The currently available therapeutic options for patients with advanced adrenocortical carcinoma are discussed and the molecular rationale behind and clinical evidence for novel and emerging therapies are detailed.
Keywords: Adrenal cortex neoplasms, Adrenal cortex hormones, Mitotane, Drug therapy, Protein kinase inhibitors, Insulin-like growth factor 2
Learning Objectives
After completing this course, the reader will be able to:
Review the role and describe the limitations of conventional therapies for adrenocortical carcinoma.
Evaluate the current preclinical molecular research contributing to the rational selection of targeted therapies for adrenocortical carcinoma.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Adrenocortical carcinoma (ACC) is a rare but aggressive malignancy with a poor prognosis. Complete surgical resection offers the only potential for cure; however, even after apparently successful excision, local or metastatic recurrence is frequent. Treatment options for advanced ACC are severely limited. Mitotane is the only recognized adrenolytic therapy available; however, response rates are modest and unpredictable whereas systemic toxicities are significant. Reported responses to conventional cytotoxic chemotherapy have also been disappointing, and the rarity of ACC had hampered the ability to undertake randomized clinical studies until the establishment of the First International Randomized Trial in Locally Advanced and Metastatic Adrenocortical Carcinoma. This yet-to-be reported study seeks to identify the most effective first- and second-line cytotoxic regimens. The past decade has also seen increasing research into the molecular pathogenesis of ACCs, with particular interest in the insulin-like growth factor signaling pathway. The widespread development of small molecule tyrosine kinase inhibitors in broader oncological practice is now allowing for the rational selection of targeted therapies to study in ACC. In this review, we discuss the currently available therapeutic options for patients with advanced ACC and detail the molecular rationale behind, and clinical evidence for, novel and emerging therapies.
Introduction
Adrenocortical carcinoma (ACC) is a rare malignancy with an estimated prevalence of 4–12 per million population [1], in contrast to benign adrenocortical adenomas that occur in at least 3% of the population aged >50 years [1, 2]. ACC follows a bimodal age distribution, with peaks in childhood and in the fourth to fifth decades of life [3, 4]. At present, early identification of this aggressive malignancy with complete surgical resection offers the only potential for cure [2, 5]. The prognosis is poor, however, and even those patients with apparently localized tumors at diagnosis frequently develop metastatic disease within 6–24 months of surgical resection [6–9].
The majority of ACCs are sporadic neoplasms of undetermined etiology; however, familial predisposition does occur. Rare hereditary syndromes known to predispose to either benign or malignant adrenocortical tumors (ACTs) include the Li-Fraumeni syndrome, Beckwith-Wiedemann syndrome, multiple endocrine neoplasia 1, Carney complex, and congenital adrenal hyperplasia. Somatic mutations in the genes involved in these syndromes have occasionally been identified in sporadic ACTs [10].
Approximately 60% of ACCs show clinical hormonal hypersecretion, whereas the remainder are considered nonfunctioning. Cushing's syndrome, resulting from cortisol hypersecretion, with or without virilization, resulting from androgen excess, is the most frequent presentation in functioning ACC [2]. Cortisol-secreting ACCs have been reported to be associated with a poorer overall prognosis, showing a higher likelihood of [11] and earlier time to [12] metastatic disease. Whether this relates to the presence of hypercortisolemia, with the resultant adverse systemic metabolic and immune sequelae, or whether cortisol secreting ACCs have an inherently more aggressive biology is currently unclear [11].
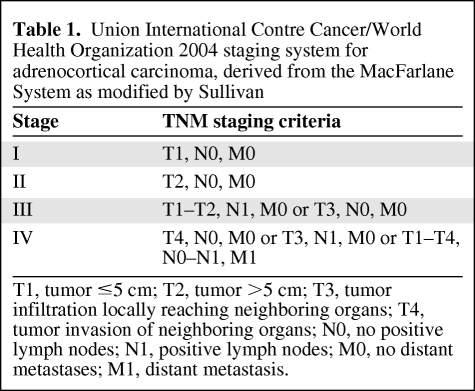
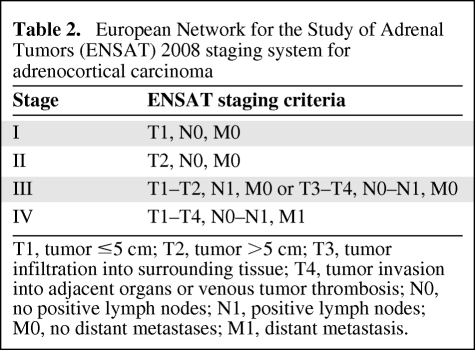
Survival is dependent on stage at presentation [3, 13, 14]. For many years, the staging of ACC was largely performed using the MacFarlane classification as modified by Sullivan [15, 16]. Modifications were used, predominantly restricting stage IV to only patients with metastatic disease [7, 8, 17]. In 2004, the World Health Organization and the Union Internationale Contre Cancer defined tumor–node–metastasis stages based on the MacFarlane/Sullivan system (Table 1) [18]. Subsequently the European Network for the Study of Adrenal Tumors (ENSAT) reported greater prognostic utility by restricting stage IV ACC to include only patients with metastatic disease and defining stage III as the presence of positive lymph node(s), any local infiltration, or venous tumor thrombosis, regardless of tumor size (Table 2) [19]. The reported 5-year survival rates in 416 patients using this classification were 82% for stage I, 61% for stage II, 50% for stage III, and 13% for stage IV. The ENSAT staging system was recently validated in an independent cohort using the North American Surveillance, Epidemiology, and End Results registry [20].
Table 1.
Union International Contre Cancer/World Health Organization 2004 staging system for adrenocortical carcinoma, derived from the MacFarlane System as modified by Sullivan
T1, tumor ≤5 cm; T2, tumor >5 cm; T3, tumor infiltration locally reaching neighboring organs; T4, tumor invasion of neighboring organs; N0, no positive lymph nodes; N1, positive lymph nodes; M0, no distant metastases; M1, distant metastasis.
Table 2.
European Network for the Study of Adrenal Tumors (ENSAT) 2008 staging system for adrenocortical carcinoma
T1, tumor ≤5 cm; T2, tumor >5 cm; T3, tumor infiltration into surrounding tissue; T4, tumor invasion into adjacent organs or venous tumor thrombosis; N0, no positive lymph nodes; N1, positive lymph nodes; M0, no distant metastases; M1, distant metastasis.
The management of patients with ACC requires a multidisciplinary approach, with initial complete surgical resection the overriding goal in limited disease. Adjuvant medical therapy or irradiation may also be considered [21, 22]. Systemic therapies for advanced ACC are limited, and there remains a need for new and effective treatments. Mitotane (1,1-dichloro-2(o-chlorophenyl)-2-(p-chlorophenyl)ethane [o,p'DDD]) has remained the only available adenolytic agent for several decades, although response rates are modest at approximately 30%. Conventional cytotoxic therapy has been disappointing, and historically, the rarity of ACC had limited large-scale clinical trials until the recent establishment of the First International Randomized Trial in Locally Advanced and Metastatic Adrenocortical Carcinoma (FIRM-ACT). That study, discussed in the section on cytotoxic chemotherapy below, sought to establish the most effective chemotherapy regimen for advanced ACC patients.
Our understanding of the molecular biology of ACC has also evolved. A number of global gene-expression profiling studies of sporadic ACCs have been completed, seeking to identify biomarkers of potential prognostic or therapeutic utility [10, 23–29]. Those studies have offered insights into the pathophysiology of adrenocortical carcinogenesis. Identification of specific molecular pathways dysregulated in ACC has the potential to allow rational testing of targeted therapies. This review describes the current therapeutic options available for adults with advanced ACC, with a focus on new and emerging therapies.
Surgery
Surgical resection remains the cornerstone of treatment for patients presenting with stage I to stage III ACC [2, 5, 30]. The aim of surgery is to achieve a complete margin-negative (R0) resection of the tumor, which may be possible even with significant local invasion in select patients [8, 31, 32]. Selection of an experienced endocrine or oncologic surgeon is critical, and prognosis is directly related to the ability of the surgeon to achieve clear margins. Patients who have a complete surgical resection have a 5-year survival rate of 40%–50%, whereas those who have an incomplete resection have a median survival duration <1 year [8, 17, 33–36]. In the event of clinical presentation with limited metastatic disease, there is evidence that complete resection of the metastatic and primary tumors provides benefit.
When a preoperative diagnosis or a high level of suspicion of ACC exists, open surgical resection is recommended [36–38]. A transabdominal approach allows maximal exposure for complete en-bloc resection, minimizes the chance of tumor spillage, and allows vascular control of the inferior vena cava (IVC), aorta, and renal vessels. En-bloc excision of the tumor may involve resection of the adrenal gland and contiguous involved organs, including the kidney, liver, spleen, pancreas, and colon. Involvement of the IVC or renal vein with tumor thrombus is not a contraindication to surgical intervention and may be managed with cardiopulmonary bypass [35, 39, 40]. Preservation of the integrity of the tumor capsule is critical in preventing tumor spillage, thereby reducing the incidence of local recurrence [35, 41].
Controversy surrounds the role of laparoscopic resection for ACC [42–45]. Since the first laparoscopic adrenalectomy performed in 1992, it has largely replaced the open technique and has become the gold standard for the management of benign adrenal pathology [46, 47]. Laparoscopic removal of large adrenal tumors can be performed safely by experienced surgeons who have the judgment to convert to an open operation if key intraoperative findings suggestive of malignancy are encountered [48]. Concerningly, retrospective studies have demonstrated more frequent or earlier locoregional recurrence and a shorter disease-free survival interval when this technique is used for the management of ACCs [5, 37, 49–53]. Postulated contributing factors include a higher risk for capsular breach and tumor spillage, a higher rate of margin-positive disease, and cell dispersion within the peritoneal cavity in association with gas insufflation. At present, laparoscopic resection is not recommended in the management of ACC [5], although a recent retrospective analysis of 152 patients from the German ACC Registry did not find this technique inferior to an open procedure in localized ACC up to 10 cm in diameter [54].
Even after an apparently complete surgical resection, 50%–80% of patients develop either locoregional or metastatic tumor recurrence [5, 55]. When complete removal is feasible, aggressive surgical resection of these deposits, with the goal of again achieving negative surgical margins, should be undertaken [7, 8, 34, 56–58]. Such patients undergoing complete surgical resection have been shown, in retrospective series, to have longer disease-free (41.5 months versus 15 months) and overall (5-year survival rate, 50% versus 8%) survival times than those patients who are not amenable for surgical resection [33]. Although the number of patients included in retrospective series is small, available data point toward adopting an aggressive surgical approach to recurrent disease.
Data to support the routine debulking of nonresectable tumors are lacking, although decision making should be individualized, with consideration given to the underlying tumor biology, including the rate of progression, length of the disease-free interval, and histological grade [5]. Debulking of tumor in advanced metastatic disease may provide relief from hormonal excess in functioning tumors and thus facilitate other therapeutic options [17, 33, 57, 59]. Overall, however, individuals with inoperable recurrent or metastatic disease have a particularly poor prognosis and are often better palliated with medical management.
Mitotane
Mitotane (o,p'DDD) has been the mainstay of medical management for advanced ACC since it was found to be adrenolytic in the 1950s [2, 60, 61]. Despite this, quality, prospective, controlled, systematic studies of its efficacy are lacking, and available studies are heterogeneous because of variable dosing regimens used, varying study endpoints, and frequent combination of mitotane with other therapies [61]. Overall, in reported retrospective series wherein mitotane was administered for advanced ACC, a partial or complete response of variable duration was seen in 33% of cases [62]. Unfortunately, at present, there is no widely accepted method for predicting the response of an individual to mitotane therapy. A tritium-release assay has been developed and is reported to correlate with the ability of the tumor to metabolize mitotane [62, 63].
Although not conclusively determined, the mechanism of action of mitotane is believed to require metabolic transformation within the tumor by an unidentified cytochrome P450 enzyme(s), with the resultant acyl chloride derivative causing cytotoxicity through covalent binding to adrenocortical macromolecules [62, 64–66]. The identity of these targets proteins has not been elucidated [61, 62]. There is also evidence that mitotane leads to oxidative damage through free radical and superoxide formation [62]. Cytotoxic changes are predominantly seen in the zona reticularis and zona fasciculata, whereas the zona glomerulosa appears relatively resistant. In addition to its potential role as a cytotoxic, mitotane inhibits steroidogenesis and thus assists in the management of hormone excess in clinically hyperfunctioning ACC [21, 61].
The tolerability of mitotane is limited by its significant systemic toxicities [2, 61]. Gastrointestinal effects are common, including nausea, vomiting, anorexia, and diarrhea, whereas neurological toxicities, including lethargy, somnolence, ataxia, confusion, and depression, are dose limiting. Abnormalities in liver function tests are common, although significant hepatotoxicity is rare. Recognized hematological abnormalities include cytopenias and prolongation of bleeding time. Skin rash and renal abnormalities, including hematuria and cystitis, have also been described [2, 21, 61]. The measurement of serum mitotane levels, targeting a range of 14–20 mg/l [67], was validated in several studies as correlating with a therapeutic response while minimizing neurological toxicity [68–70]. Variable dosing regimens have been proposed to maximize the prompt achievement of a therapeutic level while minimizing side effects [71–73]. There is significant interpatient variability in the daily dose required, and the half-life of mitotane increases with prolonged administration [2, 21, 61]. Mitotane dose titration and supportive therapies require close clinical supervision.
In addition to its adrenal effects, mitotane therapy is associated with diverse biochemical and clinical endocrinological effects. Hypercholesterolemia and hypertriglyceridemia are commonly observed. Hepatic production of hormone-binding globulins (including cortisol-, sex hormone-, thyroid-, and vitamin D-binding globulins) is augmented, thus increasing total hormone levels while impairing free hormone bioavailability [21, 74–76]. Mitotane may also influence estrogen receptor–dependent signaling [75]. Clinically, gynecomastia and sexual dysfunction may be observed in men, and androgen replacement may be required. A reduction in free thyroxine levels is also frequently observed, with recent in vitro work suggesting that mitotane also directly inhibits pituitary thyrotroph secretion and viability [77]. Thyroxine replacement may be required if clinical hypothyroidism is evident [78].
The induction of hepatic cytochrome P450 enzymes by mitotane induces the metabolism of a variety of agents, including corticosteroids. High-dose glucocorticoid replacement is therefore typically necessary, as a result of both the inhibition of adrenocortical steroidogenesis and the increased metabolic clearance of corticosteroids [21, 61, 79, 80]. Mineralocorticoid supplementation may also be necessary. Overall, the assessment of hormonal replacement is largely based on clinical assessment, with underreplacement of corticosteroids recognized to exacerbate mitotane-induced side effects [81].
Cytotoxic Chemotherapy
The rarity of ACC has limited clinical studies of cytotoxic chemotherapeutic agents. Cisplatin-containing regimens have been the most frequently studied, but as yet, no randomized trial results have been published. Most reports describe small series of advanced ACC that has progressed despite mitotane therapy. Reported response rates are generally modest, studies lack power, and comparisons between different regimens are lacking. As yet, clinical factors predictive of a cytotoxic response have not been determined [41, 73].
The presence of high levels of the multidrug-resistance (MDR) protein MDR-1/P-glycoprotein has been postulated to be responsible for the low response rates to some cytotoxics [82]. MDR-1 is found in high concentrations in ACC, where it acts as a drug efflux pump [83]. In vitro studies have demonstrated that mitotane partially reverses chemotherapy failure by inhibiting drug efflux. This forms part of the rationale for combining mitotane with standard chemotherapy; however, the role this plays in vivo remains to be fully elucidated [84–86]. Early-phase clinical trials involving targeted MDR-1 efflux pump inhibitors are discussed in the targeted therapies section below.
Expression of the excision repair crosscomplementation group 1 (ERCC-1) protein, involved in DNA repair, has been found to influence response to platinum compounds in other malignancies, particularly non-small cell lung carcinoma [87]. High ERCC-1 expression by immunohistochemistry in ACC was reported, in a retrospective tissue array analysis, to predict poor prognosis in patients treated with platinum-based chemotherapy [88].
The most encouraging results to date have come from the combination of etoposide, doxorubicin, and cisplatin with low-dose mitotane (the EDP/Italian/Berruti protocol) [12, 89]. Among 72 patients with nonresectable ACC studied prospectively, an overall response rate of 49% was achieved with this regimen [12]. A complete response was achieved in five patients, whereas a further 30 patients had disease stabilization. The median time to progression in responding patients was 18 months. The use of chemotherapy allowed 10 patients to be downstaged such that radical surgical resection was possible. Those patients had a significantly longer overall survival time, again highlighting the importance of surgical resection in this disease.
The combination of the above cytotoxic drugs and mitotane is associated with significant toxicity. A combination of streptozotocin and mitotane was studied in an attempt to achieve clinical benefit with less toxicity [90]. The combination was investigated as adjuvant therapy in 28 patients following radical resection and as active therapy in 22 patients with measurable disease. A longer disease-free interval and longer survival time were observed in those individuals who received adjuvant chemotherapy, and a complete or partial response was seen in 36% of patients with measurable disease. The associated toxicity was minimal.
The success, albeit limited, of these two protocols led to the recommendation by the International Consensus Conference on Adrenal Cancer, Ann Arbor, MI, that either the EDP plus mitotane or the streptozotocin plus mitotane regimen be used in advanced ACC patients [5]. Subsequent to this meeting, FIRM-ACT was established (http://www.firm-act.org). This prospective study is comparing both cytotoxic regimens as first- and second-line therapies in advanced ACC patients, with survival as the primary outcome. This study is now closed and is expected to report its findings in 2011.
Even with use of these regimens, it is predicted that a significant number of patients will develop progressive or recurrent disease. A need therefore exists for further therapies. A study of 11 patients who had failed streptozotocin and mitotane reported that the combination of vincristine, cisplatin, teniposide, and cyclophosphamide led to a partial response or disease stabilization in nine individuals [91]. A recent phase II clinical trial of 28 patients reported that gemcitabine plus metronomic fluoropyrimidines (5-fluorouracil or oral capecitabine), in combination with mitotane, was well tolerated as second-line therapy, resulting in a complete response in one patient, a partial response in one patient, and disease stabilization in a further 11 patients [92].
Paclitaxel was demonstrated, in preclinical studies, to produce dose-dependent inhibition of adrenal cancer cell line proliferation [93, 94]. An early-phase clinical trial reported a minor response in a single patient with ACC treated with paclitaxel and interferon-α2b [95]. Phase II studies currently under way include metronomic paclitaxel in combination with the oral multikinase inhibitor sorafenib, and docetaxel in combination with cisplatin. Another novel agent under preclinical investigation is decitabine, an inhibitor of DNA promoter methylation, reported to inhibit adrenal cancer cell proliferation, reduce cortisol secretion, and inhibit cell invasion in vitro [96].
The rarity of ACC has limited the ability of oncologists to perform large-scale clinical studies, but significant international effort has now demonstrated that adequately powered clinical studies of ACC therapy are possible. The outcome of the FIRM-ACT study is keenly awaited; however, it is acknowledged that further advances are still required, likely through the combination of conventional cytotoxics with novel targeted therapies.
Radiotherapy
Traditionally, ACC was believed to be radioresistant [6, 55, 97]. A recent retrospective review of published data combined with German ACC Registry data found a response to palliative radiotherapy in 57% (52 of 91) of patients [98]. Radiation had been given predominantly for painful bony metastases; however, a small number of local or distant recurrences were also treated. A smaller, recent, retrospective review of the Dutch ACC Registry also reported a partial tumor response in the metastatic deposits of two patients and pain relief in six patients irradiated for bony disease [99]. Taken together, this evidence suggests that palliative radiotherapy has a role in symptomatic metastatic deposits, particularly bone disease. Interestingly, in vitro work suggests that mitotane has a sensitizing effect on adrenocortical cancer cells treated with ionizing radiation [100, 101].
Radionuclide Therapy
Radiolabeled metomidate, a tracer that binds to adrenal 11β-hydroxylase (P450c11), shows promise in distinguishing neoplasms of adrenocortical origin from other lesions, such as metastatic deposits and pheochromocytoma. 11C-labeled metomidate has been used in positron emission tomography studies and as 123I-iodometomidate in single photon emission computed tomography imaging [102–104]. Iodine-labeled metomidate may, in the future, hold therapeutic potential as a specific adrenocortical radionuclide [30, 104].
Radiofrequency Ablation
Radiofrequency thermal ablation (RFA) has been reported as being effective for the short-term local control of ACCs <5 cm in size, with one study reporting that eight of 12 such tumors decreased in size and lost contrast enhancement on imaging over a mean follow-up of 10.3 months [105]. Percutaneous laser ablation was also reported as being useful for the palliative treatment of unresectable hepatic metastases and a single primary ACC in a series of four patients [106]. Other studies have reported variable outcomes, and adverse effects include bleeding, infection, and injury to adjacent structures [107, 108]. Further investigation is necessary to evaluate long-term efficacy and to identify the clinical scenarios in which RFA should be used.
Management of Hormonal Excess
Hormonal excess causes significant morbidity in advanced ACC patients. Although mitotane reduces steroidogenesis, its slow onset of action often necessitates the introduction of alternative adrenostatic drugs. Adrenal insufficiency is a potentially life-threatening risk of these therapies, and close endocrinological supervision is required to ensure that cortisol levels are kept in the desired range or to titrate adrenal hormone replacement therapy if required [21, 109, 110].
Ketoconazole
Ketoconazole is an antifungal agent that inhibits multiple adrenal steroidogenic enzymes including C17–20 desmolase (P450c17), as well as the cholesterol side-chain cleavage enzyme (P450scc) and 11β-hydroxylase (P450c11) [111–114]. It may also act as a glucocorticoid receptor antagonist [115]. The use of ketoconazole is limited by hepatotoxicity and gastrointestinal side effects. Because of its effective hepatic cytochrome enzyme inhibition, interaction with other agents can significantly increase drug toxicity [21].
Metyrapone
Metyrapone inhibits 11β-hydroxylase (P450c11 hydroxylase), blocking the final step in cortisol synthesis. The resultant increase in circulating precursors may cause adverse clinical effects, including induction of hypertension through the weak mineralocorticoid 11-deoxycortisol, and acne and hirsutism through the accumulation of androgenic precursors [116]. Like ketoconazole, metyrapone is an inhibitor of hepatic cytochrome enzymes, with a resultant high risk for drug interactions.
Aminoglutethimide
Aminoglutethimide inhibits the cholesterol side chain cleavage (P450scc) enzyme as well as the aromatization of androgens to estrogens, thus blocking the synthesis of cortisol, aldosterone, androgens, and extra-adrenal estrogen production. Adverse effects include lethargy, somnolence, headache, ataxia, nausea, and rash [117]. Interestingly, in vitro data have demonstrated that aminoglutethimide and etomidate inhibit proliferation in a human ACC cell line, in addition to their effects on steroidogenesis [118].
Etomidate
Intravenous etomidate inhibits 11β-hydroxylase (P450c11) and the cholesterol side chain cleavage (P450scc) enzyme with prompt reduction in corticosteroid levels. It therefore has a role in acute hypercortisolemia, whereby patients are unable to take oral adrenostatic medications, including the intensive care unit setting [21, 119].
Targeted Therapies
The understanding of growth factors and receptor tyrosine kinases in human malignancies has evolved in recent years. These cell surface transmembrane proteins activate intracellular signaling pathways and thus modulate biological activities, including cell proliferation, migration, and invasion. In vitro work, in combination with gene-expression profiling and immunohistochemical studies, has allowed for rational selection of targeted therapies to trial in ACC patients [73, 120]. The following section summarizes the current experience with these therapies, including those in preclinical and early-phase clinical investigations.
Insulin-Like Growth Factor 2
The most consistent molecular event identified in ACCs is overexpression of insulin-like growth factor (IGF)-2 [23, 24, 29, 121–124], known to signal predominantly through the IGF-1 receptor (IGF-1R) [125–127]. Preclinical studies have demonstrated that antagonism of IGF-1R inhibits the growth of ACC cells in vitro and in xenograft models [124, 128, 129]. A phase I study of figitumumab, an anti–IGF-1R monoclonal antibody, demonstrated stable disease in eight of 14 patients [130], and a phase I study of a small-molecule tyrosine kinase IGF-1R inhibitor (OSI-906) demonstrated a partial tumor response in one of three patients with advanced ACC [131]. Targeting the IGF signaling pathway is seen as one of the most promising therapies in advanced ACC, and further clinical studies using IGF-1R antagonists in advanced ACC patients are currently in progress (http://www.clinicaltrials.gov) [30].
Epidermal Growth Factor Receptor
Overexpression of the epidermal growth factor receptor (EGFR) protein in ACCs [132–135] has provided the rationale for the use of EGFR inhibitors. Unfortunately, results to date have been disappointing. The EGFR antagonist gefitinib failed to demonstrate any activity in 19 patients with advanced ACC [136], and a recent report of 10 patients with advanced ACC also found the EGFR inhibitor erlotinib to show limited efficacy in combination with gemcitabine. That study reported a minor response in one individual, whereas the remainder experienced progressive disease or toxicities [137].
Angiogenesis Inhibitors
Angiogenesis and neovascularization are critical for tumor growth. Vascular endothelial growth factor (VEGF) is a proangiogenic growth factor that binds to the tyrosine kinase VEGF receptor, resulting in the activation of several intracellular signaling pathways [138]. VEGF is upregulated in ACC tumor tissue [30, 139, 140]. Circulating VEGF is also elevated in the serum of patients with both benign or malignant ACTs and pheochromocytomas [141, 142], and levels fall after successful tumor resection [143, 144]. Some studies have found circulating VEGF levels to be significantly greater in patients with ACCs than in those with benign adrenal disease [141, 144]. These findings have led to interest in the targeting of VEGF signaling in ACC therapy; however, again the limited results to date have been disappointing. A study of 10 patients with advanced ACC reported no response to bevacizumab, an anti-VEGF humanized monoclonal antibody, in combination with capecitabine [145].
Thalidomide, an antiangiogenic agent that inhibits the activity of basic fibroblast growth factor-2 [138], is in widespread clinical use in multiple myeloma patients. A single case report detailed a partial response to thalidomide in a patient with advanced chemoresistant ACC [146]. Newer antiangiogenic agents for which there is no current experience in ACC therapy include the soluble VEGF receptors, including aflibercept, and the vascular-disrupting agents (reviewed in [138, 147]). The antiangiogenic multitarget tyrosine kinase inhibitors sorafenib and sunitinib are discussed below.
Multikinase Inhibitors
Sorafenib is an oral tyrosine kinase inhibitor targeting the cell proliferation molecule Raf-1, as well as the RET proto-oncogene, KIT (stem cell ligand receptor) tyrosine kinase, platelet-derived growth factor (PDGF) receptor, and VEGF receptor subtypes 2 and 3 [138]. A phase I study reported stable disease in two patients with ACC treated with sorafenib and the farnesyltransferase inhibitor tipifarnib [148], and a subsequent single case report described a sustained response to sorafenib in an advanced ACC patient [149]. A phase II study investigating sorafenib in combination with metronomic paclitaxel is ongoing.
Sunitinib also inhibits multiple receptor tyrosine kinases, including the PDGF receptor, VEGF receptor (subtypes 1, 2, and 3), KIT, RET, FLT3, and colony-stimulating factor 1 receptor [138]. A partial response to sunitinib in a single patient with advanced ACC was recently reported [150]. The results of a phase II study investigating sunitinib in patients with advanced ACC who have progressed after cytotoxic chemotherapy (Sunitinib in Refractory ACC) are awaited.
Imatinib mesylate is an oral tyrosine kinase receptor inhibitor that primarily targets the fusion protein Bcr-Abl, in addition to inhibiting the PDGF receptor and KIT tyrosine kinases. In a phase II study, imatinib was not found to produce an objective response in the four patients enrolled with advanced ACC [151].
Mammalian Target of Rapamycin Signaling
The mammalian target of rapamycin (mTOR) signaling pathway has emerged in recent years as a potential therapeutic target in cancer. Agents that inhibit mTOR signaling include the immunosuppressant rapamycin (sirolimus) and its derivatives everolimus and temsirolimus [152]. Interestingly, rapamycin was found to demonstrate antiangiogenic effects through a reduction in VEGF production and signaling in vitro [153, 154]. Rapamycin may also have a direct vascular-disrupting role, with reports of endothelial cell toxicity and thrombosis in tumor vasculature when combined with gemcitabine in a pancreatic cancer xenograft model [155]. mTOR signaling had not been investigated in ACC until a novel study recently identified everolimus as able to reduce adrenocortical tumor growth both in vitro and in vivo in a mouse xenograft model, and demonstrated that the IGF-1R and mTOR signaling cascades were regulated by specific microRNAs [156]. Temsirolimus is currently being evaluated in a phase I clinical trial in combination with the anti-IGF-1R recombinant monoclonal antibody cixutumumab in advanced malignancies, including ACC.
MDR-1/P-Glycoprotein
Data suggest that the expression of MDR-1 is associated with a poor response to chemotherapy in a number of human malignancies [157]. This led to a search for compounds that may interfere with MDR-1 function [158]. The calcium channel blocker verapamil was identified as a first-generation MDR-1 modulator, improving chemosensitivity to both vincristine and doxorubicin in leukemic and ovarian cancer cells in vitro; however, subsequent clinical studies reported conflicting results [157]. Similarly, cyclosporine showed in vitro promise as an MDR-1 inhibitor; however, trials in hematologic malignancies and colorectal carcinoma have been disappointing [157]. Preclinical studies investigating the cotreatment of primary human ACC cultures with verapamil, cyclosporine, doxorubicin, and vincristine also failed to identify a large additive effect on cytotoxicity, nor could an effect be correlated with MDR-1 expression [159]. Second-generation analogs of MDR-1 modulators include D-verapamil and valspodar (PSC833), an analog of cyclosporin D. The combination of valspodar with vinblastine and paclitaxel in early-phase clinical trials identified complex pharmacokinetic interactions with variable clinical responses and toxicities [160–162]. Third-generation MDR-1 modulators have since been developed in an attempt to avoid such drug interactions [157]. One agent, tariquidar (XR9576), a MDR-1/P-glycoprotein drug efflux pump inhibitor [158, 163], is now in clinical trial in advanced ACC patients [30, 120, 164].
Peroxisome Proliferator-Activated Receptor γ Signaling
The nuclear receptor peroxisome proliferator-activated receptor (PPAR)-γ is expressed in the normal and neoplastic adrenal cortex [165, 166]. The treatment of human adrenocortical cancer cells in vitro with rosiglitazone, a PPAR-γ ligand, led to a lower rate of proliferation and greater steroidogenesis, suggesting antineoplastic and prodifferentiation effects [165–167]. A preclinical study in a xenograft model also demonstrated less tumor growth with rosiglitazone therapy, in association with less histopathologic infiltration, neovascularization, and VEGF gene expression [168]. As yet, there are no clinical studies of PPAR-γ ligands in ACC patients, and despite in vitro promise, the use of these agents as monotherapy in advanced breast cancer, prostate cancer, and liposarcoma patients has been disappointing [169].
β-Catenin and Wnt Signaling
β-catenin gene mutations are reported in benign and malignant ACTs, resulting in activation of the Wnt signaling pathway [170–172]. Transgenic mice demonstrating constitutive activation of β-catenin in the adrenal cortex develop progressive adrenocortical cell dysplasia, hyperaldosteronism, and locoregional metastatic invasion [173]. Preclinical in vitro studies have reported that a small-molecule inhibitor of Wnt signaling (PKF115–584) reduced adrenocortical cancer cell line proliferation and increased apoptosis [174]. At present, Wnt signaling inhibitors remain in preliminary preclinical investigation.
Steroidogenic Factor-1
Steroidogenic factor (SF)-1 is a nuclear transcription factor with a primary role in adrenal and gonadal development and in the regulation of cytochrome P450 steroidogenic enzyme expression in the adrenal cortex [175]. The demonstration of SF-1 overexpression in pediatric ACTs triggered interest in a potential role in tumorigenesis [176, 177]. In vitro studies reported that higher SF-1 expression led to greater proliferation and steroidogenesis in a human adrenocortical cancer cell line, with greater SF-1 signaling, resulting in ACT formation in mice [178]. SF-1 inverse agonists have been recently identified, and studies have demonstrated that compounds of the isoquinolinone class inhibit adrenocortical cell proliferation and steroidogenesis in vitro [179]. Further work is required to explore the potential role of such agents in ACC therapeutics.
Conclusion
Advanced ACC remains an aggressive malignancy with limited therapeutic options, and the rarity of the disease has historically frustrated systematic research efforts. The establishment in the past decade of collaborative ACC networks, combining tumor banking with annotated clinical data, is overcoming this impediment, and international efforts have demonstrated that multinational clinical trials are possible. The results of FIRM-ACT will establish the standard of care chemotherapy for advanced disease; however, it is acknowledged that novel therapeutic options are still desperately required. Despite recent advances in the understanding of the molecular pathways that are dysregulated in adrenocortical tumorigenesis, these findings have yet to be translated into meaningful clinical benefit. Basic and collaborative clinical research efforts are continuing, and at present, individuals with advanced ACC are encouraged to be managed at specialized centers with access to clinical trial enrollment.
Acknowledgments
Lyndal J. Tacon is supported by an Australian Post-Graduate Award Research Scholarship and a Cancer Institute New South Wales Research Scholars Award. Stan B. Sidhu is a New South Wales Cancer Institute Fellow.
Author Contributions
Conception/Design: Lyndal J. Tacon, Ruth S. Prichard, Patsy S.H. Soon, Bruce G. Robinson, Roderick J. Clifton-Bligh, Stan B. Sidhu
Collection and/or assembly of data: Lyndal J. Tacon, Ruth S. Prichard
Data analysis and interpretation: Lyndal J. Tacon, Ruth S. Prichard
Manuscript writing: Lyndal J. Tacon, Ruth S. Prichard
Final approval of manuscript: Lyndal J. Tacon, Ruth S. Prichard, Patsy S.H. Soon, Bruce G. Robinson, Roderick J. Clifton-Bligh, Stan B. Sidhu
References
- 1.NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”) NIH Consens State Sci Statements. 2002;19:1–25. [PubMed] [Google Scholar]
- 2.Allolio B, Fassnacht M. Clinical review: Adrenocortical carcinoma: Clinical update. J Clin Endocrinol Metab. 2006;91:2027–2037. doi: 10.1210/jc.2005-2639. [DOI] [PubMed] [Google Scholar]
- 3.Wooten MD, King DK. Adrenal cortical carcinoma. Epidemiology and treatment with mitotane and a review of the literature. Cancer. 1993;72:3145–3155. doi: 10.1002/1097-0142(19931201)72:11<3145::aid-cncr2820721105>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Wajchenberg BL, Albergaria Pereira MA, Medonca BB, et al. Adrenocortical carcinoma: Clinical and laboratory observations. Cancer. 2000;88:711–736. [PubMed] [Google Scholar]
- 5.Schteingart DE, Doherty GM, Gauger PG, et al. Management of patients with adrenal cancer: Recommendations of an international consensus conference. Endocr Relat Cancer. 2005;12:667–680. doi: 10.1677/erc.1.01029. [DOI] [PubMed] [Google Scholar]
- 6.Luton JP, Cerdas S, Billaud L, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322:1195–1201. doi: 10.1056/NEJM199004263221705. [DOI] [PubMed] [Google Scholar]
- 7.Icard P, Louvel A, Chapuis Y. Survival rates and prognostic factors in adrenocortical carcinoma. World J Surg. 1992;16:753–758. doi: 10.1007/BF02067377. [DOI] [PubMed] [Google Scholar]
- 8.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: Surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 9.Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356:2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 10.Soon PS, McDonald KL, Robinson BG, et al. Molecular markers and the pathogenesis of adrenocortical cancer. The Oncologist. 2008;13:548–561. doi: 10.1634/theoncologist.2007-0243. [DOI] [PubMed] [Google Scholar]
- 11.Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: Poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91:2650–2655. doi: 10.1210/jc.2005-2730. [DOI] [PubMed] [Google Scholar]
- 12.Berruti A, Terzolo M, Sperone P, et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: A large prospective phase II trial. Endocr Relat Cancer. 2005;12:657–666. doi: 10.1677/erc.1.01025. [DOI] [PubMed] [Google Scholar]
- 13.Henley DJ, van Heerden JA, Grant CS, et al. Adrenal cortical carcinoma—a continuing challenge. Surgery. 1983;94:926–931. [PubMed] [Google Scholar]
- 14.Cohn K, Gottesman L, Brennan M. Adrenocortical carcinoma. Surgery. 1986;100:1170–1177. [PubMed] [Google Scholar]
- 15.Macfarlane DA. Cancer of the adrenal cortex; the natural history, prognosis and treatment in a study of fifty-five cases. Ann R Coll Surg Engl. 1958;23:155–186. [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan M, Boileau M, Hodges CV. Adrenal cortical carcinoma. J Urol. 1978;120:660–665. doi: 10.1016/s0022-5347(17)57317-6. [DOI] [PubMed] [Google Scholar]
- 17.Lee JE, Berger DH, el-Naggar AK, et al. Surgical management, DNA content, and patient survival in adrenal cortical carcinoma. Surgery. 1995;118:1090–1098. doi: 10.1016/s0039-6060(05)80119-9. [DOI] [PubMed] [Google Scholar]
- 18.DeLellis RA, Lloyd RV, Heitz PU, et al. Pathology and Genetics of Tumours of Endocrine Organs. Lyon, France: IARC Press; 2004. World Health Organization Classification of Tumours; pp. 1–136. [Google Scholar]
- 19.Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: Proposal for a revised TNM classification. Cancer. 2009;115:243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 20.Lughezzani G, Sun M, Perrotte P, et al. The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the International Union Against Cancer staging system: A North American validation. Eur J Cancer. 2010;46:713–719. doi: 10.1016/j.ejca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Veytsman I, Nieman L, Fojo T. Management of endocrine manifestations and the use of mitotane as a chemotherapeutic agent for adrenocortical carcinoma. J Clin Oncol. 2009;27:4619–4629. doi: 10.1200/JCO.2008.17.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berruti A, Fassnacht M, Baudin E, et al. Adjuvant therapy in patients with adrenocortical carcinoma: A position of an international panel. J Clin Oncol. 2010;28:e401–e402. doi: 10.1200/JCO.2009.27.5958. [DOI] [PubMed] [Google Scholar]
- 23.Giordano TJ, Thomas DG, Kuick R, et al. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am J Pathol. 2003;162:521–531. doi: 10.1016/S0002-9440(10)63846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Fraipont F, El Atifi M, Cherradi N, et al. Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J Clin Endocrinol Metab. 2005;90:1819–1829. doi: 10.1210/jc.2004-1075. [DOI] [PubMed] [Google Scholar]
- 25.Velázquez-Fernández D, Laurell C, Geli J, et al. Expression profiling of adrenocortical neoplasms suggests a molecular signature of malignancy. Surgery. 2005;138:1087–1094. doi: 10.1016/j.surg.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 26.de Reyniès A, Assié G, Rickman DS, et al. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol. 2009;27:1108–1115. doi: 10.1200/JCO.2008.18.5678. [DOI] [PubMed] [Google Scholar]
- 27.Giordano TJ, Kuick R, Else T, et al. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15:668–676. doi: 10.1158/1078-0432.CCR-08-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soon PS, Gill AJ, Benn DE, et al. Microarray gene expression and immunohistochemistry analyses of adrenocortical tumors identify IGF2 and Ki-67 as useful in differentiating carcinomas from adenomas. Endocr Relat Cancer. 2009;16:573–583. doi: 10.1677/ERC-08-0237. [DOI] [PubMed] [Google Scholar]
- 29.Laurell C, Velázquez-Fernández D, Lindsten K, et al. Transcriptional profiling enables molecular classification of adrenocortical tumours. Eur J Endocrinol. 2009;161:141–152. doi: 10.1530/EJE-09-0068. [DOI] [PubMed] [Google Scholar]
- 30.Fassnacht M, Kreissl MC, Weismann D, et al. New targets and therapeutic approaches for endocrine malignancies. Pharmacol Ther. 2009;123:117–141. doi: 10.1016/j.pharmthera.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Khorram-Manesh A, Ahlman H, Jansson S, et al. Adrenocortical carcinoma: Surgery and mitotane for treatment and steroid profiles for follow-up. World J Surg. 1998;22:605, 611. doi: 10.1007/s002689900442. discussion 611–612. [DOI] [PubMed] [Google Scholar]
- 32.Kendrick ML, Lloyd R, Erickson L, et al. Adrenocortical carcinoma: Surgical progress or status quo? Arch Surg. 2001;136:543–549. doi: 10.1001/archsurg.136.5.543. [DOI] [PubMed] [Google Scholar]
- 33.Crucitti F, Bellantone R, Ferrante A, et al. The Italian Registry for Adrenal Cortical Carcinoma: Analysis of a multiinstitutional series of 129 patients. The ACC Italian Registry Study Group. Surgery. 1996;119:161–170. doi: 10.1016/s0039-6060(96)80164-4. [DOI] [PubMed] [Google Scholar]
- 34.Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6:719–726. doi: 10.1007/s10434-999-0719-7. [DOI] [PubMed] [Google Scholar]
- 35.Dackiw AP, Lee JE, Gagel RF, et al. Adrenal cortical carcinoma. World J Surg. 2001;25:914–926. doi: 10.1007/s00268-001-0030-7. [DOI] [PubMed] [Google Scholar]
- 36.Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: Surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol. 2010;17:263–270. doi: 10.1245/s10434-009-0716-x. [DOI] [PubMed] [Google Scholar]
- 37.Miller BS, Ammori JB, Gauger PG, et al. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg. 2010;34:1380–1385. doi: 10.1007/s00268-010-0532-2. [DOI] [PubMed] [Google Scholar]
- 38.Leboulleux S, Deandreis D, Al Ghuzlan A, et al. Adrenocortical carcinoma: Is the surgical approach a risk factor of peritoneal carcinomatosis? Eur J Endocrinol. 2010;162:1147–1153. doi: 10.1530/EJE-09-1096. [DOI] [PubMed] [Google Scholar]
- 39.Ng L, Libertino JM. Adrenocortical carcinoma: Diagnosis, evaluation and treatment. J Urol. 2003;169:5–11. doi: 10.1016/S0022-5347(05)64023-2. [DOI] [PubMed] [Google Scholar]
- 40.Soon PS, Sidhu SB. Adrenocortical carcinoma. Cancer Treat Res. 2010;153:187–210. doi: 10.1007/978-1-4419-0857-5_11. [DOI] [PubMed] [Google Scholar]
- 41.Allolio B, Hahner S, Weismann D, et al. Management of adrenocortical carcinoma. Clin Endocrinol (Oxf) 2004;60:273–287. doi: 10.1046/j.1365-2265.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- 42.Henry JF, Sebag F, Iacobone M, et al. Results of laparoscopic adrenalectomy for large and potentially malignant tumors. World J Surg. 2002;26:1043–1047. doi: 10.1007/s00268-002-6666-0. [DOI] [PubMed] [Google Scholar]
- 43.Kebebew E, Siperstein AE, Clark OH, et al. Results of laparoscopic adrenalectomy for suspected and unsuspected malignant adrenal neoplasms. Arch Surg. 2002;137:948–951. doi: 10.1001/archsurg.137.8.948. discussion 952–953. [DOI] [PubMed] [Google Scholar]
- 44.Lombardi CP, Raffaelli M, De Crea C, et al. Role of laparoscopy in the management of adrenal malignancies. J Surg Oncol. 2006;94:128–131. doi: 10.1002/jso.20599. [DOI] [PubMed] [Google Scholar]
- 45.Palazzo FF, Sebag F, Sierra M, et al. Long-term outcome following laparoscopic adrenalectomy for large solid adrenal cortex tumors. World J Surg. 2006;30:893–898. doi: 10.1007/s00268-005-0288-2. [DOI] [PubMed] [Google Scholar]
- 46.Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med. 1992;327:1033. doi: 10.1056/NEJM199210013271417. [DOI] [PubMed] [Google Scholar]
- 47.Gill IS. The case for laparoscopic adrenalectomy. J Urol. 2001;166:429–436. [PubMed] [Google Scholar]
- 48.Soon PS, Yeh MW, Delbridge LW, et al. Laparoscopic surgery is safe for large adrenal lesions. Eur J Surg Oncol. 2008;34:67–70. doi: 10.1016/j.ejso.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Ushiyama T, Suzuki K, Kageyama S, et al. A case of Cushing's syndrome due to adrenocortical carcinoma with recurrence 19 months after laparoscopic adrenalectomy. J Urol. 1997;157:2239. [PubMed] [Google Scholar]
- 50.Zeh HJ, 3rd, Udelsman R. One hundred laparoscopic adrenalectomies: A single surgeon's experience. Ann Surg Oncol. 2003;10:1012–1017. doi: 10.1245/aso.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Prager G, Heinz-Peer G, Passler C, et al. Applicability of laparoscopic adrenalectomy in a prospective study in 150 consecutive patients. Arch Surg. 2004;139:46–49. doi: 10.1001/archsurg.139.1.46. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez RJ, Shapiro S, Sarlis N, et al. Laparoscopic resection of adrenal cortical carcinoma: A cautionary note. Surgery. 2005;138:1078–1085. doi: 10.1016/j.surg.2005.09.012. discussion 1085–1086. [DOI] [PubMed] [Google Scholar]
- 53.Cobb WS, Kercher KW, Sing RF, et al. Laparoscopic adrenalectomy for malignancy. Am J Surg. 2005;189:405–411. doi: 10.1016/j.amjsurg.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 54.Brix D, Allolio B, Fenske W, et al. Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: Surgical and oncologic outcome in 152 patients. Eur Urol. 2010;58:609–615. doi: 10.1016/j.eururo.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 55.Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;112:963–970. discussion 970–971. [PubMed] [Google Scholar]
- 56.Jensen JC, Pass HI, Sindelar WF, et al. Recurrent or metastatic disease in select patients with adrenocortical carcinoma. Aggressive resection vs chemotherapy. Arch Surg. 1991;126:457–461. doi: 10.1001/archsurg.1991.01410280059008. [DOI] [PubMed] [Google Scholar]
- 57.Icard P, Chapuis Y, Andreassian B, et al. Adrenocortical carcinoma in surgically treated patients: A retrospective study on 156 cases by the French Association of Endocrine Surgery. Surgery. 1992;112:972–979. discussion 979–980. [PubMed] [Google Scholar]
- 58.Bellantone R, Ferrante A, Boscherini M, et al. Role of reoperation in recurrence of adrenal cortical carcinoma: Results from 188 cases collected in the Italian National Registry for Adrenal Cortical Carcinoma. Surgery. 1997;122:1212–1218. doi: 10.1016/s0039-6060(97)90229-4. [DOI] [PubMed] [Google Scholar]
- 59.Zografos GC, Driscoll DL, Karakousis CP, et al. Adrenal adenocarcinoma: A review of 53 cases. J Surg Oncol. 1994;55:160–164. doi: 10.1002/jso.2930550306. [DOI] [PubMed] [Google Scholar]
- 60.Nelson AA, Woodard G. Severe adrenal cortical atrophy (cytotoxic) and hepatic damage produced in dogs by feeding 2,2-bis(parachlorophenyl)-1,1-dichloroethane (DDD or TDE) Arch Pathol (Chic) 1949;48:387–394. [PubMed] [Google Scholar]
- 61.Hahner S, Fassnacht M. Mitotane for adrenocortical carcinoma treatment. Curr Opin Investig Drugs. 2005;6:386–394. [PubMed] [Google Scholar]
- 62.Schteingart DE. Conventional and novel strategies in the treatment of adrenocortical cancer. Braz J Med Biol Res. 2000;33:1197–1200. doi: 10.1590/s0100-879x2000001000009. [DOI] [PubMed] [Google Scholar]
- 63.Pineiro-Sanchez M, Vaz A, Counsell R, et al. Synthesis of B3H-mitotane for use in a rapid assay for mitotane metabolism. J Labelled Comp Radiopharm. 1995;36:121–127. [Google Scholar]
- 64.Reif VD, Sinsheimer JE, Ward JC, et al. Aromatic hydroxylation and alkyl oxidation in metabolism of mitotane (o,p'-DDD) in humans. J Pharm Sci. 1974;63:1730–1736. doi: 10.1002/jps.2600631113. [DOI] [PubMed] [Google Scholar]
- 65.Schteingart DE, Sinsheimer JE, Counsell RE, et al. Comparison of the adrenalytic activity of mitotane and a methylated homolog on normal adrenal cortex and adrenal cortical carcinoma. Cancer Chemother Pharmacol. 1993;31:459–466. doi: 10.1007/BF00685036. [DOI] [PubMed] [Google Scholar]
- 66.Cai W, Counsell RE, Djanegara T, et al. Metabolic activation and binding of mitotane in adrenal cortex homogenates. J Pharm Sci. 1995;84:134–138. doi: 10.1002/jps.2600840203. [DOI] [PubMed] [Google Scholar]
- 67.van Slooten H, Moolenaar AJ, van Seters AP, et al. The treatment of adrenocortical carcinoma with o,p'-DDD: Prognostic implications of serum level monitoring. Eur J Cancer Clin Oncol. 1984;20:47–53. doi: 10.1016/0277-5379(84)90033-6. [DOI] [PubMed] [Google Scholar]
- 68.Haak HR, Hermans J, van de Velde CJ, et al. Optimal treatment of adrenocortical carcinoma with mitotane: Results in a consecutive series of 96 patients. Br J Cancer. 1994;69:947–951. doi: 10.1038/bjc.1994.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baudin E, Pellegriti G, Bonnay M, et al. Impact of monitoring plasma 1,1-dichlorodiphenildichloroethane (o,p'DDD) levels on the treatment of patients with adrenocortical carcinoma. Cancer. 2001;92:1385–1392. doi: 10.1002/1097-0142(20010915)92:6<1385::aid-cncr1461>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 70.Wängberg B, Khorram-Manesh A, Jansson S, et al. The long-term survival in adrenocortical carcinoma with active surgical management and use of monitored mitotane. Endocr Relat Cancer. 2010;17:265–272. doi: 10.1677/ERC-09-0190. [DOI] [PubMed] [Google Scholar]
- 71.Terzolo M, Pia A, Berruti A, et al. Low-dose monitored mitotane treatment achieves the therapeutic range with manageable side effects in patients with adrenocortical cancer. J Clin Endocrinol Metab. 2000;85:2234–2238. doi: 10.1210/jcem.85.6.6619. [DOI] [PubMed] [Google Scholar]
- 72.Faggiano A, Leboulleux S, Young J, et al. Rapidly progressing high o,p'DDD doses shorten the time required to reach the therapeutic threshold with an acceptable tolerance: Preliminary results. Clin Endocrinol (Oxf) 2006;64:110–113. doi: 10.1111/j.1365-2265.2005.02403.x. [DOI] [PubMed] [Google Scholar]
- 73.Berruti A, Ferrero A, Sperone P, et al. Emerging drugs for adrenocortical carcinoma. Exp Opin Emerg Drugs. 2008;13:497–509. doi: 10.1517/14728214.13.3.497. [DOI] [PubMed] [Google Scholar]
- 74.van Seters AP, Moolenaar AJ. Mitotane increases the blood levels of hormone-binding proteins. Acta Endocrinol (Copenh) 1991;124:526–533. doi: 10.1530/acta.0.1240526. [DOI] [PubMed] [Google Scholar]
- 75.Nader N, Raverot G, Emptoz-Bonneton A, et al. Mitotane has an estrogenic effect on sex hormone-binding globulin and corticosteroid-binding globulin in humans. J Clin Endocrinol Metab. 2006;91:2165–2170. doi: 10.1210/jc.2005-2157. [DOI] [PubMed] [Google Scholar]
- 76.Alexandraki KI, Kaltsas GA, le Roux CW, et al. Assessment of serum-free cortisol levels in patients with adrenocortical carcinoma treated with mitotane: A pilot study. Clin Endocrinol (Oxf) 2010;72:305–311. doi: 10.1111/j.1365-2265.2009.03631.x. [DOI] [PubMed] [Google Scholar]
- 77.Zatelli MC, Gentilin E, Daffara F, et al. Therapeutic concentrations of mitotane (o,p'-DDD) inhibit thyrotroph cell viability and TSH expression and secretion in a mouse cell line model. Endocrinology. 2010;151:2453–2461. doi: 10.1210/en.2009-1404. [DOI] [PubMed] [Google Scholar]
- 78.Terzolo M, Berruti A. Adjunctive treatment of adrenocortical carcinoma. Curr Opin Endocrinol Diabetes Obes. 2008;15:221–226. doi: 10.1097/MED.0b013e3282fdf4c0. [DOI] [PubMed] [Google Scholar]
- 79.Robinson BG, Hales IB, Henniker AJ, et al. The effect of o,p'-DDD on adrenal steroid replacement therapy requirements. Clin Endocrinol (Oxf) 1987;27:437–444. doi: 10.1111/j.1365-2265.1987.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 80.Hague RV, May W, Cullen DR. Hepatic microsomal enzyme induction and adrenal crisis due to o,p'DDD therapy for metastatic adrenocortical carcinoma. Clin Endocrinol (Oxf) 1989;31:51–57. doi: 10.1111/j.1365-2265.1989.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 81.Kasperlik-Zaluska AA. Clinical results of the use of mitotane for adrenocortical carcinoma. Braz J Med Biol Res. 2000;33:1191–1196. doi: 10.1590/s0100-879x2000001000008. [DOI] [PubMed] [Google Scholar]
- 82.Flynn SD, Murren JR, Kirby WM, et al. P-glycoprotein expression and multidrug resistance in adrenocortical carcinoma. Surgery. 1992;112:981–986. [PubMed] [Google Scholar]
- 83.Haak HR, van Seters AP, Moolenaar AJ, et al. Expression of P-glycoprotein in relation to clinical manifestation, treatment and prognosis of adrenocortical cancer. Eur J Cancer. 1993;29A:1036–1038. doi: 10.1016/s0959-8049(05)80219-9. [DOI] [PubMed] [Google Scholar]
- 84.Bates SE, Shieh CY, Mickley LA, et al. Mitotane enhances cytotoxicity of chemotherapy in cell lines expressing a multidrug resistance gene (mdr-1/P-glycoprotein) which is also expressed by adrenocortical carcinomas. J Clin Endocrinol Metab. 1991;73:18–29. doi: 10.1210/jcem-73-1-18. [DOI] [PubMed] [Google Scholar]
- 85.Feller N, Hoekman K, Kuiper CM, et al. A patient with adrenocortical carcinoma: Characterization of its biological activity and drug resistance profile. Clin Cancer Res. 1997;3:389–394. [PubMed] [Google Scholar]
- 86.Abraham J, Bakke S, Rutt A, et al. A phase II trial of combination chemotherapy and surgical resection for the treatment of metastatic adrenocortical carcinoma: Continuous infusion doxorubicin, vincristine, and etoposide with daily mitotane as a P-glycoprotein antagonist. Cancer. 2002;94:2333–2343. doi: 10.1002/cncr.10487. [DOI] [PubMed] [Google Scholar]
- 87.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 88.Ronchi CL, Sbiera S, Kraus L, et al. Expression of excision repair cross complementing group 1 and prognosis in adrenocortical carcinoma patients treated with platinum-based chemotherapy. Endocr Relat Cancer. 2009;16:907–918. doi: 10.1677/ERC-08-0224. [DOI] [PubMed] [Google Scholar]
- 89.Berruti A, Terzolo M, Pia A, et al. Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer. Cancer. 1998;83:2194–2200. [PubMed] [Google Scholar]
- 90.Khan TS, Imam H, Juhlin C, et al. Streptozocin and o,p'DDD in the treatment of adrenocortical cancer patients: Long-term survival in its adjuvant use. Ann Oncol. 2000;11:1281–1287. doi: 10.1023/a:1008377915129. [DOI] [PubMed] [Google Scholar]
- 91.Khan TS, Sundin A, Juhlin C, et al. Vincristine, cisplatin, teniposide, and cyclophosphamide combination in the treatment of recurrent or metastatic adrenocortical cancer. Med Oncol. 2004;21:167–177. doi: 10.1385/MO:21:2:167. [DOI] [PubMed] [Google Scholar]
- 92.Sperone P, Ferrero A, Daffara F, et al. Gemcitabine plus metronomic 5-fluorouracil or capecitabine as a second-/third-line chemotherapy in advanced adrenocortical carcinoma: A multicenter phase II study. Endocr Relat Cancer. 2010;17:445–453. doi: 10.1677/ERC-09-0281. [DOI] [PubMed] [Google Scholar]
- 93.Fallo F, Pilon C, Barzon L, et al. Paclitaxel is an effective antiproliferative agent on the human NCI-H295 adrenocortical carcinoma cell line. Chemotherapy. 1998;44:129–134. doi: 10.1159/000007104. [DOI] [PubMed] [Google Scholar]
- 94.Montoya M, Brown JW, Fishman LM. Comparative effects of chemotherapeutic agents on the growth and survival of human adrenal carcinoma cells in culture. Horm Metab Res. 2008;40:302–305. doi: 10.1055/s-2008-1073139. [DOI] [PubMed] [Google Scholar]
- 95.Schneider B, Fukunaga A, Murry D, et al. A phase I, pharmacokinetic and pharmacodynamic dose escalation trial of weekly paclitaxel with interferon-alpha2b in patients with solid tumors. Cancer Chemother Pharmacol. 2007;59:261–268. doi: 10.1007/s00280-006-0264-z. [DOI] [PubMed] [Google Scholar]
- 96.Suh I, Weng J, Fernandez-Ranvier G, et al. Antineoplastic effects of decitabine, an inhibitor of DNA promoter methylation, in adrenocortical carcinoma cells. Arch Surg. 2010;145:226–232. doi: 10.1001/archsurg.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schulick RD, Brennan MF. Adrenocortical carcinoma. World J Urol. 1999;17:26–34. doi: 10.1007/s003450050101. [DOI] [PubMed] [Google Scholar]
- 98.Polat B, Fassnacht M, Pfreundner L, et al. Radiotherapy in adrenocortical carcinoma. Cancer. 2009;115:2816–2823. doi: 10.1002/cncr.24331. [DOI] [PubMed] [Google Scholar]
- 99.Hermsen IG, Groenen YE, Dercksen MW, et al. Response to radiation therapy in adrenocortical carcinoma. J Endocrinol Invest. 2010;33:712–714. doi: 10.1007/BF03346675. [DOI] [PubMed] [Google Scholar]
- 100.Cerquetti L, Bucci B, Marchese R, et al. Mitotane increases the radiotherapy inhibitory effect and induces G2-arrest in combined treatment on both H295R and SW13 adrenocortical cell lines. Endocrine-Related Cancer. 2008;15:623–634. doi: 10.1677/erc.1.1315. [DOI] [PubMed] [Google Scholar]
- 101.Cerquetti L, Sampaoli C, Amendola D, et al. Mitotane sensitizes adrenocortical cancer cells to ionizing radiations by involvement of the cyclin B1/CDK complex in G2 arrest and mismatch repair enzymes modulation. Int J Oncol. 2010;37:493–501. doi: 10.3892/ijo_00000698. [DOI] [PubMed] [Google Scholar]
- 102.Khan TS, Sundin A, Juhlin C, et al. 11C-metomidate PET imaging of adrenocortical cancer. Eur J Nucl Med Mol Imaging. 2003;30:403–410. doi: 10.1007/s00259-002-1025-9. [DOI] [PubMed] [Google Scholar]
- 103.Hennings J, Lindhe O, Bergström M, et al. [11C]metomidate positron emission tomography of adrenocortical tumors in correlation with histopathological findings. J Clin Endocrinol Metab. 2006;91:1410–1414. doi: 10.1210/jc.2005-2273. [DOI] [PubMed] [Google Scholar]
- 104.Hahner S, Stuermer A, Kreissl M, et al. [123 I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. J Clin Endocrinol Metab. 2008;93:2358–2365. doi: 10.1210/jc.2008-0050. [DOI] [PubMed] [Google Scholar]
- 105.Wood BJ, Abraham J, Hvizda JL, et al. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer. 2003;97:554–560. doi: 10.1002/cncr.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pacella CM, Stasi R, Bizzarri G, et al. Percutaneous laser ablation of unresectable primary and metastatic adrenocortical carcinoma. Eur J Radiol. 2008;66:88–94. doi: 10.1016/j.ejrad.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 107.Bauditz J, Quinkler M, Wermke W. Radiofrequency thermal ablation of hepatic metastases of adrenocortical cancer—a case report and review of the literature. Exp Clin Endocrinol Diabetes. 2009;117:316–319. doi: 10.1055/s-0028-1087178. [DOI] [PubMed] [Google Scholar]
- 108.Venkatesan AM, Locklin J, Dupuy DE, et al. Percutaneous ablation of adrenal tumors. Tech Vasc Interv Radiol. 2010;13:89–99. doi: 10.1053/j.tvir.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shalet S, Mukherjee A. Pharmacological treatment of hypercortisolism. Curr Opin Endocrinol Diabetes Obes. 2008;15:234–238. doi: 10.1097/MED.0b013e3282fc7025. [DOI] [PubMed] [Google Scholar]
- 110.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23:273–289. doi: 10.1016/j.beem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 111.Pont A, Williams PL, Loose DS, et al. Ketoconazole blocks adrenal steroid synthesis. Ann Intern Med. 1982;97:370–372. doi: 10.7326/0003-4819-97-3-370. [DOI] [PubMed] [Google Scholar]
- 112.Kowal J. The effect of ketoconazole on steroidogenesis in cultured mouse adrenal cortex tumor cells. Endocrinology. 1983;112:1541–1543. doi: 10.1210/endo-112-4-1541. [DOI] [PubMed] [Google Scholar]
- 113.Loose DS, Kan PB, Hirst MA, et al. Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes. J Clin Invest. 1983;71:1495–1499. doi: 10.1172/JCI110903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van Tyle JH. Ketoconazole. Mechanism of action, spectrum of activity, pharmacokinetics, drug interactions, adverse reactions and therapeutic use. Pharmacotherapy. 1984;4:343–373. doi: 10.1002/j.1875-9114.1984.tb03398.x. [DOI] [PubMed] [Google Scholar]
- 115.Loose DS, Stover EP, Feldman D. Ketoconazole binds to glucocorticoid receptors and exhibits glucocorticoid antagonist activity in cultured cells. J Clin Invest. 1983;72:404–408. doi: 10.1172/JCI110982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Verhelst JA, Trainer PJ, Howlett TA, et al. Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing's syndrome. Clin Endocrinol (Oxf) 1991;35:169–178. doi: 10.1111/j.1365-2265.1991.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 117.Misbin RI, Canary J, Willard D. Aminoglutethimide in the treatment of Cushing's syndrome. J Clin Pharmacol. 1976;16:645–651. doi: 10.1002/j.1552-4604.1976.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 118.Fassnacht M, Hahner S, Beuschlein F, et al. New mechanisms of adrenostatic compounds in a human adrenocortical cancer cell line. Eur J Clin Invest. 2000;30(suppl 3):76–82. doi: 10.1046/j.1365-2362.2000.0300s3076.x. [DOI] [PubMed] [Google Scholar]
- 119.Wagner RL, White PF, Kan PB, et al. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415–1421. doi: 10.1056/NEJM198405313102202. [DOI] [PubMed] [Google Scholar]
- 120.Kirschner LS. Emerging treatment strategies for adrenocortical carcinoma: A new hope. J Clin Endocrinol Metab. 2006;91:14–21. doi: 10.1210/jc.2005-1739. [DOI] [PubMed] [Google Scholar]
- 121.Gicquel C, Bertagna X, Schneid H, et al. Rearrangements at the 11p15 locus and overexpression of insulin-like growth factor-II gene in sporadic adrenocortical tumors. J Clin Endocrinol Metab. 1994;78:1444–1453. doi: 10.1210/jcem.78.6.7911125. [DOI] [PubMed] [Google Scholar]
- 122.Gicquel C, Bertagna X, Gaston V, et al. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res. 2001;61:6762–6767. [PubMed] [Google Scholar]
- 123.Schmitt A, Saremaslani P, Schmid S, et al. IGFII and MIB1 immunohistochemistry is helpful for the differentiation of benign from malignant adrenocortical tumours. Histopathology. 2006;49:298–307. doi: 10.1111/j.1365-2559.2006.02505.x. [DOI] [PubMed] [Google Scholar]
- 124.Almeida MQ, Fragoso MC, Lotfi CF, et al. Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab. 2008;93:3524–3531. doi: 10.1210/jc.2008-0065. [DOI] [PubMed] [Google Scholar]
- 125.Weber MM, Fottner C, Wolf E. The role of the insulin-like growth factor system in adrenocortical tumourigenesis. Eur J Clin Invest. 2000;30(suppl 3):69–75. doi: 10.1046/j.1365-2362.2000.0300s3069.x. [DOI] [PubMed] [Google Scholar]
- 126.Tao Y, Pinzi V, Bourhis J, et al. Mechanisms of disease: Signaling of the insulin-like growth factor 1 receptor pathway—therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4:591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
- 127.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: Early clinical trial results and future directions. Oncogene. 2009;28:3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 128.Logié A, Boulle N, Gaston V, et al. Autocrine role of IGF-II in proliferation of human adrenocortical carcinoma NCI H295R cell line. J Mol Endocrinol. 1999;23:23–32. doi: 10.1677/jme.0.0230023. [DOI] [PubMed] [Google Scholar]
- 129.Barlaskar FM, Spalding AC, Heaton JH, et al. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab. 2009;94:204–212. doi: 10.1210/jc.2008-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Haluska P, Worden F, Olmos D, et al. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol. 2010;65:765–773. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carden CP, Frentzas S, Langham M, et al. Preliminary activity in adrenocortical tumor (ACC) in phase I dose escalation study of intermittent oral dosing of OSI-906, a small-molecule insulin-like growth factor-1 receptor (IGF-1R) tyrosine kinase inhibitor in patients with advanced solid tumors. J Clin Oncol. 2009;27(15 suppl):3544. [Google Scholar]
- 132.Kamio T, Shigematsu K, Sou H, et al. Immunohistochemical expression of epidermal growth factor receptors in human adrenocortical carcinoma. Hum Pathol. 1990;21:277–282. doi: 10.1016/0046-8177(90)90227-v. [DOI] [PubMed] [Google Scholar]
- 133.Sasano H, Suzuki T, Shizawa S, et al. Transforming growth factor alpha, epidermal growth factor, and epidermal growth factor receptor expression in normal and diseased human adrenal cortex by immunohistochemistry and in situ hybridization. Mod Pathol. 1994;7:741–746. [PubMed] [Google Scholar]
- 134.Edgren M, Eriksson B, Wilander E, et al. Biological characteristics of adrenocortical carcinoma: A study of p53, IGF, EGF-r, Ki-67 and PCNA in 17 adrenocortical carcinomas. Anticancer Res. 1997;17:1303–1309. [PubMed] [Google Scholar]
- 135.Nakamura M, Miki Y, Akahira J, et al. An analysis of potential surrogate markers of target-specific therapy in archival materials of adrenocortical carcinoma. Endocr Pathol. 2009;20:17–23. doi: 10.1007/s12022-009-9058-2. [DOI] [PubMed] [Google Scholar]
- 136.Samnotra V, Vassilopoulou-Sellin R, Fojo AT, et al. A phase II trial of gefitinib monotherapy in patients with unresectable adrenocortical carcinoma (ACC) J Clin Oncol. 2007;25(18 suppl):15527. [Google Scholar]
- 137.Quinkler M, Hahner S, Wortmann S, et al. Treatment of advanced adrenocortical carcinoma with erlotinib plus gemcitabine. J Clin Endocrinol Metab. 2008;93:2057–2062. doi: 10.1210/jc.2007-2564. [DOI] [PubMed] [Google Scholar]
- 138.Eichholz A, Merchant S, Gaya AM. Anti-angiogenesis therapies: Their potential in cancer management. Onco Targets Ther. 2010;3:69–82. doi: 10.2147/ott.s5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Fraipont F, El Atifi M, Gicquel C, et al. Expression of the angiogenesis markers vascular endothelial growth factor-A, thrombospondin-1, and platelet-derived endothelial cell growth factor in human sporadic adrenocortical tumors: Correlation with genotypic alterations. J Clin Endocrinol Metab. 2000;85:4734–4741. doi: 10.1210/jcem.85.12.7012. [DOI] [PubMed] [Google Scholar]
- 140.Bernini GP, Moretti A, Bonadio AG, et al. Angiogenesis in human normal and pathologic adrenal cortex. J Clin Endocrinol Metab. 2002;87:4961–4965. doi: 10.1210/jc.2001-011799. [DOI] [PubMed] [Google Scholar]
- 141.Zacharieva S, Atanassova I, Orbetzova M, et al. Circulating vascular endothelial growth factor and active renin concentrations and prostaglandin E2 urinary excretion in patients with adrenal tumours. Eur J Endocrinol. 2004;150:345–349. doi: 10.1530/eje.0.1500345. [DOI] [PubMed] [Google Scholar]
- 142.Britvin TA, Kazantseva IA, Kalinin AP, et al. Vascular endothelium growth factor in the sera of patients with adrenal tumors. Bull Exp Biol Med. 2005;140:228–230. doi: 10.1007/s10517-005-0452-6. [DOI] [PubMed] [Google Scholar]
- 143.Kolomecki K, Stepien H, Narebski JM. Vascular endothelial growth factor and basic fibroblast growth factor evaluation in blood serum of patients with hormonally active and inactive adrenal gland tumours. Cytobios. 2000;101:55–64. [PubMed] [Google Scholar]
- 144.Kolomecki K, Stepien H, Bartos M, et al. Usefulness of VEGF, MMP-2, MMP-3 and TIMP-2 serum level evaluation in patients with adrenal tumours. Endocr Regul. 2001;35:9–16. [PubMed] [Google Scholar]
- 145.Wortmann S, Quinkler M, Ritter C, et al. Bevacizumab plus capecitabine as a salvage therapy in advanced adrenocortical carcinoma. Eur J Endocrinol. 2010;162:349–356. doi: 10.1530/EJE-09-0804. [DOI] [PubMed] [Google Scholar]
- 146.Chacón R, Tossen G, Loria FS, et al. CASE 2. Response in a patient with metastatic adrenal cortical carcinoma with thalidomide. J Clin Oncol. 2005;23:1579–1580. doi: 10.1200/JCO.2005.03.195. [DOI] [PubMed] [Google Scholar]
- 147.Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat Rev. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hong DS, Sebti SM, Newman RA, et al. Phase I trial of a combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in advanced malignancies. Clin Cancer Res. 2009;15:7061–7068. doi: 10.1158/1078-0432.CCR-09-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Butler C, Butler WM, Rizvi AA. Sustained remission with the kinase inhibitor sorafenib in stage IV metastatic adrenocortical carcinoma. Endocr Pract. 2010;16:441–445. doi: 10.4158/EP09295.CR. [DOI] [PubMed] [Google Scholar]
- 150.Lee JO, Lee KW, Kim CJ, et al. Metastatic adrenocortical carcinoma treated with sunitinib: A case report. Jpn J Clin Oncol. 2009;39:183–185. doi: 10.1093/jjco/hyn146. [DOI] [PubMed] [Google Scholar]
- 151.Gross DJ, Munter G, Bitan M, et al. The role of imatinib mesylate (Glivec) for treatment of patients with malignant endocrine tumors positive for c-kit or PDGF-R. Endocr Relat Cancer. 2006;13:535–540. doi: 10.1677/erc.1.01124. [DOI] [PubMed] [Google Scholar]
- 152.Del Bufalo D, Ciuffreda L, Trisciuoglio D, et al. Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006;66:5549–5554. doi: 10.1158/0008-5472.CAN-05-2825. [DOI] [PubMed] [Google Scholar]
- 153.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 154.Humar R, Kiefer FN, Berns H, et al. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J. 2002;16:771–780. doi: 10.1096/fj.01-0658com. [DOI] [PubMed] [Google Scholar]
- 155.Bruns CJ, Koehl GE, Guba M, et al. Rapamycin-induced endothelial cell death and tumor vessel thrombosis potentiate cytotoxic therapy against pancreatic cancer. Clin Cancer Res. 2004;10:2109–2119. doi: 10.1158/1078-0432.ccr-03-0502. [DOI] [PubMed] [Google Scholar]
- 156.Doghman M, El Wakil A, Cardinaud B, et al. Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res. 2010;70:4666–4675. doi: 10.1158/0008-5472.CAN-09-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Takara K, Sakaeda T, Okumura K. An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr Pharm Des. 2006;12:273–286. doi: 10.2174/138161206775201965. [DOI] [PubMed] [Google Scholar]
- 158.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 159.Fridborg H, Larsson R, Juhlin C, et al. P-glycoprotein expression and activity of resistance modifying agents in primary cultures of human renal and adrenocortical carcinoma cells. Anticancer Res. 1994;14:1009–1016. [PubMed] [Google Scholar]
- 160.Bates S, Kang M, Meadows B, et al. A phase I study of infusional vinblastine in combination with the P-glycoprotein antagonist PSC 833 (valspodar) Cancer. 2001;92:1577–1590. doi: 10.1002/1097-0142(20010915)92:6<1577::aid-cncr1484>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 161.Bates SE, Bakke S, Kang M, et al. A phase I/II study of infusional vinblastine with the P-glycoprotein antagonist valspodar (PSC 833) in renal cell carcinoma. Clin Cancer Res. 2004;10:4724–4733. doi: 10.1158/1078-0432.CCR-0829-03. [DOI] [PubMed] [Google Scholar]
- 162.Carlson RW, O'Neill AM, Goldstein LJ, et al. A pilot phase II trial of valspodar modulation of multidrug resistance to paclitaxel in the treatment of metastatic carcinoma of the breast (E1195): A trial of the Eastern Cooperative Oncology Group. Cancer Invest. 2006;24:677–681. doi: 10.1080/07357900600981349. [DOI] [PubMed] [Google Scholar]
- 163.Fox E, Bates SE. Tariquidar (XR9576): A P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther. 2007;7:447–459. doi: 10.1586/14737140.7.4.447. [DOI] [PubMed] [Google Scholar]
- 164.Kuruba R, Gallagher SF. Current management of adrenal tumors. Curr Opin Oncol. 2008;20:34–46. doi: 10.1097/CCO.0b013e3282f301fd. [DOI] [PubMed] [Google Scholar]
- 165.Ferruzzi P, Ceni E, Tarocchi M, et al. Thiazolidinediones inhibit growth and invasiveness of the human adrenocortical cancer cell line H295R. J Clin Endocrinol Metab. 2005;90:1332–1339. doi: 10.1210/jc.2004-0978. [DOI] [PubMed] [Google Scholar]
- 166.Betz MJ, Shapiro I, Fassnacht M, et al. Peroxisome proliferator-activated receptor-gamma agonists suppress adrenocortical tumor cell proliferation and induce differentiation. J Clin Endocrinol Metab. 2005;90:3886–3896. doi: 10.1210/jc.2004-1267. [DOI] [PubMed] [Google Scholar]
- 167.Cantini G, Lombardi A, Piscitelli E, et al. Rosiglitazone inhibits adrenocortical cancer cell proliferation by interfering with the IGF-IR intracellular signaling. PPAR Res. 2008;2008:904041. doi: 10.1155/2008/904041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Luconi M, Mangoni M, Gelmini S, et al. Rosiglitazone impairs proliferation of human adrenocortical cancer: Preclinical study in a xenograft mouse model. Endocr Relat Cancer. 2010;17:169–177. doi: 10.1677/ERC-09-0170. [DOI] [PubMed] [Google Scholar]
- 169.Ondrey F. Peroxisome proliferator-activated receptor gamma pathway targeting in carcinogenesis: Implications for chemoprevention. Clin Cancer Res. 2009;15:2–8. doi: 10.1158/1078-0432.CCR-08-0326. [DOI] [PubMed] [Google Scholar]
- 170.Tissier F, Cavard C, Groussin L, et al. Mutations of beta-catenin in adrenocortical tumors: Activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65:7622–7627. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 171.Gaujoux S, Tissier F, Groussin L, et al. Wnt/beta-catenin and 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A signaling pathways alterations and somatic beta-catenin gene mutations in the progression of adrenocortical tumors. J Clin Endocrinol Metab. 2008;93:4135–4140. doi: 10.1210/jc.2008-0631. [DOI] [PubMed] [Google Scholar]
- 172.Tadjine M, Lampron A, Ouadi L, et al. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol (Oxf) 2008;68:264–270. doi: 10.1111/j.1365-2265.2007.03033.x. [DOI] [PubMed] [Google Scholar]
- 173.Berthon A, Sahut-Barnola I, Lambert-Langlais S, et al. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010;19:1561–1576. doi: 10.1093/hmg/ddq029. [DOI] [PubMed] [Google Scholar]
- 174.Doghman M, Cazareth J, Lalli E. The T cell factor/beta-catenin antagonist PKF115–584 inhibits proliferation of adrenocortical carcinoma cells. J Clin Endocrinol Metab. 2008;93:3222–3225. doi: 10.1210/jc.2008-0247. [DOI] [PubMed] [Google Scholar]
- 175.Schimmer BP, White PC. Minireview: Steroidogenic factor 1: Its roles in differentiation, development, and disease. Mol Endocrinol. 2010;24:1322–1337. doi: 10.1210/me.2009-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Figueiredo BC, Cavalli LR, Pianovski MA, et al. Amplification of the steroidogenic factor 1 gene in childhood adrenocortical tumors. J Clin Endocrinol Metab. 2005;90:615–619. doi: 10.1210/jc.2004-0942. [DOI] [PubMed] [Google Scholar]
- 177.Pianovski MA, Cavalli LR, Figueiredo BC, et al. SF-1 overexpression in childhood adrenocortical tumours. Eur J Cancer. 2006;42:1040–1043. doi: 10.1016/j.ejca.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 178.Doghman M, Karpova T, Rodrigues GA, et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007;21:2968–2987. doi: 10.1210/me.2007-0120. [DOI] [PubMed] [Google Scholar]
- 179.Doghman M, Cazareth J, Douguet D, et al. Inhibition of adrenocortical carcinoma cell proliferation by steroidogenic factor-1 inverse agonists. J Clin Endocrinol Metab. 2009;94:2178–2183. doi: 10.1210/jc.2008-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]