The article examines whether or not the incidence of brain metastasis in hepatocellular carcinoma patients has increased relative to the extended survival duration in these patients resulting from treatment with molecular targeted agents.
Keywords: Antiangiogenic therapy, Brain metastasis, Hepatocellular carcinoma
Learning Objectives
After completing this course, the reader will be able to:
Identify clinicopathologic parameters associated with development of brain metastasis to help stratify advanced HCC patients for appropriate care.
Describe the potential benefits of aggressive surgical intervention in selected advanced HCC patients with brain metastases.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Aim.
Brain metastasis was regarded, until recently, as a rare and late-stage event in patients with hepatocellular carcinoma (HCC). With the prolongation of survival in patients with advanced HCC by molecular targeted agents, this may have changed. We aimed to examine whether or not the incidence of brain metastasis in these patients has increased.
Methods.
Between June 2005 and May 2009, 158 advanced HCC patients in total with either metastatic or locally advanced disease untreatable by locoregional therapies were enrolled in clinical trials of first-line antiangiogenic therapies. The clinicopathologic features and survival times of those who developed brain metastasis were analyzed.
Results.
Eleven (7%) of 158 advanced HCC patients, with a median follow-up of 26.6 months, were diagnosed with brain metastasis as a result of compatible symptoms, confirmed by brain imaging. All 11 patients had extrahepatic metastasis upon enrollment, and 10 of them had lung metastasis. The median time to brain metastasis was 9.6 months (range, 0.6–19.6 months). The median overall survival (OS) time after diagnosis of brain metastasis was 4.6 months (range, 0.7–12.6 months). Four patients received brain tumor excision, and their survival duration after brain metastasis tended to be longer than that of those who did not (median OS time, 6.1 months versus 3.1 months).
Conclusions.
In the era of antiangiogenic targeted therapy, the importance of brain metastasis for advanced HCC patients may have increased.
Introduction
Brain metastasis occurring in patients with hepatocellular carcinoma (HCC) was, until recently, considered to be a rare event. Previously, long-term retrospective studies spanning decades reported that 0.2%–2.2% of all HCC patients developed brain metastasis [1–4]. These patients were reported to have an extremely poor prognosis, with median survival duration as short as 3–7 weeks [4–7]. Thus, brain metastasis was believed to be a terminal event that occurred late in the disease course of HCC.
Recently, the systemic therapy of HCC, especially in the advanced stage untreatable by surgery or other locoregional therapies, was revolutionized by molecular targeted therapy. Sorafenib, a multikinase inhibitor with antiangiogenic activity, was demonstrated to delay tumor progression and improve the overall survival (OS) time of patients with advanced HCC in two large, placebo-controlled, randomized phase III studies [8, 9]. Several other antiangiogenic agents showed similar antitumor effects in patients with advanced HCC [10].
With the prolongation of survival in patients with advanced HCC, it is likely that late complications (such as brain metastasis), which used to be rare, will arise more frequently. We thus retrospectively reviewed the medical records of patients with advanced HCC who had been enrolled in clinical trials of antiangiogenic therapies at our center. The data indicate that the incidence of brain metastasis may be increasing in patients with advanced HCC in the era of molecular targeted therapy.
Patients and Methods
We reviewed the medical records of all advanced HCC patients who were enrolled in clinical trials for first-line systemic therapy at National Taiwan University Hospital, Taipei, Taiwan, between June 2005 and May 2009 [11–15]. These patients had metastatic or locally advanced HCC not amenable to locoregional therapy. The clinical trials tested the efficacy of various types of antiangiogenic targeted therapy, including sorafenib, sorafenib plus tegafur/uracil, sunitinib, bevacizumab plus capecitabine, bevacizumab plus erlotinib, and thalidomide plus tegafur/uracil [11–15]. Brain imaging was not routinely performed unless the patient developed compatible symptoms. Cases of brain metastasis detected by computed tomography or magnetic resonance imaging were included in the current analysis.
The data collected included clinicopathologic characteristics upon enrollment and upon detection of brain metastasis, symptoms and signs of brain metastasis, treatment methods, follow-up time, and treatment outcomes. The difference between groups with or without brain metastasis was evaluated by χ2 tests for nominal clinicopathologic variables and by independent t-tests for continuous variables. Time to brain metastasis and survival time were estimated by Kaplan–Meier analysis. The survival of patients with brain tumor excision was compared with that of patients without brain tumor excision by the log-rank test. Two-sided p values <.05 were regarded as statistically significant, and .05 ≤p-value <.10 was regarded as borderline significant.
Results
From June 2005 to May 2009, in total, 158 patients with advanced HCC were enrolled in clinical trials of various first-line antiangiogenic therapies. Among them, 83 (53%) patients had extrahepatic metastasis upon enrollment, and the lung was the most common metastatic site (54 patients, 65%). With a median follow-up of 26.6 months, the median progression-free survival (PFS) and OS times for all 158 patients were 3.7 months and 6.6 months, respectively.
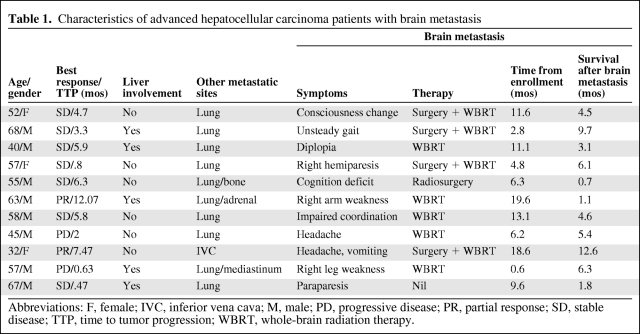
Eleven (7%) patients (8 men, 3 women; median age, 57 years; range, 32–68 years) developed brain metastasis 0.6–19.6 months (median, 9.6 months) after enrollment. Their clinical characteristics are listed in Table 1. Upon enrollment, all 11 patients who later developed brain metastasis had extrahepatic metastasis; 10 (91%) of them had lung metastasis. Brain metastasis developed in four patients during first-line treatment (including three patients treated with sunitinib and one patient treated with thalidomide plus tegafur/uracil) and in another seven patients after their first-line therapy had been stopped as a result of disease progression. Because brain imaging was not routinely arranged, all patients had symptoms upon diagnosis of brain metastasis. Symptoms were diverse, varying from motor dysfunction and cognition deficits to consciousness disturbance. Three patients had brain metastasis complicated with intracranial hemorrhage. Of the five patients who developed an isolated or limited number of metastatic lesions, four underwent surgery plus whole-brain radiation therapy (WBRT) and one underwent radiosurgery. Five patients received WBRT only, and one patient received best supportive care because of a poor general condition.
Table 1.
Characteristics of advanced hepatocellular carcinoma patients with brain metastasis
Abbreviations: F, female; IVC, inferior vena cava; M, male; PD, progressive disease; PR, partial response; SD, stable disease; TTP, time to tumor progression; WBRT, whole-brain radiation therapy.
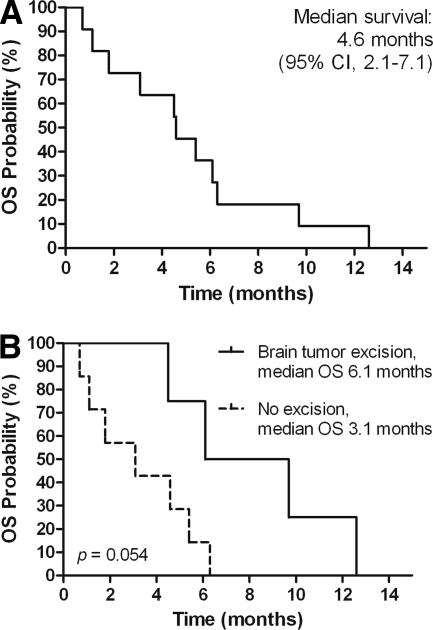
The median PFS and OS times of the 11 patients from the time of enrollment in the trials were 5.8 months and 12.4 months, respectively. The median survival duration after the development of brain metastasis was 4.6 months (95% confidence interval [CI], 2.1–7.1 months) (Fig. 1A). Of the four patients who survived >6 months after diagnosis of brain metastasis, three had their brain tumors excised. Survival tended to be longer for those who underwent surgery for brain metastasis than for those who did not (median OS time, 6.1 months versus 3.1 months; p = .054) (Fig. 1B).
Figure 1.
Kaplan–Meier analysis of overall survival (OS) after brain metastasis of advanced hepatocellular carcinoma patients with brain metastasis, showing the whole group (A), and grouped by patients receiving brain tumor excision or not (B).
Abbreviation: CI, confidence interval.
Potential clinical parameters associated with the development of brain metastasis were examined. Compared with those without brain metastasis, these 11 patients were significantly more likely to have any extrahepatic metastasis (p < .001) or lung metastasis (p < .001) at the time of the enrollment. Although the median PFS interval after first-line therapy was longer in patients who developed brain metastasis (5.8 months; 95% CI, 4.5–7.1 months) than in those who did not (3.7 months; 95% CI, 3.0–4.4 months), the difference was not statistically significant (p = .968).
Discussion
In the current study, 11 patients with advanced HCC developed symptomatic brain metastasis during or after antiangiogenic therapy. No such case series have ever been reported for this unique patient group. This report preliminarily assesses the significance of brain metastasis in advanced HCC patients undergoing antiangiogenic targeted therapy.
The incidence of brain metastasis from HCC was higher in our study (7%, 11 of 158 patients) than in previously reported studies (0.2%–2.2%) [1–4]. One reason for such a significant difference is potential patient selection bias. Previous studies enrolled patients at all stages of HCC, but the current study enrolled only patients in advanced stages. However, the greater longevity of patients with advanced stage disease resulting from these targeted agents may also contribute to a higher risk for brain metastasis. The median time to brain metastasis of these 11 patients in our report was 9.6 months. The median OS time was 12.4 months, which is markedly longer than the 2- to 4-months OS time seen in the historical control data for advanced HCC patients diagnosed in the Asia-Pacific region and treated with supportive care [8]. Similarly, with the greater number of active systemic therapies including molecularly targeted therapy, the incidence of brain metastasis was found to have increased in patients with breast, lung, and colorectal cancer over the past decade [16–20]. Notably, all 11 patients who eventually developed symptomatic brain metastasis had extrahepatic involvement at the time of diagnosis of advanced HCC. Moreover, lung metastasis was present in 91% of these patients, which is compatible with another report [4]. This may imply that the tumors of these patients with brain metastasis had very high metastatic potential. On the other hand, two recent animal studies pointed out that antiangiogenic therapy might elicit tumor invasiveness and accelerate distant metastasis despite control of the primary tumor [21, 22]. Importantly, this metastasis-accelerating phenotype tended to occur in mice treated with short-term antiangiogenic therapy, rather than in those treated with sustained treatment [22]. In our cohort, six of the 11 (55%) patients who developed brain metastasis later had certain periods of treatment delay during antiangiogenic therapy. In contrast, 44% of patients who had no brain metastasis had a treatment delay. Whether or not the discontinuous dosing of antiangiogenic therapy potentiates the invasiveness and metastasis of human HCC warrants further investigation.
The median OS time after brain metastasis development was obviously longer for our patients (4.6 months) than for the historical control (3–7 weeks) [4–7]. Close and regular follow-up of our patients, who had been enrolled into prospective clinical studies, facilitates early diagnosis and prompt treatment. All but one patient received therapy for the brain metastasis, and four patients had brain tumor excision followed by WBRT. Our analysis, though it included only a limited number of patients, showed that surgical treatment for brain metastasis tended to prolong survival. Our data support the desirability of aggressive intervention for patients in good general condition who have low tumor volume in order to improve their neurologic recovery and quality of life, and prolong their survival.
In conclusion, in the era of antiangiogenic therapy, brain metastasis of advanced HCC is not as rare as previously reported. Aggressive treatment and supportive care for such patients may improve their outcome.
Acknowledgment
This work was supported by grants DOH98-TD-B-111-001 and DOH99-TD-B-111-001.
Author Contributions
Conception/Design: Chih-Hung Hsu, Yu-Yun Shao
Provision of study material or patients: Chih-Hung Hsu, Ann-Lii Cheng
Collection and/or assembly of data: Yu-Yun Shao, Li-Chun Lu
Data analysis and interpretation: Yu-Yun Shao
Manuscript writing: Yu-Yun Shao
Final approval of manuscript: Chih-Hung Hsu
References
- 1.Murakami K, Nawano S, Moriyama N, et al. Intracranial metastases of hepatocellular carcinoma: CT and MRI. Neuroradiology. 1996;38(suppl 1):S31–S35. doi: 10.1007/BF02278115. [DOI] [PubMed] [Google Scholar]
- 2.Kim M, Na DL, Park SH, et al. Nervous system involvement by metastatic hepatocellular carcinoma. J Neurooncol. 1998;36:85–90. doi: 10.1023/a:1005716408970. [DOI] [PubMed] [Google Scholar]
- 3.Chen SF, Tsai NW, Lui CC, et al. Hepatocellular carcinoma presenting as nervous system involvement. Eur J Neurol. 2007;14:408–412. doi: 10.1111/j.1468-1331.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- 4.Choi HJ, Cho BC, Sohn JH, et al. Brain metastases from hepatocellular carcinoma: Prognostic factors and outcome: Brain metastasis from HCC. J Neurooncol. 2009;91:307–313. doi: 10.1007/s11060-008-9713-3. [DOI] [PubMed] [Google Scholar]
- 5.Chang L, Chen YL, Kao MC. Intracranial metastasis of hepatocellular carcinoma: Review of 45 cases. Surg Neurol. 2004;62:172–177. doi: 10.1016/j.surneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh MJ, Lu CH, Tsai NW, et al. Prediction, clinical characteristics and prognosis of intracerebral hemorrhage in hepatocellular carcinoma patients with intracerebral metastasis. J Clin Neurosci. 2009;16:394–398. doi: 10.1016/j.jocn.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Yen FS, Wu JC, Lai CR, et al. Clinical and radiological pictures of hepatocellular carcinoma with intracranial metastasis. J Gastroenterol Hepatol. 1995;10:413–418. doi: 10.1111/j.1440-1746.1995.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 10.Zhu AX. Beyond sorafenib: Novel targeted therapies for advanced hepatocellular carcinoma. Expert Opin Investig Drugs. 2010;19:663–672. doi: 10.1517/13543781003767426. [DOI] [PubMed] [Google Scholar]
- 11.Hsu C, Chang D, Lin Z, et al. Thalidomide plus tegafur/uracil for the treatment of advanced/metastatic hepatocellular carcinoma (HCC): A phase II single-arm study. J Clin Oncol. 2008;26(15 suppl):15598. [Google Scholar]
- 12.Hsu CH, Shen YC, Lin ZZ, et al. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol. 2010;53:126–131. doi: 10.1016/j.jhep.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CH, Yang TS, Hsu C, et al. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;102:981–986. doi: 10.1038/sj.bjc.6605580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao YY, Lin ZZ, Hsu C, et al. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer. 2010;116:4590–4596. doi: 10.1002/cncr.25257. [DOI] [PubMed] [Google Scholar]
- 15.Hsu C, Kang Y, Yang T, et al. A phase II study of bevacizumab (B) and erlotinib (E) in combination for Asian patients (pts) with advanced/meta-static hepatocellular carcinoma (HCC): An interim safety report. J Clin Oncol. 2009;27(15 suppl):4585. [Google Scholar]
- 16.Clayton AJ, Danson S, Jolly S, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91:639–643. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundermeyer ML, Meropol NJ, Rogatko A, et al. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer. 2005;5:108–113. doi: 10.3816/ccc.2005.n.022. [DOI] [PubMed] [Google Scholar]
- 18.Weil RJ, Palmieri DC, Bronder JL, et al. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167:913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen AM, Jahan TM, Jablons DM, et al. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced nonsmall-cell lung cancer: Clinical implications for the subsequent management of the brain. Cancer. 2007;109:1668–1675. doi: 10.1002/cncr.22565. [DOI] [PubMed] [Google Scholar]
- 20.Ricciardi S, de Marinis F. Multimodality management of non-small cell lung cancer patients with brain metastases. Curr Opin Oncol. 2010;22:86–93. doi: 10.1097/CCO.0b013e3283350106. [DOI] [PubMed] [Google Scholar]
- 21.Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]