Results of a meta-analysis to determine the effects of supervised exercise training on peak oxygen consumption in adults with cancer are reported.
Keywords: Aerobic training, Resistance training, Peak oxygen consumption, Aerobic capacity, Randomized controlled trials
Abstract
Background.
We conducted a meta-analysis to determine the effects of supervised exercise training on peak oxygen consumption (VO2peak) in adults with cancer.
Methods.
A literature review using Ovid MEDLINE (1950–2010), the Cochrane Central Register of Controlled Trials (1991–2010), AMED (1985–2010), Embase (1988–2010), PubMed (1966–2010), Scopus (1950–2010), and Web of Science (1950–2010) was performed to identify randomized controlled trials examining the effects of supervised exercise training on measurement of VO2peak (via gas exchange analysis) in adults with cancer. Studies were selected using predetermined criteria, and two independent reviewers extracted data. Weighted mean differences (WMDs) were calculated using random effect models.
Results.
Six studies evaluated VO2peak involving a total of 571 adult cancer patients (exercise, n = 344; usual care control, n = 227). Pooled data indicated that exercise training was associated with a statistically significant increase in VO2peak (WMD, 2.90 ml·kg−1·min−1; 95% confidence interval [CI], 1.16–4.64); however, significant heterogeneity was evident in this estimate (I2, 87%). Usual care (control) was associated with a significant decline in VO2peak from baseline to postintervention (WMD, −1.02 ml·kg−1·min−1; 95% CI, −1.46 to −0.58; I2, 22%). Sensitivity analyses indicated superior improvements in VO2peak for studies conducted for a shorter duration (<4 months) and following the completion of adjuvant therapy (p-values < .001). Exercise training was not associated with a higher incidence of adverse events, although safety was not rigorously monitored or reported.
Conclusions.
Supervised exercise training is associated with significant improvements in VO2peak following a diagnosis of early-stage cancer, with minimal adverse events.
Introduction
It is well established that cardiorespiratory fitness as well as change in cardiorespiratory fitness are powerful predictors of mortality in healthy adults as well as those with cardiovascular disease (CVD), even after controlling for traditional CVD risk factors [1–5]. Maximal or peak oxygen consumption (VO2peak) provides the gold standard measurement of cardiorespiratory fitness and is used widely in numerous clinical and research applications [6].
Emerging evidence indicates that VO2peak also may be a parameter of central importance following a diagnosis of cancer. Prior to surgical resection, VO2peak is a strong predictor of perioperative or postoperative complication risk in patients with non-small cell lung cancer (NSCLC) [7–10]. VO2peak is also centrally implicated in the etiology of certain cancer therapy–induced late effects. Specifically, VO2peak is a predictor of anthracycline and trastuzumab-induced left ventricular dysfunction and CVD risk profile (e.g., blood pressure, lipid profile, c-reactive protein) as well as global quality of life (QOL) and fatigue in patients with solid malignancies [11–13]. Finally, there is evidence from one report that VO2peak is also a strong independent predictor of survival in NSCLC patients even after controlling for traditional prognostic factors [14].
Unfortunately, cancer patients have marked reductions in VO2peak. In a series of studies by our group spanning the entire cancer survivorship continuum (i.e., diagnosis to palliation), we observed that VO2peak is consistently ∼30% below that of age- and sex-matched sedentary individuals without a history of cancer [15, 16]. The precise causes of a poor VO2peak remain to be elucidated but likely reflect normal age-related exercise limitation together with additional direct (injury to the cardiovascular system) and indirect (toxicities secondary to treatment) effects of cytotoxic therapy that, in combination, adversely impact the organ components that govern exercise tolerance [17].
Numerous studies report that structured exercise training is associated with significant improvements in measures of cardiorespiratory fitness and related outcomes across a broad range of oncology settings [18–21]. However, the current evidence base is fraught with important methodological limitations, including nonrandomized designs, small sample sizes, different exercise training modes (aerobic and/or resistance training), and determination of cardiorespiratory fitness using non-VO2peak measures. To clarify this issue, we employed the meta-analysis approach to determine the effect of exercise training on VO2peak in adult cancer patients. A secondary aim was to examine whether the effects of exercise on VO2peak differed as a function of exercise intervention (e.g., type, intensity, duration) or clinical characteristics (e.g., cancer type, treatment status).
Methods
Search Strategy and Inclusion Criteria
A comprehensive literature review was conducted using Ovid MEDLINE (1950–2010), the Cochrane Central Register of Controlled Trials (1991–2010), AMED (1985–2010), Embase (1988–2010), PubMed (1966–2010), Scopus (1950–2010), and Web of Science (1950–2010) with the following Medical Subject Heading terms and text words: oncology, cancer, neoplasms, malignancies, exercise, exercise therapy, and exercise training. Relevant reference lists were also manually searched.
Randomized controlled trials (RCTs) involving adult patients with histologically confirmed cancer that allocated subjects to a supervised exercise training or concurrent nonexercise control group were deemed eligible. Supervised exercise training was defined as interventions consisting of aerobic, resistance, or the combination of aerobic and resistance training as opposed to unsupervised or home-based interventions. Additionally, all eligible studies were required to report a measurement of cardiorespiratory fitness via a cardiopulmonary exercise test (CPET) with gas exchange analysis (to permit assessment of VO2peak). Studies with a participant mean age <18 years, that were not written in English, that were a review article only, and that did not assess the independent effects of exercise training were excluded.
Study Selection, Data Extraction, and Quality Assessment
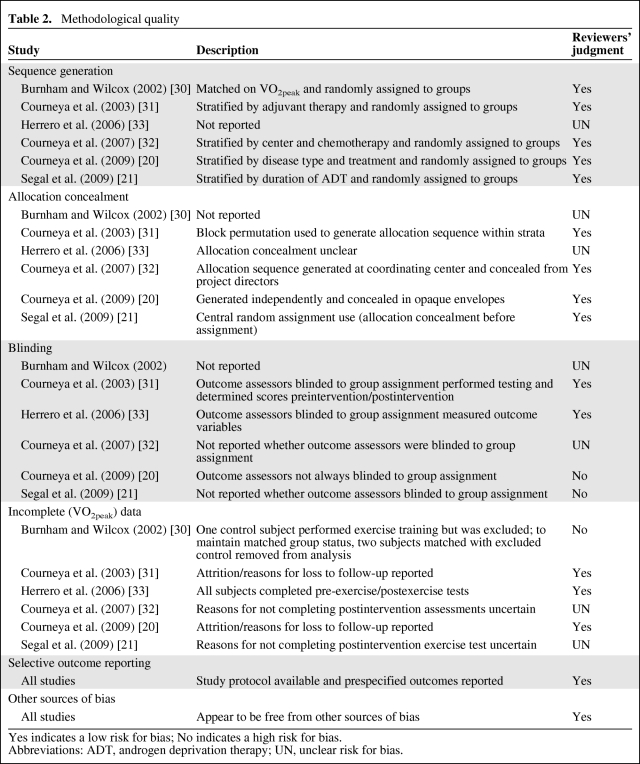
Two authors (C.L.B. and E.N.P.) independently evaluated study eligibility by reviewing the titles and abstracts of all potential citations according to the inclusion criteria. The same authors independently performed data extraction using standardized data abstraction forms. Disagreements were resolved by consensus in discussion with a third independent author (M.H.). When required, the primary authors were contacted to clarify ambiguous experimental procedures and/or results or provide additional data not provided in the published manuscript. Methodological quality of eligible studies was assessed using the Oxford quality scoring system and Schulz approach to allocation concealment [22]. The risk for bias was assessed with the Cochrane criteria [23].
Data Synthesis and Analysis
For each eligible study, the effect size of exercise training was calculated using the change in VO2peak (ml·kg−1·min−1) from baseline to postintervention for the exercise and nonexercise control groups. In circumstances when the change from baseline data or corresponding standard deviations were not available, these values were calculated using standard statistical methods assuming a correlation of 0.50 between the baseline and postintervention scores within each subject [24]. Data from all eligible studies were combined as weighted mean differences (WMDs) with 95% confidence intervals (CIs) using the random effects model. Statistical analyses were conducted using Review Manager Software (RevMan 5.0; The Cochrane Collaboration, Oxford, UK). The primary analysis compared the effect of exercise training, regardless of exercise prescription characteristics, with that of the nonexercise control on VO2peak. Sensitivity analyses were performed to investigate whether the effects of exercise on VO2peak differed as a function of exercise intervention or clinical characteristics (e.g., cancer type, treatment status).
Heterogeneity was quantified using the I2 statistic. I2 evaluates the percentage of total variation across included studies attributed to heterogeneity as opposed to chance. A value >50% is considered substantial heterogeneity [25]. The Deeks' χ2 test was conducted to test for significant heterogeneity reduction in partitioned subgroups [26]. The following subgroup analyses were conducted to investigate possible sources of heterogeneity: (a) intervention length (≤4 months versus >4 months), (b) gender/primary cancer diagnosis (all females/breast cancer versus all males/prostate cancer versus mixed), and (c) treatment status (postsurgery and completion of adjuvant therapy versus postsurgery and during adjuvant therapy versus mixed). Publication bias was tested visually using a funnel plot [27] and quantitatively using the Begg adjusted-rank correlation test [28] and Egger regression asymmetry test [29]. Tests were performed using Stata 11.0 (Stata Corporation, College Station, TX).
Results
In total, 2,855 potential citations were identified; after initial review; 35 papers were deemed eligible and underwent full review (Fig. 1). The major reasons for exclusion were: (a) inclusion of participants without a histological diagnosis of cancer, (b) absence of an exercise intervention, and (c) review article. Upon further review, 29 papers were further excluded; reasons for exclusion were: (a) studies did not perform a supervised exercise intervention or did not conduct a direct measure of VO2peak, (b) insufficient data were presented in the paper, (c) exercise training was combined with a concurrent complementary intervention, and (d) no “usual care” control group. Thus, six trials were deemed eligible [20, 21, 30–33] and included in the primary analysis.
Figure 1.
Selection process of eligible studies.
Study Characteristics
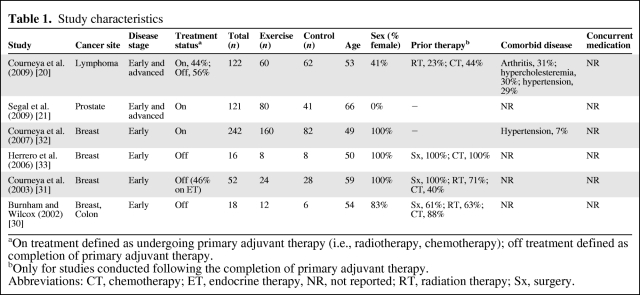
Study characteristics are provided in Table 1. Three studies performed a two-arm RCT (aerobic training versus control [20, 31] or the combination of aerobic and resistance training versus control [33]) and three conducted a three-arm RCT comparing either different types of exercise (aerobic training versus resistance training) [21, 32] or intensities of aerobic training (low intensity versus moderate intensity) [30]. Three of the six studies were conducted in women with early-stage breast cancer [31–33]; the other studies were conducted among patients with prostate cancer (n = 1) [21], non-Hodgkin's lymphoma (n = 1) [20], and a combination of colon or breast cancer (n = 1) [30]. Three studies were conducted following the completion of definitive adjuvant therapy (i.e., chemotherapy or radiation) [30, 31, 33], two were conducted during definitive cytotoxic therapy [21, 32], and one included patients both receiving and following the completion of therapy [20]. No studies reported performing continuous electrocardiogram monitoring during CPET whereas two studies reported monitoring heart rate during exercise training sessions [21, 30]. The methodological quality of trials is presented in Table 2.
Table 1.
Study characteristics
aOn treatment defined as undergoing primary adjuvant therapy (i.e., radiotherapy, chemotherapy); off treatment defined as completion of primary adjuvant therapy.
bOnly for studies conducted following the completion of primary adjuvant therapy.
Abbreviations: CT, chemotherapy; ET, endocrine therapy, NR, not reported; RT, radiation therapy; Sx, surgery.
Table 2.
Methodological quality
Yes indicates a low risk for bias; No indicates a high risk for bias.
Abbreviations: ADT, androgen deprivation therapy; UN, unclear risk for bias.
Exercise Intervention Characteristics
The exercise intervention characteristics of included studies are provided in Table 3. Intervention lengths were in the range of 8–24 weeks. In all studies, exercise was prescribed 3 times per week, with session durations in the range, on average, of 14–45 minutes. All studies reported prescribing “moderate-to-high intensity” exercise, defined as 40%–80% of peak heart rate, heart rate reserve, or VO2peak obtained from the baseline cardiopulmonary exercise test, whereas one prescribed “low-intensity” training (25%–40% of baseline heart rate reserve). Aerobic training alone was the form of exercise training in three studies [20, 31, 32], two compared aerobic training only with resistance training only [21, 32], and one tested the combination of aerobic and resistance training [33].
Table 3.
Exercise intervention characteristics
Abbreviations: BP, blood pressure; CE, cycle ergometry; ET, elliptical trainer; HR, heart rate; HRmax, heart rate maximum; HRR, heart rate reserve; INT, interval; LE, lower extremity; NR, not reported; RM, repetition maximum; SC, stair climber; TM, treadmill; UE, upper extremity; x1, one time/wk of interval training.
Effect of Exercise Training on VO2peak
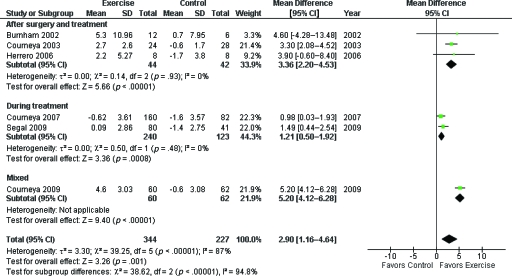
Six studies examined the effect of exercise training on VO2peak, with 344 participants in the exercise groups and 227 participants in the nonexercise groups. The baseline mean VO2peak was not different between groups in any study (p > .05). Pooled data indicated that exercise training was associated with a statistically significant increase in VO2peak (WMD, 2.90 ml·kg−1·min−1; 95% CI, 1.16–4.64); however, significant heterogeneity was evident in this estimate (I2, 87%) (Fig. 2). Usual care (control) was associated with a significant decline in VO2peak from baseline to postintervention (WMD, −1.02 ml·kg−1·min−1; 95% CI, −1.46 to −0.58; I2, 22%) There was no evidence of publication bias (Begg adjusted-rank correlation test, p = .71; Egger regression asymmetry test, p = .69).
Figure 2.
Pooled effects of supervised exercise training, compared with usual care (control), on cardiorespiratory fitness (peak oxygen consumption, VO2peak).
Abbreviations: CI, confidence interval; SD, standard deviation.
Effect of Exercise Training on VO2peak by Exercise Intervention or Clinical Characteristics
Sensitivity analyses were conducted to investigate whether the effects of exercise on VO2peak differed as a function of exercise intervention or clinical characteristics. However, given the small number of eligible studies, it was only feasible to conduct sensitivity analyses based on intervention length (<4 months versus ≥4 months), therapy status (during versus following adjuvant therapy), and exercise modality (aerobic only versus resistance only). Concerning intervention length, for studies conducted for ≥4 months, the overall effect size was 1.21 ml·kg−1·min−1 (two studies, 363 patients; WMD, 1.21 ml·kg−1·min−1; 95% CI, 0.50–1.92; I2, 0%) favoring exercise training. The corresponding pooled effect size for studies <4 months was 4.26 ml·kg−1·min−1 (four studies, 208 patients; WMD, 4.26 ml·kg−1·min−1; 95% CI, 2.92–5.60) favoring exercise training, although moderate heterogeneity was evident in this estimate (I2, 43%). The difference between subgroups was significant (χ2, 33.49; p < .001) favoring studies conducted for <4 months. For therapy status, in studies conducted during adjuvant therapy, the pooled effect size was 1.21 ml·kg−1·min−1 (two studies, 363 patients; WMD, 1.21 ml·kg−1·min−1; 95% CI, 0.50–1.92; I2, 0%) favoring exercise training. The corresponding pooled effect size for studies conducted following the completion of therapy was 3.36 ml·kg−1·min−1 (three studies, 86 patients; WMD, 3.36 ml·kg−1·min−1; 95% CI, 2.20–4.53; I2, 0%) favoring exercise training. The difference between subgroups was significant (χ2, 38.62; p < .001) favoring studies conducted after therapy completion (Fig. 3). Only two studies directly compared aerobic training with resistance training, with contrasting results. Overall, there was no significant difference in VO2peak as a function of exercise modality (WMD, −0.78 ml·kg−1·min−1; 95% CI, −2.45 to 0.88; I2, 75%).
Figure 3.
Pooled effects of supervised exercise training, compared with usual care (control), on cardiorespiratory fitness (peak oxygen consumption, VO2peak) by treatment status.
Abbreviations: CI, confidence interval; SD, standard deviation.
Adherence, Loss to Follow-Up, and Adverse Events
The mean lost-to-follow-up rate was 8.1% ± 7.2% (range, 0%−20%); there was no evidence for different lost-to-follow-up rates between the exercise and control groups. The mean adherence rate was 88.7% ± 10.1% (range, 70.2%−98.4%); five of six studies reported an adherence rate ≥80% [20, 21, 30, 31, 33]. Finally, all studies reported that adverse events (AEs) were monitored during study conduct. Two AEs were reported during cardiopulmonary exercise testing, nine were reported during exercise training, and two were reported in control participants; a total patient AE rate of 13 per 571 adult patients (2.3%) was found. The most serious AE was a myocardial infarction during aerobic training [21].
Discussion
The principal finding of this meta-analysis was that relatively short-term, structured, moderate-intensity exercise training is associated with significant improvements in the VO2peak in select curative-intent cancer patients both during and following adjuvant therapy. Specifically, the WMD in VO2peak was 2.91 ml·kg−1·min−1 from baseline to postintervention, favoring exercise training. The magnitude of change is similar to that reported in a prior meta-analysis that included three studies (two were unpublished dissertations) in women with early breast cancer; McNeely et al. [34] found that VO2peak increased 3.39 ml·kg−1·min−1 with exercise training, involving 95 patients in total. The prognostic relevance of this improvement in adult cancer patients is not yet known; however, Myers et al. [1] and Gulati et al. [35] found that the Framingham Risk Score−adjusted mortality risk decreased by 12% and 17% for every 1-MET (3.5 ml·kg−1·min−1) difference in aerobic capacity among asymptomatic men and women, respectively. It is noteworthy that the beneficial effects of exercise training were observed with minimal AEs. In total, 13 AEs were reported across six studies, for a total AE rate of 2.3%. However, the performance (monitoring) of VO2peak assessment did not comply with CPET recommendations for clinical populations [6] or cancer patients [36]. In addition, no study adopted a standardized method for monitoring or reporting nonexercise-related AEs. As such, it is not clear whether the low incidence of AEs reflects the true safety of CPET/exercise training in cancer patients or less than optimal monitoring/reporting of AEs. Cancer is a heterogeneous disease varying considerably in location, pathogenesis, and therapeutic management; thus, the risk for an exercise-related AE is likely highly dependent on these factors. Unfortunately, given the low incidence of AEs, it was not possible to investigate this question. We stress that future studies should strive to comprehensively monitor and report AEs when conducting exercise intervention studies in the oncology setting [36].
A finding of major importance is the significant decline in VO2peak among patients assigned to the usual care control groups. In cross-sectional studies, we found that the VO2peak of cancer patients was consistently ∼30% below that of age- and sex-matched sedentary but otherwise healthy individuals [13, 15, 16, 37]. The present finding suggests that, without exercise training, VO2peak will remain low or become even further impaired, particularly during adjuvant therapy. The clinical importance of this finding cannot be overstated. First, VO2peak is a strong, independent predictor of mortality in humans with and without CVD [1–5]. Recent work by our group found that, relative to patients with a low VO2peak (<13 ml·kg−1·min−1), moderate (13.9–16.9 ml·kg−1·min−1) and high (≥17 ml·kg−1·min−1) VO2peak levels were associated with a 21%−24% lower all-cause mortality rate in presurgical NSCLC patients. A 1.0 ml·kg−1·min−1 decrease in VO2peak, a reduction similar to that observed in patients randomized to the nonexercise control groups, was associated with a 4% greater mortality rate [14]. Second, Paterson et al. [38] demonstrated that a minimum VO2peak of ∼15 ml·kg−1·min−1 in women and ∼18 ml·kg−1·min−1 in men aged 85 years was necessary for full and independent living (e.g., garden activities, walking up stairs). Alarmingly, a large proportion of adult cancer patients do not meet this minimum threshold, further highlighting the critical importance of exercise-based rehabilitation following diagnosis. Finally, VO2peak is associated with a broad range of relevant outcomes in cancer patients, including surgical complication risk, certain therapy late effects, global QOL, and fatigue [9, 11–13, 15, 16, 39].
With only six eligible trials, sensitivity analyses were difficult, although significant differences were indicated for two parameters: exercise length and therapy status. Surprisingly, shorter duration exercise interventions (<4 months) were associated with superior VO2peak improvements than in those of longer duration (≥4 months). This finding may be an artifact of when the longer duration studies were conducted as opposed to real differences in intervention length per se. Longer duration studies were, for the most part, conducted during cytotoxic therapy, when smaller improvements in VO2peak are expected. The sensitivity analysis indicating superior VO2peak improvements following rather than during adjuvant therapy supports this notion. Structured exercise interventions in healthy (nondiseased) adult populations typically report an ∼15% increase in VO2peak with aerobic-based training following traditional prescription guidelines (3–5 days per week at 50%−75% of baseline VO2peak for 12–15 weeks) [40, 41]. Despite exercise studies in cancer patients employing similar exercise prescriptions, the magnitude of the VO2peak improvement appears lower, suggesting that the use of cytotoxic therapy may attenuate normal cardiovascular and/or skeletal muscle adaptations to exercise training [32, 37]. The reasons for these divergent findings are not known but likely relate to differences in the extent and causes of exercise limitation between healthy adults and those with cancer. In addition to the normal effects of aging, cancer patients are also subject to cytotoxic therapy−induced injury together with profound deconditioning that dramatically depletes the compensatory abilities of the cardiovascular reserve [17]. In addition, these effects are further compounded by treatment-associated weight gain, which also impacts VO2peak [42]. Studies investigating the limitations to exercise, and underlying molecular mechanisms, in cancer patients both during and following therapy are warranted to ensure the optimal efficacy of exercise in the oncology setting.
Caution is warranted when interpreting the present results given the significant heterogeneity evident in the primary and sensitivity analyses. In an effort to minimize heterogeneity, we only selected RCTs that included a measurement of VO2peak via expired gas exchange analysis. Upon closer inspection, the significant heterogeneity is not surprising given the stark between-study differences in cancer diagnosis, cytotoxic therapy, disease stage, and exercise prescription characteristics. There is little doubt that the field of exercise oncology has made significant progress over the past decade; however, findings of our meta-analysis, and prior reviews [34, 43], clearly demonstrate that the current evidence base is emergent, with many fundamental questions (e.g., optimal prescription, timing, and setting of exercise, effects of exercise on tumor biology, and therapeutic efficacy) remaining to be addressed. A major goal of exercise oncology research is to establish evidence-based exercise rehabilitation/physical activity guidelines to maximize the health and longevity of persons following a cancer diagnosis. Clearly, more studies are required to inform such guidelines, but simply increasing the absolute number will not address the current limitations. Instead, in order to advance the field, it is critical that the next generation of studies logically build on and extend current scientific knowledge in homogeneous patient populations/settings applying rigorous RCT methodology. Such an approach will permit definitive conclusions regarding the efficacy of exercise in oncology management. Additionally, as we move into the era of “personalized medicine” in oncology, it will be increasingly important to match the exercise prescription to the clinical/treatment characteristics of a patient subgroup or individual patient. Such a goal is not trivial and will only be achieved by adopting a translational (bed-to-benchside) approach to inform mechanistically driven phase III trials in conjunction with rational correlative science studies to ensure the optimal safety and efficacy of exercise [44].
In conclusion, there is promising evidence that supervised exercise training, compared with usual care (control), is associated with significant improvements in VO2peak following a diagnosis of select early-stage cancer with minimal AEs, although significant heterogeneity is evident. Limited evidence is currently available to suggest that the exercise−VO2peak relationship is different based on exercise intervention or clinical patient characteristics.
Acknowledgments
L.W.J. is supported by NIH CA143254, CA142566, CA138634, CA133895, CA125458, and George and Susan Beischer.
Author Contributions
Conception/Design: Lee Jones
Provision of study material or patients: Claudio Battaglini, Edith Pituskin, Whitney Hornsby
Collection and/or assembly of data: Lee Jones, Claudio Battaglini, Edith Pituskin, Whitney Hornsby
Data analysis and interpretation: Lee Jones, Mark Haykowsky, Jessica Scott, Yuanyuan Liang
Manuscript writing: Lee Jones, Jessica Scott, Yuanyuan Liang
Final approval of manuscript: Lee Jones, Claudio Battaglini, Mark Haykowsky, Edith Pituskin, Jessica Scott, Yuanyuan Liang, Whitney Hornsby
References
- 1.Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 2.Gulati M, Black HR, Shaw LJ, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005;353:468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 3.Ekelund LG, Haskell WL, Johnson JL, et al. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med. 1988;319:1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 4.Sandvik L, Erikssen J, Thaulow E, et al. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med. 1993;328:533–537. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- 5.Erikssen G, Liestøl K, Bjørnholt J, et al. Changes in physical fitness and changes in mortality. Lancet. 1998;352:759–762. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society; American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 7.Beckles MA, Spiro SG, Colice GL, et al. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest. 2003;123(1 suppl):105S–114S. doi: 10.1378/chest.123.1_suppl.105s. [DOI] [PubMed] [Google Scholar]
- 8.Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 suppl):161S–77S. doi: 10.1378/chest.07-1359. [DOI] [PubMed] [Google Scholar]
- 9.Brunelli A, Belardinelli R, Refai M, et al. Peak oxygen consumption during cardiopulmonary exercise test improves risk stratification in candidates to major lung resection. Chest. 2009;135:1260–1267. doi: 10.1378/chest.08-2059. [DOI] [PubMed] [Google Scholar]
- 10.Loewen GM, Watson D, Kohman L, et al. Preoperative exercise VO2 measurement for lung resection candidates: Results of Cancer and Leukemia Group B Protocol 9238. J Thorac Oncol. 2007;2:619–625. doi: 10.1097/JTO.0b013e318074bba7. [DOI] [PubMed] [Google Scholar]
- 11.Herrero F, Balmer J, San Juan AF, et al. Is cardiorespiratory fitness related to quality of life in survivors of breast cancer? J Strength Cond Res. 2006;20:535–540. doi: 10.1519/r-18215.1. [DOI] [PubMed] [Google Scholar]
- 12.Jones LW, Haykowsky M, Peddle CJ, et al. Cardiovascular risk profile of patients with HER2/neu-positive breast cancer treated with anthracycline-taxane-containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Biomarkers Prev. 2007;16:1026–1031. doi: 10.1158/1055-9965.EPI-06-0870. [DOI] [PubMed] [Google Scholar]
- 13.Jones LW, Haykowsky M, Pituskin EN, et al. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor–positive operable breast cancer. The Oncologist. 2007;12:1156–1164. doi: 10.1634/theoncologist.12-10-1156. [DOI] [PubMed] [Google Scholar]
- 14.Jones LW, Douglas PS, Eves ND, et al. Rationale and design of the exercise intensity trial (EXCITE): A randomized trial comparing the effects of moderate versus moderate to high-intensity aerobic training in women with operable breast cancer. BMC Cancer. 2010;10:531. doi: 10.1186/1471-2407-10-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haykowsky MJ, Mackey JR, Thompson RB, et al. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res. 2009;15:4963–4967. doi: 10.1158/1078-0432.CCR-09-0628. [DOI] [PubMed] [Google Scholar]
- 16.Jones LW, Eves ND, Mackey JR, et al. Safety and feasibility of cardiopulmonary exercise testing in patients with advanced cancer. Lung Cancer. 2007;55:225–232. doi: 10.1016/j.lungcan.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Jones LW, Eves ND, Haykowsky M, et al. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 18.Adamsen L, Quist M, Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomised controlled trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daley AJ, Crank H, Saxton JM, et al. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25:1713–1721. doi: 10.1200/JCO.2006.09.5083. [DOI] [PubMed] [Google Scholar]
- 20.Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27:4605–4612. doi: 10.1200/JCO.2008.20.0634. [DOI] [PubMed] [Google Scholar]
- 21.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 22.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.2 [updated September 2009] The Cochrane Collaboration. 2009. [accessed December 29, 2010]. Available at www.cochrane-handbook.org.
- 24.Follmann D, Elliott P, Suh I, et al. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman D, editors. Systematic Reviews in Health Care: Metaanalysis in Context. Second Edition. London, UK: BMJ Books; 2001. p. 300. [Google Scholar]
- 27.Light RJ, Pillemer DB. Summing Up. The Science of Reviewing Research. Cambridge, MA: Harvard University Press; 1984. [Google Scholar]
- 28.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc. 2002;34:1863–1867. doi: 10.1097/00005768-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Courneya KS, Mackey JR, Bell GJ, et al. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 32.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 33.Herrero F, San Juan AF, Fleck SJ, et al. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int J Sports Med. 2006;27:573–580. doi: 10.1055/s-2005-865848. [DOI] [PubMed] [Google Scholar]
- 34.McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: The St James Women Take Heart Project. Circulation. 2003;108:1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 36.Jones LW, Eves ND, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 37.Jones LW, Eves ND, Peterson BL, et al. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical nonsmall cell lung cancer patients: A pilot study. Cancer. 2008;113:3430–3439. doi: 10.1002/cncr.23967. [DOI] [PubMed] [Google Scholar]
- 38.Paterson DH, Cunningham DA, Koval JJ, et al. Aerobic fitness in a population of independently living men and women aged 55–86 years. Med Sci Sports Exerc. 1999;31:1813–1820. doi: 10.1097/00005768-199912000-00018. [DOI] [PubMed] [Google Scholar]
- 39.Jones LW, Eves ND, Mackey JR, et al. Systemic inflammation, cardiorespiratory fitness, and quality of life in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:194–195. doi: 10.1097/JTO.0b013e318160f36b. [DOI] [PubMed] [Google Scholar]
- 40.Warburton DE, Nicol CW, Bredin SS. Prescribing exercise as preventive therapy. CMAJ. 2006;174:961–974. doi: 10.1503/cmaj.1040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: The evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rock CL, Flatt SW, Newman V, et al. Factors associated with weight gain in women after diagnosis of breast cancer. Women's Healthy Eating and Living Study Group. J Am Diet Assoc. 1999;99:1212–1221. doi: 10.1016/s0002-8223(99)00298-9. [DOI] [PubMed] [Google Scholar]
- 43.Speck RM, Courneya KS, Mâsse LC, et al. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 44.Jones LW, Peppercorn J. Exercise research: Early promise warrants further investment. Lancet Oncol. 2010;11:408–410. doi: 10.1016/S1470-2045(10)70094-2. [DOI] [PubMed] [Google Scholar]