV malignant melanoma [114]. First clinical results have also been reported for the MEK inhibitor GSK1120212 in 20 patients with BRAF mutant melanoma showing six partial responses and two complete responses (40% OR). Interestingly, two partial responses have likewise been observed in 22 BRAF wild-type melanoma patients [115].
Tasisulam
Tasisulam (Eli Lilly and Company, Indianapolis) is a novel antiproliferative and cytotoxic drug that induces apoptosis through the mitochondrial cell death pathway. In addition to the apoptotic activity, a loss of mitochondrial membrane potential and the induction of reactive oxygen species appear to be the relevant anticancer mechanisms. Interim data of a phase II trial showed an overall response rate of 12% and disease stabilization in an additional 35% of patients [116]. Recently, a phase III trial comparing tasisulam versus paclitaxel alone was initiated, recruiting 800 patients worldwide [90].
ABI-007 (Abraxane)
ABI-007 (Abraxane, Abraxis BioScience Inc., Los Angeles) is an albumin-bound paclitaxel that is approved for the treatment of metastatic breast cancer and is now being investigated in phase III compared with dacarbazine in previously untreated patients with advanced melanoma. Abraxane has important tolerance benefits compared to solvent-based paclitaxel, which has a high risk of Cremophor EL-related hypersensitivity reactions [91].
Adoptive Cell Therapy
To date, adoptive cell therapy that has been developed by Rosenberg and colleagues has yielded some of the most dramatic responses among patients with metastatic melanoma. Objective response rates in highly selected patients enrolled in this series have been stated to range between 49% and 72%. Adoptive cell therapy as undertaken by this group based at the National Cancer Institute is complex and costly, involving multiple steps: first, specifically sensitized antitumor lymphocytes must be isolated from the patient's tumor or stimulated in vitro with autologous melanoma cells. For this purpose, tumor-infiltrating lymphocytes are cultured in vitro. These are grown in IL-2, exhibiting major histocompatibility complex–restricted recognition of the autologous melanoma cells. Second, the antitumor lymphocytes have to be expanded in vitro to large numbers. The efficacy of adoptive cell therapy depends on the presence of large numbers of antitumor lymphocytes capable of recognizing the melanoma cells and destroying the cancer cells in vivo. The ideal number for the adoptive transfer is >1011 cells. Objective clinical responses were associated with cells that were cultured for shorter time periods, and a protocol for rapid expansion has therefore been developed. The in vitro expansion was performed with use of IL-2 and anti-CD3-antibodies in the presence of irradiated allogeneic feeder cells. Cells were harvested 14 days after in vitro expansion [117]. Third, lymphodepletion has been performed as preparation of the host before adoptive cell transfer. Seven days before adoptive transfer, a nonmyeloablative lymphodepleting regimen consisting of cyclophosphamide and fludarabine has been applied. It has been suggested that this has to be supplemented by total body irradiation in single fractions of 2 Gy or with 12 Gy administered as 2 Gy b.i.d. for 3 days. Fourth, the adoptive cell transfer accompanied by a high-dose treatment with IL-2 for 3 days is performed. The tumor-infiltrating lymphocytes were applied as a bolus intravenous infusion over 0.5–1 hours, and the high-dose IL-2 treatment was started within 24 hours. Patients who received total body irradiation additionally received autologous purified cryopreserved CD34+ hematopoietic stem cells from a granulocyte colony-stimulating factor-mobilized pheresis [118]. After nonmyeloablative but lymphodepleting chemotherapy, adoptive cell transfer therapy (N = 35) resulted in objective response rates of 51% [119]. By intensifying the lymphodepleting therapy (N = 25) through the addition of total body irradiation with a total dose of 12 Gy, the response rate could be increased to 72% [118].
It is important to mention that this therapeutic approach is in general not available for metastatic melanoma patients. First, this particular therapeutic approach has exclusively been established at the surgery branch, National Cancer Institute in Bethesda, Maryland. There are very few groups worldwide that developed therapeutic approaches with lymphodepletion and adoptive cell transfer [120–122]. Second, this procedure is very complex and has several critical steps, such as the isolation of the tumor-infiltrating lymphocytes and their in vitro expansion, which is labor-intensive as well as costly. Third, the selected patients must have an excellent performance status with no other severe concomitant disease. Therefore, only a few patients per year have been included in phase II studies, and to date, no phase III study has been initiated. Nevertheless, tumor remissions accomplished by such a strategy seem to be durable and may result in cancer cure.
Conclusions
Despite decades of clinical research, patients with advanced melanoma continue to have a poor prognosis, and no agents have shown statistically significant improvement in overall survival in a phase III trial in patients with metastatic melanoma. For high-risk, resected disease, adjuvant therapy with IFN-α has been shown to consistently increase relapse-free survival, as well as overall survival in some studies. Standard off-protocol treatment for patients with metastatic melanoma is evolving, and where mutations can be documented in BRAF (V600E) or the c-Kit gene, there exist promising new approaches to targeted therapy that have altered the paradigm of systemic therapy. Apart from these, or for patients who are symptomatic and unable to consider the pursuit of new investigational trials, chemotherapy offers transient, palliative efficacy. Advances in the understanding of the mechanism of chemotherapy resistance offer the hope for improved results with chemotherapy, and the triumvirate of more effective chemotherapy, immunotherapy, and targeted therapy are likely to be combined with one another for significant advances in melanoma over the coming few years. Because of the potential benefits of new targeted drugs and of immunotherapies, treatment guidelines for melanoma recommend the inclusion of patients with metastatic melanoma in clinical trials.
Several new immunotherapies have demonstrated promising antitumor activity with manageable side effects in patients with advanced melanoma. These include the anti-CTLA4 mAb's tremelimumab and ipilimumab, and the targeted agents RG7204 (BRAF V600E), AZD6244 (BRAF V600E), and the novel proapoptotic agent tasisulam. Although early clinical trials have not indicated that any of these offers a “breakthrough” in terms of antitumor activity for all patients, each will likely offer incremental improvements over standard care. Complex immunotherapies with adoptive T-cell transfer after nonmyeloablative lymphodepletion suggest response rates that are extraordinary, but we must remember that these results are derived from highly selected patient samples, without large multicenter phase III trials to date. Ongoing clinical trials will hopefully elucidate the therapeutic mechanisms of these approaches and provide survival benefit to patients with melanoma.
Acknowledgments
Claus Garbe and Thomas K. Eigentler contributed equally to this manuscript.
Claus Garbe, as corresponding author, had full access to all data in the review and had final responsibility for the decision to submit for publication. The authors thank Tamara Fink, Ph.D. (ProEd Communications, Inc., Beachwood, Ohio), supported by Pfizer Inc. (New York), for her assistance in language editing for this manuscript.
Author Contributions
Conception/Design: Claus Garbe, Thomas K. Eigentler
Collection and/or assembly of data: Claus Garbe, Thomas K. Eigentler, Axel Hauschild
Data analysis and interpretation: Claus Garbe, Thomas K. Eigentler
Manuscript writing: Claus Garbe, Thomas K. Eigentler, Ulrich Keilholz, Axel Hauschild, John M. Kirkwood
Final approval of manuscript: Claus Garbe, Thomas K. Eigentler, Ulrich Keilholz, Axel Hauschild, John M. Kirkwood
References
- 1.Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–185. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- 2.Molife R, Hancock BW. Adjuvant therapy of malignant melanoma. Crit Rev Oncol Hematol. 2002;44:81–102. doi: 10.1016/s1040-8428(02)00014-8. [DOI] [PubMed] [Google Scholar]
- 3.Wheatley K, Ives N, Hancock B, et al. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev. 2003;29:241–252. doi: 10.1016/s0305-7372(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 4.Mocellin S, Pasquali S, Rossi CR, et al. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 5.Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer. 2004;40:1825–1836. doi: 10.1016/j.ejca.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 7.Bajetta E, Del Vecchio M, Nova P, et al. Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-alpha2b in metastatic melanoma. Ann Oncol. 2006;17:571–577. doi: 10.1093/annonc/mdl007. [DOI] [PubMed] [Google Scholar]
- 8.Ridolfi R, Chiarion-Sileni V, Guida M, et al. Cisplatin, dacarbazine with or without subcutaneous interleukin-2, and interferon alpha-2b in advanced melanoma outpatients: results from an Italian multicenter phase III randomized clinical trial. J Clin Oncol. 2002;20:1600–1607. doi: 10.1200/JCO.2002.20.6.1600. [DOI] [PubMed] [Google Scholar]
- 9.Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): a trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26:5748–5754. doi: 10.1200/JCO.2008.17.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis KD, Robinson WA, McCarter M, et al. Phase II multicenter study of neoadjuvant biochemotherapy for patients with stage III malignant melanoma. J Clin Oncol. 2006;24:3157–3163. doi: 10.1200/JCO.2005.04.5344. [DOI] [PubMed] [Google Scholar]
- 11.Eigentler TK, Caroli UM, Radny P, et al. Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol. 2003;4:748–759. doi: 10.1016/s1470-2045(03)01280-4. [DOI] [PubMed] [Google Scholar]
- 12.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma: European consensus-based interdisciplinary guideline. Eur J Cancer. 2010;46:270–283. doi: 10.1016/j.ejca.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250–275. doi: 10.6004/jnccn.2009.0020. [DOI] [PubMed] [Google Scholar]
- 15.Thomas JM, Newton-Bishop J, A'Hern R, et al. Excision margins in high-risk malignant melanoma. N Engl J Med. 2004;350:757–766. doi: 10.1056/NEJMoa030681. [DOI] [PubMed] [Google Scholar]
- 16.Balch CM, Soong S, Ross MI, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7:87–97. doi: 10.1007/s10434-000-0087-9. [DOI] [PubMed] [Google Scholar]
- 17.Shah GD, Chapman PB. Adjuvant therapy of melanoma. Cancer J. 2007;13:217–222. doi: 10.1097/PPO.0b013e318074dfd4. [DOI] [PubMed] [Google Scholar]
- 18.Fecher LA, Flaherty KT. Where are we with adjuvant therapy of stage III and IV melanoma in 2009? J Natl Compr Canc Netw. 2009;7:295–304. doi: 10.6004/jnccn.2009.0022. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 20.Creagan ET, Dalton RJ, Ahmann DL, et al. Randomized, surgical adjuvant clinical trial of recombinant interferon alfa-2a in selected patients with malignant melanoma. J Clin Oncol. 1995;13:2776–2783. doi: 10.1200/JCO.1995.13.11.2776. [DOI] [PubMed] [Google Scholar]
- 21.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 22.Eggermont AM, Suciu S, MacKie R, et al. Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet. 2005;366:1189–1196. doi: 10.1016/S0140-6736(05)67482-X. [DOI] [PubMed] [Google Scholar]
- 23.Hancock BW, Wheatley K, Harris S, et al. Adjuvant interferon in high-risk melanoma: the AIM HIGH Study–United Kingdom Coordinating Committee on Cancer Research randomized study of adjuvant low-dose extended-duration interferon Alfa-2a in high-risk resected malignant melanoma. J Clin Oncol. 2004;22:53–61. doi: 10.1200/JCO.2004.03.185. [DOI] [PubMed] [Google Scholar]
- 24.Pehamberger H, Soyer HP, Steiner A, et al. Adjuvant interferon alfa-2a treatment in resected primary stage II cutaneous melanoma. Austrian Malignant Melanoma Cooperative Group. J Clin Oncol. 1998;16:1425–1429. doi: 10.1200/JCO.1998.16.4.1425. [DOI] [PubMed] [Google Scholar]
- 25.Cascinelli N, Belli F, MacKie RM, et al. Effect of long-term adjuvant therapy with interferon alpha-2a in patients with regional node metastases from cutaneous melanoma: a randomised trial. Lancet. 2001;358:866–869. doi: 10.1016/S0140-6736(01)06068-8. [DOI] [PubMed] [Google Scholar]
- 26.Garbe C, Radny P, Linse R, et al. Adjuvant low-dose interferon α2a with or without dacarbazine compared with surgery alone: a prospective-randomized phase III DeCOG trial in melanoma patients with regional lymph node metastasis. Ann Oncol. 2008;19:1195–1201. doi: 10.1093/annonc/mdn001. [DOI] [PubMed] [Google Scholar]
- 27.Grob JJ, Dreno B, de la SP, et al. Randomised trial of interferon alpha-2a as adjuvant therapy in resected primary melanoma thicker than 1.5 mm without clinically detectable node metastases. French Cooperative Group on Melanoma. Lancet. 1998;351:1905–1910. doi: 10.1016/s0140-6736(97)12445-x. [DOI] [PubMed] [Google Scholar]
- 28.Eggermont AM, Gore M. Randomized adjuvant therapy trials in melanoma: surgical and systemic. Semin Oncol. 2007;34:509–515. doi: 10.1053/j.seminoncol.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Cameron DA, Cornbleet MC, MacKie RM, et al. Adjuvant interferon alpha 2b in high risk melanoma—the Scottish study. Br J Cancer. 2001;84:1146–1149. doi: 10.1054/bjoc.2000.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lens MB, Dawes M. Interferon alfa therapy for malignant melanoma: a systematic review of randomized controlled trials. J Clin Oncol. 2002;20:1818–1825. doi: 10.1200/JCO.2002.07.070. [DOI] [PubMed] [Google Scholar]
- 31.Garbe C, Eigentler TK. Diagnosis and treatment of cutaneous melanoma: state of the art 2006. Melanoma Res. 2007;17:117–127. doi: 10.1097/CMR.0b013e328042bb36. [DOI] [PubMed] [Google Scholar]
- 32.Wheatley K, et al. Interferon-{alpha} as adjuvant therapy for melanoma: an individual patient data meta-analysis of randomised trials. J Clin Oncol. 2007;25(18 suppl) Abstract 8526. [Google Scholar]
- 33.Eggermont AM, Suciu S, Santinami M, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–126. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 34.Chiarion-Sileni V, Del Bianco P, Romanini A, et al. Tolerability of intensified intravenous interferon alfa-2b versus the ECOG 1684 schedule as adjuvant therapy for stage III melanoma: a randomized phase III Italian Melanoma Intergroup trial. BMC Cancer. 2006;6:44. doi: 10.1186/1471-2407-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. National Institutes of Health. Pegylated Interferon-Alpha-2a in Patients With Malignant Melanoma Stage IIA–IIIB. [Accessed June 10, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00204529.
- 36.U.S. National Institutes of Health. PegIntron Versus IntronA in CMAJCC Stage II (EADO 2001/CMII Trial) [Accessed June 10, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00221702.
- 37.Grob JJ, et al. Adjuvant therapy with pegylated interferon alfa-2b (36 months) versus low-dose interferon alfa-2b (18 months) in melanoma patients without macro-metastatic nodes: EADO trial. J Clin Oncol. 2010;28(15 suppl) Abstract LBA8506. [Google Scholar]
- 38.Pectasides D, Dafni U, Bafaloukos D, et al. Randomized phase III study of 1 month versus 1 year of adjuvant high-dose interferon alfa-2b in patients with resected high-risk melanoma. J Clin Oncol. 2009;27:939–944. doi: 10.1200/JCO.2008.16.3121. [DOI] [PubMed] [Google Scholar]
- 39.Hauschild A, Weichenthal M, Rass K, et al. Prospective randomized multicenter adjuvant dermatologic cooperative oncology group trial of low-dose interferon alfa-2b with or without a modified high-dose interferon alfa-2b induction phase in patients with lymph node-negative melanoma. J Clin Oncol. 2009;27:3496–3502. doi: 10.1200/JCO.2008.21.3892. [DOI] [PubMed] [Google Scholar]
- 40.Moschos SJ, Edington HD, Land SR, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 41.Yurkovetsky ZR, Kirkwood JM, Edington HD, et al. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13:2422–2428. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
- 42.Kim CJ, Dessureault S, Gabrilovich D, et al. Immunotherapy for melanoma. Cancer Control. 2002;9:22–30. doi: 10.1177/107327480200900104. [DOI] [PubMed] [Google Scholar]
- 43.Zarour HM, Kirkwood JM. Melanoma vaccines: early progress and future promises. Semin Cutan Med Surg. 2003;22:68–75. doi: 10.1053/sder.2003.50006. [DOI] [PubMed] [Google Scholar]
- 44.Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 45.Isreal RJ, et al. Naturally occuring and GMK-induced antibodies to GM2 in high-risk melanoma patients in study E1694/S9512/CALGB 509801. J Clin Oncol. 2001;20(suppl) Abstract 1078. [Google Scholar]
- 46.Morton DL, et al. An international, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. J Clin Oncol. 2007;25(18 suppl) Abstract 8508. [Google Scholar]
- 47.Eggermont A, et al. Randomized phase III trial comparing postoperative adjuvant ganglioside GM2-KLH/QS-21 vaccination versus observation in stage II (T3–T4N0M0) melanoma: final results of study EORTC 18961. J Clin Oncol. 2010;28(15 suppl) doi: 10.1200/JCO.2012.47.9303. Abstract 8505. [DOI] [PubMed] [Google Scholar]
- 48.U.S. National Institutes of Health. A Phase III Study to Test the Benefit of a New Kind of Anti-cancer Treatment in Patients With Melanoma, After Surgical Removal of Their Tumor. [Accessed June 10, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00796445.
- 49.Kirkwood JM, Lee S, Moschos SJ, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/-granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gogas H, Ioannovich J, Dafni U, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–718. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- 51.U.S. National Institutes of Health. Efficacy Study of Ipilimumab Versus Placebo to Prevent Recurrence After Complete Resection of High Risk Stage III Melanoma. [Accessed June 10, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00636168.
- 52.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22:1118–1125. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 53.Middleton M, Hauschild A, Thomson D, et al. Results of a multicenter randomized study to evaluate the safety and efficacy of combined immunotherapy with interleukin-2, interferon-{alpha}2b and histamine dihydrochloride versus dacarbazine in patients with stage IV melanoma. Ann Oncol. 2007;18:1691–1697. doi: 10.1093/annonc/mdm331. [DOI] [PubMed] [Google Scholar]
- 54.Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 55.Schadendorf D, Ugurel S, Schuler-Thurner B, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17:563–570. doi: 10.1093/annonc/mdj138. [DOI] [PubMed] [Google Scholar]
- 56.Ranson M, Hersey P, Thompson D, et al. Randomized trial of the combination of lomeguatrib and temozolomide compared with temozolomide alone in chemotherapy naive patients with metastatic cutaneous melanoma. J Clin Oncol. 2007;25:2540–2545. doi: 10.1200/JCO.2007.10.8217. [DOI] [PubMed] [Google Scholar]
- 57.Kaufmann R, Spieth K, Leiter U, et al. Temozolomide in combination with interferon-alfa versus temozolomide alone in patients with advanced metastatic melanoma: a randomized, phase III, multicenter study from the Dermatologic Cooperative Oncology Group. J Clin Oncol. 2005;23:9001–9007. doi: 10.1200/JCO.2005.01.1551. [DOI] [PubMed] [Google Scholar]
- 58.Bafaloukos D, Tsoutsos D, Kalofonos H, et al. Temozolomide and cisplatin versus temozolomide in patients with advanced melanoma: a randomized phase II study of the Hellenic Cooperative Oncology Group. Ann Oncol. 2005;16:950–957. doi: 10.1093/annonc/mdi190. [DOI] [PubMed] [Google Scholar]
- 59.Danson S, Lorigan P, Arance A, et al. Randomized phase II study of temozolomide given every 8 hours or daily with either interferon alfa-2b or thalidomide in metastatic malignant melanoma. J Clin Oncol. 2003;21:2551–2557. doi: 10.1200/JCO.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 60.McDermott DF, Sosman JA, Gonzalez R, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol. 2008;26:2178–2185. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 61.Atzpodien J, Neuber K, Kamanabrou D, et al. Combination chemotherapy with or without s.c. IL-2 and IFN-alpha: results of a prospectively randomized trial of the Cooperative Advanced Malignant Melanoma Chemoimmunotherapy Group (ACIMM) Br J Cancer. 2002;86:179–184. doi: 10.1038/sj.bjc.6600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keilholz U, Punt CJ, Gore M, et al. Dacarbazine, cisplatin, and interferon-alfa-2b with or without interleukin-2 in metastatic melanoma: a randomized phase III trial (18951) of the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol. 2005;23:6747–6755. doi: 10.1200/JCO.2005.03.202. [DOI] [PubMed] [Google Scholar]
- 63.Punt CJ, Suciu S, Gore MA, et al. Chemoimmunotherapy with dacarbazine, cisplatin, interferon-alpha2b and interleukin-2 versus two cycles of dacarbazine followed by chemoimmunotherapy in patients with metastatic melanoma: a randomised phase II study of the European Organization for Research and Treatment of Cancer Melanoma Group. Eur J Cancer. 2006;42:2991–2995. doi: 10.1016/j.ejca.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Richtig E, Hofmann-Wellenhof R, Pehamberger H, et al. Temozolomide and interferon alpha 2b in metastatic melanoma stage IV. Br J Dermatol. 2004;151:91–98. doi: 10.1111/j.1365-2133.2004.06019.x. [DOI] [PubMed] [Google Scholar]
- 65.Glover D, Ibrahim J, Kirkwood J, et al. Phase II randomized trial of cisplatin and WR-2721 versus cisplatin alone for metastatic melanoma: an Eastern Cooperative Oncology Group Study (E1686) Melanoma Res. 2003;13:619–626. doi: 10.1097/00008390-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 66.Vuoristo MS, Hahka-Kemppinen M, Parvinen LM, et al. Randomized trial of dacarbazine versus bleomycin, vincristine, lomustine and dacarbazine (BOLD) chemotherapy combined with natural or recombinant interferon-alpha in patients with advanced melanoma. Melanoma Res. 2005;15:291–296. doi: 10.1097/00008390-200508000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Reichle A, Vogt T, Coras B, et al. Targeted combined anti-inflammatory and angiostatic therapy in advanced melanoma: a randomized phase II trial. Melanoma Res. 2007;17:360–364. doi: 10.1097/CMR.0b013e3282f1d2c8. [DOI] [PubMed] [Google Scholar]
- 68.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 69.Maio M, Mackiewicz A, Testori A, et al. Large randomized study of thymosin alpha 1, interferon alfa, or both in combination with dacarbazine in patients with metastatic melanoma. J Clin Oncol. 2010;28:1780–1787. doi: 10.1200/JCO.2009.25.5208. [DOI] [PubMed] [Google Scholar]
- 70.Lens MB, Eisen TG. Systemic chemotherapy in the treatment of malignant melanoma. Expert Opin Pharmacother. 2003;4:2205–2211. doi: 10.1517/14656566.4.12.2205. [DOI] [PubMed] [Google Scholar]
- 71.Patel P, et al. Extended schedule, escalated dose Temozolomide versus Dacarbazine in Stage IV malignant melanoma: Final results of the randomised phase III study (EORTC 18032) Ann Oncol. 2008;19(8 suppl) doi: 10.1016/j.ejca.2011.04.030. Abstract LBA8. [DOI] [PubMed] [Google Scholar]
- 72.Parkinson DR, Abrams JS, Wiernik PH, et al. Interleukin-2 therapy in patients with metastatic malignant melanoma: a phase II study. J Clin Oncol. 1990;8:1650–1656. doi: 10.1200/JCO.1990.8.10.1650. [DOI] [PubMed] [Google Scholar]
- 73.Agarwala S. Improving survival in patients with high-risk and metastatic melanoma: immunotherapy leads the way. Am J Clin Dermatol. 2003;4:333–346. doi: 10.2165/00128071-200304050-00004. [DOI] [PubMed] [Google Scholar]
- 74.Atkins MB. Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res. 2006;12:2353s–2358s. doi: 10.1158/1078-0432.CCR-05-2503. [DOI] [PubMed] [Google Scholar]
- 75.Keilholz U, Stoter G, Punt CJ, et al. Recombinant interleukin-2-based treatments for advanced melanoma: the experience of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. Cancer J Sci Am. 1997;3(Suppl 1):S22–28. [PubMed] [Google Scholar]
- 76.Balmer CM. Clinical use of biologic response modifiers in cancer treatment: an overview. Part II. Colony-stimulating factors and interleukin-2. DICP. 1991;25:490–498. doi: 10.1177/106002809102500509. [DOI] [PubMed] [Google Scholar]
- 77.Bruton JK, Koeller JM. Recombinant interleukin-2. Pharmacotherapy. 1994;14:635–656. [PubMed] [Google Scholar]
- 78.Schwartzentruber DJ, et al. A phase III multi-institutional randomized study of immunization with the gp100: 209–217(210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma. J Clin Oncol. 2009;27(18 suppl) Abstract CRA9011. [Google Scholar]
- 79.U.S. National Institutes of Health. M-Vax + Low Dose Interleukin-2 Versus Placebo Vaccine in Metastatic Melanoma in Patients With Stage IV Melanoma. [Accessed June 10, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00477906.
- 80.U.S. National Institutes of Health. Chemotherapy With or Without Immunotherapy in Treating Patients With Stage III or Stage IV Melanoma. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00003647.
- 81.Hauschild A, Garbe C, Stolz W, et al. Dacarbazine and interferon alpha with or without interleukin 2 in metastatic melanoma: a randomized phase III multicentre trial of the Dermatologic Cooperative Oncology Group (DeCOG) Br J Cancer. 2001;84:1036–1042. doi: 10.1054/bjoc.2001.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ives NJ, Stowe RL, Lorigan P, et al. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2,621 patients. J Clin Oncol. 2007;25:5426–5434. doi: 10.1200/JCO.2007.12.0253. [DOI] [PubMed] [Google Scholar]
- 83.O'Day SJ, Atkins MB, Boasberg P, et al. Phase II multicenter trial of maintenance biotherapy after induction concurrent Biochemotherapy for patients with metastatic melanoma. J Clin Oncol. 2009;27:6207–6212. doi: 10.1200/JCO.2008.20.3075. [DOI] [PubMed] [Google Scholar]
- 84.U.S. National Institutes of Health. Dacarbazine and Ipilimumab vs. Dacarbazine With Placebo in Untreated Unresectable Stage III or IV Melanoma. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00324155.
- 85.U.S. National Institutes of Health. Elesclomol (STA-4783) With Paclitaxel Versus Paclitaxel Alone in Melanoma. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00522834.
- 86.U.S. National Institutes of Health. Trial of Dacarbazine With or Without Genasense in Advanced Melanoma. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00518895.
- 87.Hodi FS, O'Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.U.S. National Institutes of Health. Carboplatin and Paclitaxel With or Without Sorafenib in Treating Patients With Unresectable Stage III or Stage IV Melanoma. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00110019.
- 89.U.S. National Institutes of Health. A Study of RO5185426 in Comparison With Dacarbazine in Previously Untreated Patients With Metastatic Melanoma. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT01006980.
- 90.U.S. National Institutes of Health. A Study of Tasisulam Versus Paclitaxel as Treatment for Metastatic Melanoma. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT01006252.
- 91.U.S. National Institutes of Health. A Trial of ABI-007 Versus Dacarbazine in Previously Untreated Patients With Metastatic Malignant Melanoma. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00864253.
- 92.Ribas A, et al. Phase III, open-label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide [TMZ] or dacarbazine [DTIC]) in patients with advanced melanoma. J Clin Oncol. 2008;26(15 suppl) Abstract LBA9011. [Google Scholar]
- 93.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 94.Ribas A, Hanson DC, Noe DA, et al. Tremelimumab (CP-675,206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. The Oncologist. 2007;12:873–883. doi: 10.1634/theoncologist.12-7-873. [DOI] [PubMed] [Google Scholar]
- 95.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gomez-Navarro J, et al. Survival of patients (pts) with metastatic melanoma treated with the anti-CTLA4 monoclonal antibody (mAb) CP-675,206 in a phase I/II study. J Clin Oncol. 2007;25(18 suppl) Abstract 8524. [Google Scholar]
- 98.Weber JS, et al. The efficacy and safety of ipilimumab (MDX-010) in patients with unresectable stage III or stage IV malignant melanoma. J Clin Oncol. 2007;25(18 suppl) Abstract 8523. [Google Scholar]
- 99.Kirkwood JM, et al. A phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. J Clin Oncol. 2008;26(15 suppl) doi: 10.1158/1078-0432.CCR-09-2033. Abstract 9023. [DOI] [PubMed] [Google Scholar]
- 100.O'Day SJ, et al. Efficacy and safety of ipilimumab induction and maintenance dosing in patients with advanced melanoma who progressed on one or more prior therapies. J Clin Oncol. 2008;26(15 suppl) Abstract 9021. [Google Scholar]
- 101.Hamid O, et al. Kinetics of response to ipilimumab (MDX-010) in patients with stage III/IV melanoma. J Clin Oncol. 2007;25(18 suppl) Abstract 8525. [Google Scholar]
- 102.Flaherty KT. Chemotherapy and targeted therapy combinations in advanced melanoma. Clin Cancer Res. 2006;12:2366s–2370s. doi: 10.1158/1078-0432.CCR-05-2505. [DOI] [PubMed] [Google Scholar]
- 103.Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- 104.Amaravadi R, et al. Updated results of a randomized phase II study comparing two schedules of temozolomide in combination with sorafenib in patients with advanced melanoma. J Clin Oncol. 2007;25(18 suppl) Abstract 8527. [Google Scholar]
- 105.Flaherty K, et al. Final results of E2603: a double-blind, randomized phase III trial comparing carboplatin (C)/paclitaxel (P) with or without sorafenib (S) in metastatic melanoma. J Clin Oncol. 2010;28(15 suppl) Abstract 8511. [Google Scholar]
- 106.Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–586. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Flaherty K, et al. Phase I study of PLX4032: proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J Clin Oncol. 2009;27(15 suppl) Abstract 9000. [Google Scholar]
- 108.McArthur G, et al. Early FDG-PET responses to PLX4032 in BRAF-mutant advanced melanoma. J Clin Oncol. 2010;28(15 suppl) Abstract 8529. [Google Scholar]
- 109.U.S. National Institutes of Health. A Phase I Study to Investigate the Safety, Pharmacokinetics, and Pharmacodynamics of GSK2118436 in Subjects With Solid Tumors. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00880321.
- 110.U.S. National Institutes of Health. A Study to Evaluate RAF265, an Oral Drug Administered to Subjects With Locally Advanced or Metastatic Melanoma. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00304525.
- 111.Kefford R, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol. 2010;28(15 suppl) Abstract 8503. [Google Scholar]
- 112.Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–2219. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 113.Dummer R, et al. AZD6244 (ARRY-142886) vs temozolomide (TMZ) in patients (pts) with advanced melanoma: an open-label, randomized, multicenter, phase II study. J Clin Oncol. 2008;26(15 suppl) Abstract 9033. [Google Scholar]
- 114.U.S. National Institutes of Health. Comparison of AZD6244 in Combination With Dacarbazine Versus (vs) Dacarbazine Alone in BRAF Mutation Positive Melanoma Patients. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00936221.
- 115.Infante JR, et al. Safety and efficacy results from the first-in-human study of the oral MEK 1/2 inhibitor GSK1120212. J Clin Oncol. 2010;28(15 Suppl) Abstract 2503. [Google Scholar]
- 116.Kirkwood J, et al. A phase II study of tasisulam sodium (LY573636) as second-line treatment for patients with unresectable or metastatic melanoma. J Clin Oncol. 2010;28(15 Suppl) doi: 10.1002/cncr.26068. Abstract 8541. [DOI] [PubMed] [Google Scholar]
- 117.Dudley ME, Wunderlich JR, Shelton TE, et al. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Minimally cultured or selected autologous tumor-infiltrating lymphocytes after a lympho-depleting chemotherapy regimen in metastatic melanoma patients. J Immunother. 2009;32:415–423. doi: 10.1097/CJI.0b013e31819c8bda. [DOI] [PubMed] [Google Scholar]
- 121.Koike N, Pilon-Thomas S, Mule JJ. Nonmyeloablative chemotherapy followed by T-cell adoptive transfer and dendritic cell-based vaccination results in rejection of established melanoma. J Immunother. 2008;31:402–412. doi: 10.1097/CJI.0b013e31816cabbb. [DOI] [PubMed] [Google Scholar]
- 122.Wallen H, Thompson JA, Reilly JZ, et al. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS One. 2009;4:e4749. doi: 10.1371/journal.pone.0004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hauschild A, et al. Phase III, randomized, double-blind study of elesclomol and paclitaxel versus paclitaxel alone in stage IV metastatic melanoma (MM) J Clin Oncol. 2009;27(18 suppl) doi: 10.1200/JCO.2008.17.1579. Abstract LBA9012. [DOI] [PubMed] [Google Scholar]
- 124.Bedikian AY, et al. Results of pooled analyses from two phase III trials of 1,085 patients (pts) with advanced melanoma: oblimersen (OBL) plus dacarbazine (DTIC) versus DTIC alone. J Clin Oncol. 2010;28(15 suppl) Abstract 8573. [Google Scholar]
- 125.Cancer Research, U.K. A trial looking at bevacizumab after surgery for melanoma skin cancer (AVAST-M) [Accessed June 11, 2010]. Available at http://www.cancerhelp.org.uk/trials/a-trial-looking-at-bevacizumab-after-surgery-for-melanoma-skin-cancer.
- 126.U.S. National Institutes of Health. Efficacy and Safety Study of OncoVEXGM-CSF Compared to GM-CSF in Melanoma. [Accessed June 11, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00769704.