A better identification of patients who are more likely to benefit from vascular endothelial growth factor–targeted therapy is warranted in metastatic renal cell carcinoma. High VFA could be a predictive biomarker from shorter survival in patients given first-line antiangiogenic agents for metastatic renal cell carcinoma.
Keywords: Antiangiogenic agents, Renal cell carcinoma, Visceral fat
Abstract
Purpose.
A better identification of patients who are more likely to benefit from vascular endothelial growth factor–targeted therapy is warranted in metastatic renal cell carcinoma (mRCC). As adipose tissue releases angiogenic factors, we determined whether parameters such as visceral fat area (VFA) were associated with outcome in these patients.
Experimental Design.
In 113 patients with mRCC who received antiangiogenic agents (bevacizumab, sunitinib, or sorafenib) (n = 64) or cytokines (n = 49) as first-line treatment, we used computed tomography to measure VFA and subcutaneous fat area (SFA). We evaluated associations linking body mass index (BMI), SFA, and VFA to time to progression (TTP) and overall survival (OS).
Results.
High SFA and VFA values were significantly associated with shorter TTP and OS. By multivariate analysis, high VFA was independently associated with shorter TTP and OS. These results were internally validated using bootstrap analysis. By contrast, VFA was not associated with survival in the cytokine group. In the whole population, interaction between VFA and treatment group was significant for TTP and OS, thereby confirming the results.
Conclusion.
Our study provides the first evidence that high VFA could be a predictive biomarker from shorter survival in patients given first-line antiangiogenic agents for mRCC.
Introduction
Renal cell carcinoma (RCC) is diagnosed in >120,000 patients in Europe and the United States every year and causes approximately 60,000 deaths annually. Approximately one third of patients with initially localized disease relapse distantly after nephrectomy, whereas 30% of patients present with metastatic disease at diagnosis [1]. Because RCC is highly resistant to chemotherapy, immunotherapy such as interferon α (INF-α) combined or not with interleukin-2 (IL-2) was previously the sole treatment available.
Recent advances in the understanding of the molecular pathogenesis of clear-cell RCC have highlighted the importance of the overexpression of growth factors such as vascular endothelial growth factor (VEGF), resulting in tumor progression and tumor neoangiogenesis [2]. Consequently, VEGF inhibition has become an attractive therapeutic target in patients with metastatic RCC (mRCC), and pharmacological agents targeting the VEGF, or VEGF receptor signaling pathway, such as sunitinib, sorafenib, or bevacizumab have revolutionized the treatment of mRCC and have displaced immunotherapy as first-line standard of care [3–6]. The introduction of these novel agents targeting the VEGF pathway makes understanding and identifying new clinical, biological, and molecular features affecting outcome an important consideration for clinical trial design and evaluation of new treatments for mRCC. Many authors have identified factors associated with outcome in patients with mRCC treated with cytokines. The most widely used prognostic factor model is from the Memorial Sloan-Kettering Cancer Center (MSKCC) [7], and groups patients into categories (favorable, intermediate, and poor), according to the number of risk factors predictive of a short survival. Nonetheless, whether the same prognostic factors are still relevant for patients treated with VEGF-targeted therapy remains unclear.
Obesity is a risk factor for the development of several types of cancer [8, 9], including renal cell carcinoma [10, 11], and a risk factor of worsened outcome for many cancer types [12]. Explanations are incompletely understood, but may involve the production by adipose tissue of adipokines and proangiogenic cytokines such as VEGF [13, 14] that may promote cancer growth, and dysregulated angiogenesis. Although VEGF serum levels have been previously correlated with shorter survival in localized or mRCC [15, 16], to date, there is no current predictive biomarker for the efficacy of VEGF-targeted therapy in terms of survival improvement. We recently reported that high visceral fat area (VFA) measured by computed tomography independently predicts a poorer outcome in patients given first-line bevacizumab-based therapy for metastatic colon cancer [17]. Whether VFA at treatment initiation predicts outcomes in patients with mRCC has not been investigated. Therefore, we designed this retrospective study to assess whether VFA, and other factors related to obesity, could predict outcome of patients given either VEGF-targeted, or cytokine-based therapy, as first-line treatment for mRCC.
Patients and Methods
Patient Population
On the basis of a retrospective cohort study, 113 consecutive patients with mRCC treated with first-line cytokines or VEGF-targeted therapy (sunitinib, sorafenib, or bevacizumab) between June 2001 and June 2009 at the Georges François Leclerc Center (Dijon, France) were included in this study. Only patients with histologically proven metastatic clear cell RCC were included. Patients received either immunotherapy (INF-α alone, or with IL-2) (cytokine group) or VEGF-targeted therapy (antiangiogenic group). Patients initially treated with temsirolimus, or an investigational agent, were excluded. Baseline demographic, clinical, and laboratory data including those previously found to have prognostic value [18–21] were collected retrospectively in all patients using uniform database templates to ensure consistent data collection. Laboratory values were standardized against institutional upper limit of normal (ULN) and lower limit of normal (LLN) values when appropriate. Outcome data on time to progression (TTP) and overall survival (OS) were collected from patient charts. This study received institutional review board approval from our center and all patients gave written informed consent.
Measurement of Visceral and Subcutaneous Fat
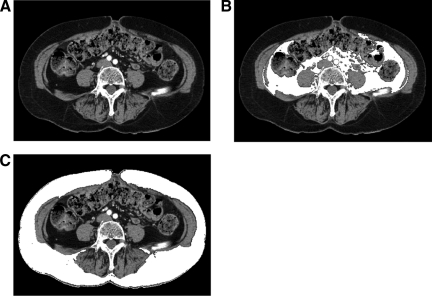
VFA and subcutaneous fat area (SFA) were measured retrospectively on the available computed tomography scans performed before treatment initiation, as previously described [22], at the level of the umbilicus with the patient in the supine position. Briefly, we used ImageJ software (http://rsb.info.nih.gov/ij/) to measure pixels with densities in the −190 Hounsfield units (HU) to −30 HU range to delineate the subcutaneous and visceral compartments and to compute the cross-sectional area of each in cm2 (Fig. 1). These measurements were performed by a radiologist blinded to patient information and treatment.
Figure 1.
Measurement of visceral and subcutaneous fat area. (A) Computed tomography, axial section through the umbilicus in a 72 year old woman. (B) Visceral fat area (VFA) (in white) was 7208 mm2 (lower than the median in the overall population). (C) Subcutaneous fat area (SFA) (in white) was 21,541 mm2 (higher than the median in the overall population).
Statistical Analysis
The primary aim of our study was to demonstrate the predictive value of body mass index (BMI), SFA, and VFA for TTP and OS in patients treated with VEGF-targeted therapy. Given the absence of normative data on VFA or SFA in the literature, SFA and VFA were dichotomized using the median of observed distribution as the cutoff. For BMI, patients were categorized into underweight/normal weight (BMI <25 kg/m2), overweight (BMI 25–30 kg/m2), or obese (BMI >30 kg/m2). Analyses were first performed in all included patients whatever treatment received. Interactions between VEGF-targeted therapy administration versus cytokines, and respectively BMI, SFA, and VFA were tested in the whole population. In the case of significant interaction, we considered subgroup analyses to confirm the results. Analyses were then repeated among patients treated with VEGF-targeted therapy and with cytokines to check if in these subgroups BMI, SFA, and VFA were associated or not with TTP or OS. OS was defined as the time from the first day of treatment to death (all causes). Survivors were censored at the last follow-up. TTP was defined as the time from the first day of treatment to the first recorded evidence of progression. Alive patients without progression were censored at last follow-up. Median follow-up with its 95% confidence interval (CI) was calculated using the reverse Kaplan-Meier method. Survival curves were estimated using the Kaplan-Meier method and compared using log-rank tests. Univariate Cox proportional-hazards models of all potential baseline predictors including the MSKCC prognostic model (interval from diagnosis to treatment from <1 year, Karnofsky performance status <80%, serum LDH >1.5 times the ULN, hemoglobin less than the LLN, and corrected serum calcium greater than the ULN) were built to compute the hazard ratios (HRs) with their 95% CIs. Multivariate Cox models for TTP and OS were constructed including VFA or SFA or BMI, or interaction between one of these variables with treatment. According to Harrell rules, we have limited the included variables for constructing multivariate models to 1 variable for 10 events both in whole population and in subgroup analyses [23]. We computed the Akaike information criterion for goodness of fit of multivariate models and Harrell's C statistic for discrimination (a Harrell's C index equal to 0.5 indicates no predictive discrimination and a Harrell's C index equal to 1.0 indicates perfect separation of patients) of each variable and for final multivariate Cox models. For multivariate analyses, to prevent multicollinearity, we retained only one interaction with BMI, or SFA or VFA, according to the better Harrell's C index. The multivariate models were internally validated using bootstrapping (150 replications). All analyses were performed using Stata V11 software (StataCorp LP, College Station, TX). p-values were two-tailed and considered significant when <.05.
Results
Patient Characteristics and Clinical Outcomes
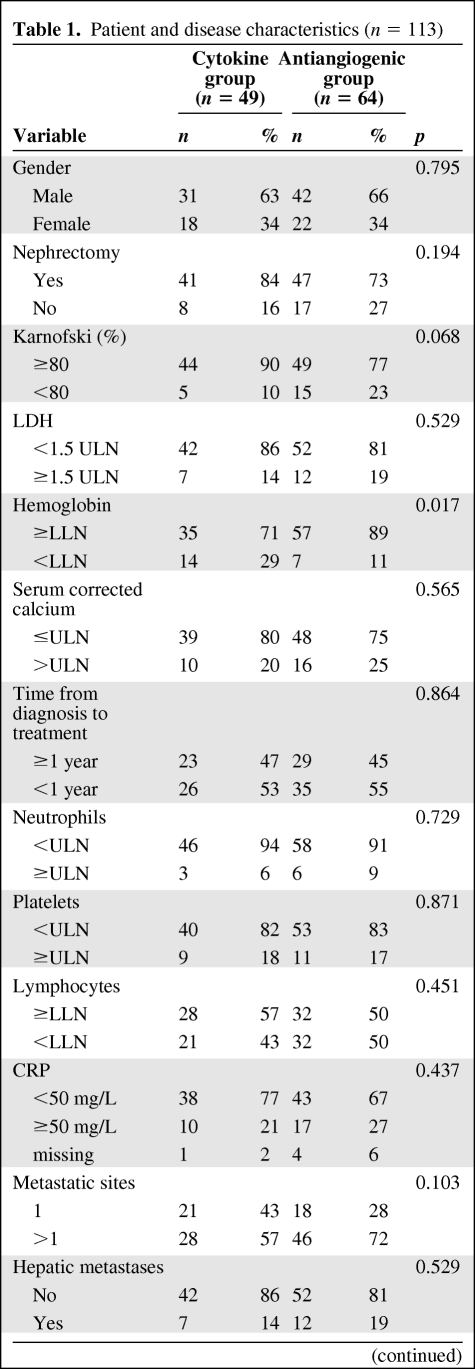
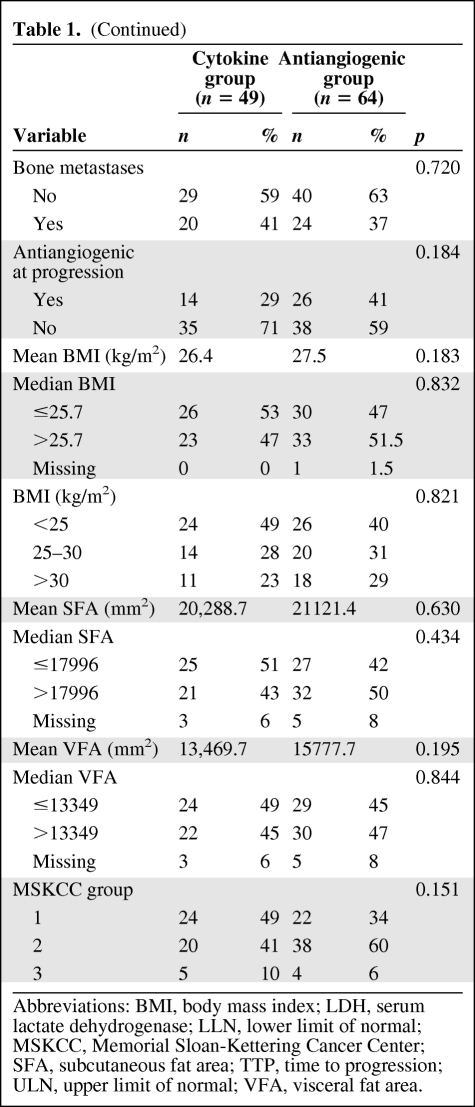
Baseline characteristics of the 113 patients are listed in Table 1 according to the treatment group (cytokine or antiangiogenic). There was no difference between the two groups, except for hemoglobin less than the LLN, which was more frequent in patients treated with cytokines. Proportions between men and women were not different between the two groups. No significant difference between fat parameter distribution between men and women was noted. Mean and median BMI, SFA, or VFA values did not differ significantly between the two groups of treatment. BMI was poorly correlated with VFA (R2 = 0.58). Within the cytokine group, 7 (14%) patients were treated with the combination of IL-2 and INF-α and 42 (86%) received INF-α alone. Within the antiangiogenic group, 10 patients (15%) received sorafenib, whereas 54 patients received either sunitinib (n = 46; 72%) or bevacizumab (n = 8; 13%) as first-line therapy. The median follow-up time after treatment initiation was 35.7 months (95% CI [24.8–39.1]); 35.4% of patients received additional therapy after disease progression (28.6% in the cytokine group and 40.6% in the antiangiogenic group).
Table 1.
Patient and disease characteristics (n = 113)
Table 1.
(Continued)
Abbreviations: BMI, body mass index; LDH, serum lactate dehydrogenase; LLN, lower limit of normal; MSKCC, Memorial Sloan-Kettering Cancer Center; SFA, subcutaneous fat area; TTP, time to progression; ULN, upper limit of normal; VFA, visceral fat area.
Whole Population Analysis
Time to Progression
At data cutoff, 86 patients had experienced progression. The median TTP was 4.4 months in the cytokine group (95% CI [2.9–5.8]) and 10.2 months (95% CI [6.3–18.3]) in the antiangiogenic group (HR: 0.44, 95% CI [0.29–0.68], log-rank p < .0001). By univariate Cox regression, factors predicting shorter TTP were LDH >1.5 ULN, hemoglobin less than the LLN, treatment with cytokines, MSKCC group, and high BMI and VFA values (supplemental online Table 1). Interaction tests revealed significant interaction between BMI, SFA, VFA, and treatment group (p = .0005, p = .0002, and p = .0002, respectively). The multivariate Cox regression model revealed that only the MSKCC group was an independent prognostic factor of shorter TTP (supplemental online Table 1). Interaction between VFA and treatment showed that as compared with patients treated with cytokines with VFA ≤ 13,349 hazard ratio for TTP was 0.25 (95% CI [0.12–0.49]) for patients treated with antiangiogenics with VFA ≤13,349 independently of the MSKCC group. Results were internally validated by bootstrapping (supplemental online Table 1).
Overall Survival
At data cutoff, 70 patients had died. The median OS was 15.4 months (95% CI [7.1–23.0]) in the cytokine group and 23.1 (95% CI [12.5–26.5]) months in the antiangiogenic group (HR: 0.81, 95% CI [0.50–1.32], log-rank p = .401). By univariate analysis, factors associated with shorter OS were absence of nephrectomy, Karnofsky index (IK) <80, LDH >1.5 ULN, hemoglobin <LLN, corrected calcemia >ULN, number of metastatic sites >1, CRP >50 mg/L, platelets >ULN, absence of VEGF-targeted therapy at disease progression, and MSKCC group (supplemental online Table 2). Interestingly, interaction tests between treatment groups and respectively BMI, SFA, and VFA highlighted significant interaction only for VFA (p = .0113). Multivariate analysis for OS revealed that MSKCC group, absence of VEGF-targeted therapy at disease progression, and number of metastatic sites >1 were independent prognostic factors of shorter OS (supplemental online Table 2). Interaction between VFA and treatment showed that as compared with patients treated with cytokines with VFA ≤13,349 hazard ratio for OS was 0.25 (95% CI [0.11–0.60]) for patients treated with antiangiogenics with VFA ≤13,349 independent of other prognostic variables. Results were internally validated by bootstrapping.
Analysis According to Treatment Group
Time to Progression
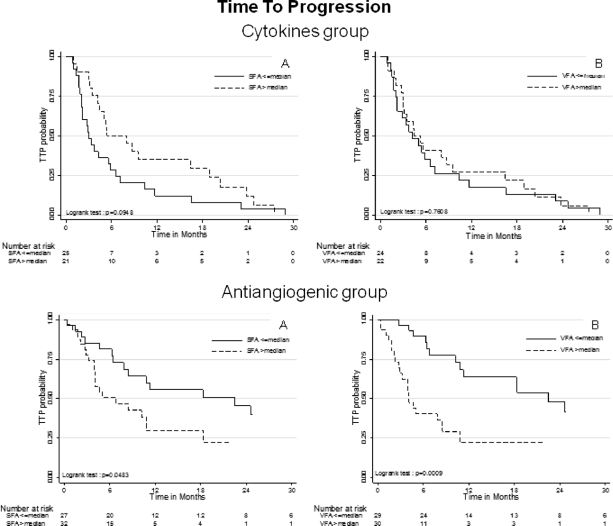
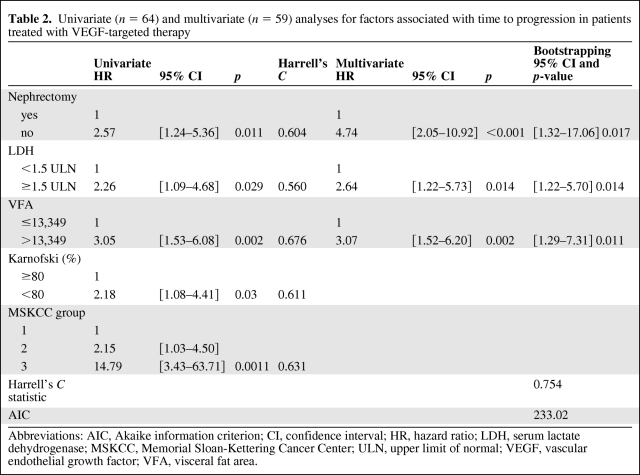
In patients treated with VEGF-targeted therapy, TTP was significantly shorter in patients with high SFA and VFA values than in patients with low values (log-rank tests p = .048 and p = .0009, respectively) (Fig. 2). Factors that predicted shorter TTP by univariate Cox regression were high VFA, absence of nephrectomy, IK <80, LDH >1.5 ULN, and MSKCC group (Table 2). Using multivariate Cox regression model, absence of nephrectomy (HR: 3.21 [1.51–6.83]), LDH >1.5 ULN (HR: 2.64 [1.22–5.73]), and high VFA (HR: 3.22 [1.60–6.50]) were independently associated with shorter TTP. Harrell's C statistic was equal to 0.754, indicating good discriminant capability by the multivariate model for predicting TTP. Moreover, univariate analysis of VFA showed that Harrell's C statistic was equal to 0.676, suggesting that high VFA was the main discriminant parameter for predicting TTP compared with other variables for which univariate Harrell's C statistic ranged from 0.56 to 0.63 (Table 2). The stability of the multivariate Cox model was assessed by bootstrapping, performed using 150 replications generated from the original sample, and confirmed that high VFA independently predicted shorter TTP (p = .013). By contrast, in patients treated with cytokines, neither SFA nor VFA were significantly associated with TTP (Fig. 2). Only LDH >1.5 ULN was associated with shorter TTP in this population (univariate HR: 2.80, 95% CI [1.16–6.76]).
Figure 2.
For each of the two groups—cytokine group (top) and antiangiogenic group (bottom)—TTP was compared according to SFA (left) and VFA (right) dichotomized to the median (Kaplan-Meier estimates). Abbreviations: SFA, subcutaneous fat area; TTP, time to progression; VFA, visceral fat area.
Table 2.
Univariate (n = 64) and multivariate (n = 59) analyses for factors associated with time to progression in patients treated with VEGF-targeted therapy
Abbreviations: AIC, Akaike information criterion; CI, confidence interval; HR, hazard ratio; LDH, serum lactate dehydrogenase; MSKCC, Memorial Sloan-Kettering Cancer Center; ULN, upper limit of normal; VEGF, vascular endothelial growth factor; VFA, visceral fat area.
Overall Survival
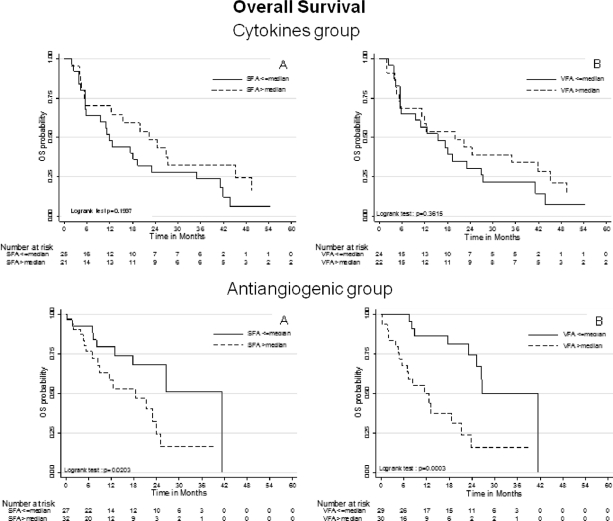
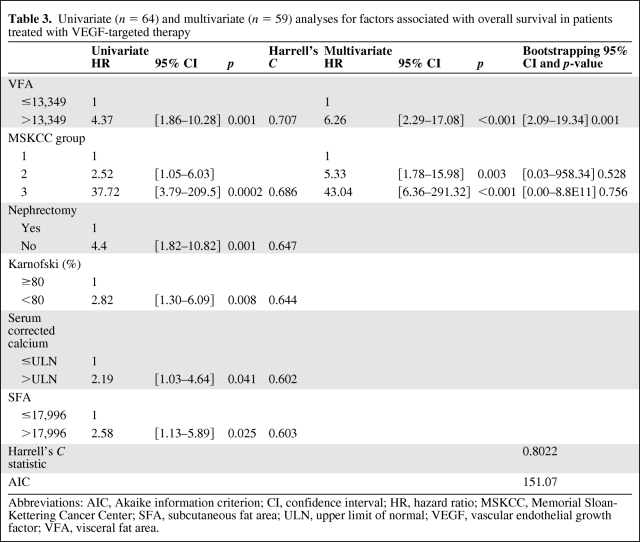
In patients treated with VEGF-targeted therapy, OS was significantly shorter in patients with high SFA and VFA values than in patients with low values (log-rank tests p = .0203 and p = .0003, respectively) (Fig. 3). Factors significantly associated with shorter OS by univariate Cox regression were high SFA, high VFA, absence of nephrectomy, IK <80, corrected calcemia >ULN, and MSKCC group (Table 3). In these patients, only high VFA (HR: 6.26 [2.29–17.08]) and MSKCC group (group 2: HR: 5.33 [1.78–15.98]; group 3: HR: 43.04 [6.36–291.32]) were independently associated with shorter OS by multivariate Cox regression (Table 3). Harrell's C statistic was equal to 0.802, indicating good discriminant capability of the multivariate model for predicting OS. Moreover, univariate analysis of VFA showed that Harrell's C statistic was equal to 0.707, suggesting that high VFA was the main discriminant parameter for predicting OS as compared with other variables for which univariate Harrell's C statistic ranged from 0.60 to 0.68 (Table 3). Bootstrapping also confirms the stability of the multivariate Cox model, and that only high VFA independently predicted shorter OS (p = .001). Thus, we also confirmed for OS that VFA constituted a predictive factor, and not a prognostic factor, because in patients treated with cytokines, OS differed neither in patients with high SFA nor in patients with high VFA values versus patients with low values (Fig. 3). Factors predicting shorter OS in patients treated with cytokines were reported in supplemental online Table 3.
Figure 3.
For each of the two groups—cytokine group (top) and antiangiogenic group (bottom)—OS was compared according to SFA (left) and VFA (right) dichotomized to the median (Kaplan-Meier estimates). Abbreviations: OS, overall survival; SFA, subcutaneous fat area; VFA, visceral fat area.
Table 3.
Univariate (n = 64) and multivariate (n = 59) analyses for factors associated with overall survival in patients treated with VEGF-targeted therapy
Abbreviations: AIC, Akaike information criterion; CI, confidence interval; HR, hazard ratio; MSKCC, Memorial Sloan-Kettering Cancer Center; SFA, subcutaneous fat area; ULN, upper limit of normal; VEGF, vascular endothelial growth factor; VFA, visceral fat area.
Discussion
VEGF-targeted therapies have recently changed the therapeutic landscape in mRCC, offering an opportunity to better individualize patient treatment. Because of multiple treatment options, improving the accuracy of current prognostic models is important to better stratify patients in clinical trials and for making relevant individualized treatment recommendations in mRCC. For patients treated with cytokines, an initial prognostic model was developed at MSKCC [7]. Recently, a prognostic model proposed by Heng et al. validates four components of the MSKCC model, with the addition of platelet and neutrophil counts [24] for patients treated with VEGF-targeted therapy. However, to date, there is no current biomarker predictive for the efficacy of VEGF-targeted therapy in terms of survival improvement for patients with mRCC.
Obesity is a well-established risk factor for developing several types of cancer [8, 9, 12], including RCC [10, 11, 25]. Mortality rates from RCC increase with increasing body mass in a prospective study conducted by the American Cancer Society [26]. Reasons for these possibly worsened outcomes remain unclear, but may involve the production by adipose tissue of adipokines that may promote cancer growth, and dysregulated angiogenesis [13, 14, 27]. Indeed, adipose tissue should be considered as an endocrine and paracrine organ that releases cytokine-like polypeptides responsible for widespread biological effects [28]. In particular, adipocytes produce insulin-like growth factors, which are known to have cancer-promoting effects on renal cells [29, 30] and multiple angiogenic factors including VEGF and leptin [27]. Leptin exerts direct angiogenic effects [27, 28] and upregulates VEGF mRNA expression [31]. Increased leptin levels have been associated with RCC invasion and progression [32, 33]. However, adiponectin, a polypeptide secreted by adipose tissue, has tumor-suppressive effects and important antiangiogenic activities [34]. Its circulating levels inversely correlate with body weight and have been found to be associated with higher tumor size and metastatic progression in patients with RCC [35]. Inflammatory cells infiltrating the adipose tissue also contribute significantly to VEGF production [27, 36], thereby explaining why higher serum VEGF levels have been found in obese patients [13, 14].
It is noteworthy that the definition of obesity is controversial since it is unclear whether BMI is the most appropriate measure of obesity. Several studies suggest that BMI is a crude measure of body fat distribution that fails to distinguish between visceral and subcutaneous fat [37]. In a recent study, Chatterjee et al. demonstrated in RCC patients that visceral obesity directly correlated with metastatic progression, whereas BMI did not [38]. Moreover, an increasing body of evidence indicates that the cytokine production profile differs between subcutaneous fat and visceral fat [39, 40]. Interestingly, computed tomography can be easily used to accurately assess intra-abdominal fat [22] via measurements of SFA and VFA at the level of umbilicus. Visceral obesity has been found to be associated with lower plasma adiponectin levels and higher incidence of metastatic disease in patients with RCC [38]. Moreover, the level of VEGF production is higher in omental fat than in fat located at any other site in the body [27]. These differences may explain why obesity-associated metabolic disorders, serum VEGF levels [13], and RCC prognosis correlate better with VFA than with SFA, or BMI. Recent phase III trials showed that VEGF-targeted therapy improved progression-free survival in patients with mRCC [3–6]. High VEGF serum levels have been reported to predict higher stage, higher tumor grade, and shorter OS in patients with localized RCC [15, 16]. In a study of 302 patients treated with cytokines, high pretreatment VEGF blood level was independently prognostic for lower OS and DFS in multivariate analysis [41]. However, the prognostic significance of VEGF blood level was not confirmed in other studies performed on patients treated with VEGF-targeted therapy [42], and thus cannot be used currently to improve patient's risk stratification. Interestingly, obese animals proved resistance to anti-VEGF therapy [43]. Moreover, we recently demonstrated that VFA measured before starting first-line bevacizumab-based therapy was likely to be a useful predictive biomarker of treatment's efficacy in metastatic colorectal cancer patients [17]. Altogether, these data could support the hypothesis that a large amount of visceral fat may be associated with high proangiogenic factor levels and therefore with resistance to VEGF-targeted therapy in patients with mRCC. Until now and despite extensive investigation, there are no validated predictive biomarkers of the efficacy of VEGF-targeted therapy. Our study provides the first evidence that a large amount of visceral fat is independently associated with worse outcomes in patients given first-line VEGF-targeted therapy for mRCC. VFA could be considered as a predictive factor of benefit from VEGF-targeted therapy, as it was not associated with a modified outcome in patients treated with cytokines.
Limitations of our study include the relative small number of patients, the single-center patient recruitment, and the retrospective design. Moreover, this patient population included patients treated with sunitinib, sorafenib, or bevacizumab, and it will be of first interest to validate this new predictive biomarker in cohorts of mRCC patients treated homogeneously. However, despite these limitations, classic prognostic factors derived from the MSKCC model remain associated with the outcome of our patients. Of note, neither neutrophil count greater than the ULN nor platelet count greater than the ULN (which have been recently described as prognostic factors associated with outcome in patients treated with VEGF-targeted therapies for RCC [24]) was associated with poorer TTP or OS in univariate analysis. This could be due to the small proportion of patients with these adverse features in our cohort (9% and 17%, respectively). Because of the small sample size, bootstrapping was performed to internally validate the results and prevent overfitting. The results obtained by bootstrapping highlighted that high VFA remains a major independent predictor of short survival. Further studies are ongoing to validate our findings in a different data set and to determine the optimal cutoff for VFA.
In conclusion, our results provide the first evidence that VFA measured before starting first-line VEGF-targeted therapy is likely to be a simple predictive biomarker in mRCC patients. Further studies may help us to determine whether the predictive effect of high VFA is related to either a larger volume of distribution of VEGF-targeted therapies or the production of high levels of VEGF by visceral fat or both preceding hypotheses. Consequently, patients with high VFA could either not benefit from VEGF-targeted therapy or perhaps require a higher dosage. If further validation studies corroborate our results, the measurement of VFA will have to be incorporated into clinical patient care as well as into stratification schema for future clinical trials with VEGF-targeted therapies, thereby taking into account not only pathologic parameters related to tumor burden but also physiologic parameters related to the patient himself.
Supplementary Material
Author Contributions
Conception/design: Sylvain Ladoire, Franck Bonnetain, Mélanie Gauthier, Sylvie Zanetta, Jean Michel Petit, Séverine Guiu, Luc Cormier, François Ghiringhelli, Boris Guiu
Provision of study material or patients: Sylvain Ladoire, Sylvie Zanetta, Isabelle Kermarrec, Eric Mourey, Frederic Michel, Denis Krause, Patrick Hillon, Luc Cormier, François Ghiringhelli, Boris Guiu
Collection/assembly of data: Sylvain Ladoire, Franck Bonnetain, Mélanie Gauthier, Jean Michel Petit, Séverine Guiu, Isabelle Kermarrec, Eric Mourey, Frederic Michel, Denis Krause, Patrick Hillon, François Ghiringhelli, Boris Guiu
Data analysis and interpretation: Sylvain Ladoire, Franck Bonnetain, Mélanie Gauthier, Jean Michel Petit, Séverine Guiu, Luc Cormier, François Ghiringhelli, Boris Guiu
Manuscript writing: Sylvain Ladoire, Franck Bonnetain, Mélanie Gauthier, Luc Cormier, François Ghiringhelli, Boris Guiu
Final approval of manuscript: Sylvain Ladoire, Franck Bonnetain, Mélanie Gauthier, Sylvie Zanetta, Jean Michel Petit, Séverine Guiu, Isabelle Kermarrec, Eric Mourey, Frederic Michel, Denis Krause, Patrick Hillon, Luc Cormier, François Ghiringhelli, Boris Guiu
References
- 1.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 2.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, H S, Rosenberg JE, et al. CALGB 90206: A phase III trial of bevacizumab plus interferon alpha versus interferon alpha monotherapy in metastatic renal cell carcinoma. Presented at the Genitourinary Cancers Symposium; February 14–16, 2008; San Francisco CA. [Google Scholar]
- 7.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 8.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 9.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 10.Bergstrom A, Hsieh CC, Lindblad P, et al. Obesity and renal cell cancer–a quantitative review. Br J Cancer. 2001;85:984–990. doi: 10.1054/bjoc.2001.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow WH, McLaughlin JK, Mandel JS, et al. Obesity and risk of renal cell cancer. Cancer Epidemiol Biomarkers Prev. 1996;5:17–21. [PubMed] [Google Scholar]
- 12.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 13.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, et al. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46:1483–1488. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 14.Silha JV, Krsek M, Sucharda P, et al. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29:1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen J, Rasmuson T, Grankvist K, et al. Vascular endothelial growth factor as prognostic factor in renal cell carcinoma. J Urol. 2000;163:343–347. [PubMed] [Google Scholar]
- 16.Rioux-Leclercq N, Fergelot P, Zerrouki S, et al. Plasma level and tissue expression of vascular endothelial growth factor in renal cell carcinoma: a prospective study of 50 cases. Hum Pathol. 2007;38:1489–1495. doi: 10.1016/j.humpath.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Guiu B, Petit JM, Bonnetain F, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based therapy in metastatic colorectal cancer. Gut. 2010;59(3):341–347. doi: 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alpha as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 19.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23:832–841. doi: 10.1200/JCO.2005.05.179. [DOI] [PubMed] [Google Scholar]
- 20.Choueiri TK, Garcia JA, Elson P, et al. Clinical factors associated with outcome in patients with metastatic clear-cell renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Cancer. 2007;110:543–550. doi: 10.1002/cncr.22827. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Bukowski RM, Figlin RA, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2008;113:1552–1558. doi: 10.1002/cncr.23776. [DOI] [PubMed] [Google Scholar]
- 22.Yoshizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 25.Bjorge T, Tretli S, Engeland A. Relation of height and body mass index to renal cell carcinoma in two million Norwegian men and women. Am J Epidemiol. 2004;160:1168–1176. doi: 10.1093/aje/. [DOI] [PubMed] [Google Scholar]
- 26.Lew EA, Garfinkel L. Variations in mortality by weight among 750,000 men and women. J Chronic Dis. 1979;32:563–576. doi: 10.1016/0021-9681(79)90119-x. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baillargeon J, Rose DP. Obesity, adipokines, and prostate cancer (review) Int J Oncol. 2006;28:737–745. [PubMed] [Google Scholar]
- 29.Rosendahl A, Forsberg G. Influence of IGF-IR stimulation or blockade on proliferation of human renal cell carcinoma cell lines. Int J Oncol. 2004;25:1327–1336. [PubMed] [Google Scholar]
- 30.Kellerer M, von Eye Corleta H, Muhlhofer A, et al. Insulin- and insulin-like growth-factor-I receptor tyrosine-kinase activities in human renal carcinoma. Int J Cancer. 1995;62:501–507. doi: 10.1002/ijc.2910620502. [DOI] [PubMed] [Google Scholar]
- 31.Suganami E, Takagi H, Ohashi H, et al. Leptin stimulates ischemia-induced retinal neovascularization: possible role of vascular endothelial growth factor expressed in retinal endothelial cells. Diabetes. 2004;53:2443–2448. doi: 10.2337/diabetes.53.9.2443. [DOI] [PubMed] [Google Scholar]
- 32.Horiguchi A, Sumitomo M, Asakuma J, et al. Increased serum leptin levels and over expression of leptin receptors are associated with the invasion and progression of renal cell carcinoma. J Urol. 2006;176:1631–1635. doi: 10.1016/j.juro.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 33.Attoub S, Noe V, Pirola L, et al. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. Faseb J. 2000;14:2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- 34.Brakenhielm E, Veitonmaki N, Cao R, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinthus JH, Kleinmann N, Tisdale B, et al. Lower plasma adiponectin levels are associated with larger tumor size and metastasis in clear-cell carcinoma of the kidney. Eur Urol. 2008;54:866–873. doi: 10.1016/j.eururo.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 36.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 37.Kuk JL, Lee S, Heymsfield SB, et al. Waist circumference and abdominal adipose tissue distribution: influence of age and sex. Am J Clin Nutr. 2005;81:1330–1334. doi: 10.1093/ajcn/81.6.1330. [DOI] [PubMed] [Google Scholar]
- 38.Chatterjee S, Kleinman N, Gharajeh A, et al. Computerized tomography measurement of visceral adiposity predicts plasma adiponectin levels and metastatic disease in patients with clear cell renal cell carcinoma. Curr Urol. 2008;2:188–193. [Google Scholar]
- 39.Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim Biophys Acta. 2000;1500:88–96. doi: 10.1016/s0925-4439(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 40.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, et al. Roles of degree of fat deposition and its localization on VEGF expression in adipocytes. Am J Physiol Endocrinol Metab. 2005;288:E1128–E1136. doi: 10.1152/ajpendo.00003.2004. [DOI] [PubMed] [Google Scholar]
- 41.Negrier S, Chabaud S, Escudier B, et al. Serum level of vascular endothelial growth factor (VEGF) as an independent prognostic factor in metastatic renal cell carcinoma (MRCC) J Clin Oncol. 2007;25(suppl):5044. [Google Scholar]
- 42.Bukowski R, Heng D, Eisen T, et al. Sorafenib in advanced renal cell carcinoma (RCC): survival and biomarker results from a phase III trial. Eur Urol. 2008;7(suppl):245. [Google Scholar]
- 43.Rupnick MA, Panigrahy D, Zhang CY, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.