Bisphosphonate-associated osteonecrosis of the jaw is a growing clinical concern that affects treatment decisions because of its potential negative impact on quality of life. It significantly affects the quality of life of patients with cancer and may be an important consideration for intravenous and oral bisphosphonate treatment decisions by patients, clinicians, and policy makers.
Keywords: Quality of life, Osteonecrosis, Bisphosphonates, Zoledronic acid, Pamidronate, Utility
Abstract
Purpose.
Potentially debilitating, osteonecrosis of the jaw (ONJ) is an emerging complication of bisphosphonates. However, its effect on quality of life (QoL) is unknown. We determined the ONJ-related QoL decline in a cancer patient cohort.
Patients and Methods.
Thirty-four cancer patients with bisphosphonate-associated ONJ completed a telephone survey (October 2007 through May 2008). The Oral Health Impact Profile 14 (OHIP) retrospectively assessed participant oral health–related QoL before and after ONJ. Standardized ONJ descriptions were developed in a multidisciplinary, iterative process and were evaluated with three frequently used preference-based QoL measurement methods on a 0 (death) to 1 (perfect health) scale: Visual Analogue Scale (VAS), Time Trade-Off (TTO), and EQ-5D.
Results.
ONJ significantly (p < .001) increased OHIP scores (worse QoL) for additive (3.56–16.53) and weighted (7.0–17.5) methods. Seven individual OHIP items significantly increased (Bonferroni correction p < .0035): pain, eating discomfort, self-consciousness, unsatisfactory diet, interrupted meals, irritability, and decreased life satisfaction. Mean preference-based QoL values significantly decreased (p < .001) with worsening ONJ stage (VAS, TTO, and EQ-5D): no ONJ (0.76, 0.86, 0.82), ONJ stage 1 (0.69, 0.82, 0.78), ONJ stage 2 (0.51, 0.67, 0.55), and ONJ stage 3 (0.37, 0.61, 0.32). As ONJ worsened, EQ-5D domain scores significantly increased (p < .001). Pain/discomfort and anxiety/depression contributed most to declining QoL.
Conclusions.
ONJ significantly affects QoL, a detriment that increases with worsening ONJ. QoL impairments for ONJ stages 2 and 3 are similar to other treatment side effects that influence decision-making. Bisphosphonate-associated ONJ QoL is an important consideration for patients, clinicians, and policy makers.
Introduction
Bisphosphonate-associated osteonecrosis of the jaw (ONJ) is a growing clinical concern that affects treatment decisions because of its potential negative impact on quality of life (QoL) [1–9]. A potentially painful and debilitating condition and the subject of ongoing litigations, [10–12] ONJ is defined as exposed necrotic maxillofacial bone with complications ranging from pain to fractures [9, 12–24]. The reported risk of ONJ in cancer patients treated with intravenous bisphosphonates varies [25]: frequency estimates range from 0.7% [13] to 12% [18] and cumulative risk estimates range from a 4.8% cumulative incidence for a cohort of breast cancer patients treated for >5 years with intravenous bisphosphonates [13] to a 40% cumulative risk at 36 months for a cohort of multiple myeloma patients [25, 26]. Although less common, ONJ has been reported with oral bisphosphonates for osteoporosis [27–35] and is a priority Food and Drug Administration Targeted Post-Marketing Surveillance complication [36]. Furthermore, the cancer patients at risk for ONJ is sizeable: in 2004, >3 million individuals worldwide and one third of Americans with advanced breast cancer had received intravenous bisphosphonates such as zoledronic acid and pamidronate [37]. In 2009, sales of zoledronic acid reached nearly $1.5 billion [38]. Additionally, five recent trials may expand the role of intravenous bisphosphonates beyond advanced cancers to patients potentially cured from cancer [39–43]. Intravenous bisphosphonates improve QoL [44] by decreasing skeletal-related events (SREs) (fracture, spinal cord compression, need for radiation or surgery, and hypercalcemia) in cancer patients with bone involvement (breast or prostate cancer metastatic to the bone and multiple myeloma) [43, 45–47]. In contrast, ONJ may decrease QoL because of infected and painful necrotic jaw bone; ulcerated, painful, and swollen oral mucosa; chronic sinus tracts and facial disfigurement; impaired speech, swallowing, and eating; and/or frequent medical and dental evaluations and treatments [48–51]. Therefore, the population at risk for ONJ is large and expanding, and the public health implications may be substantial.
Despite the extent of ONJ complications and evidence that oral health QoL affects overall QoL [52–59], the effect of ONJ on QoL has not been determined [43–47]. A search of PubMED and a QoL publication registry [60] identified only one oral health cancer therapy complication QoL study that used methods suitable for comprehensive economic evaluation [61]. However, this prior study evaluated short-term (7 day) stomatitis rather than a serious long-term complication such as ONJ, which is needed for full assessment of bisphosphonate benefit-risk trade-offs.
Accurate ONJ QoL data are needed to inform bisphosphonate treatment decision-making: patients and physicians must determine whether potential negative ONJ-related QoL effects outweigh the potential benefit of bisphosphonates while policy makers need to know the magnitude of ONJ-related QoL decline to evaluate the comparative value of bisphosphonates [62–65]. If the negative QoL impact of ONJ is substantially large, the favorable comparative value recently attributed to intravenous bisphosphonates may be reduced or, according to Hillner et al., may be reversed [66, 67]. In addition, ONJ QoL findings may be important for other drugs such as denosumab (a fully human monoclonal antibody against receptor activator of nuclear factor κB [RANK] ligand) that may be superior to zoledronic acid in delaying or preventing SREs but may also have a higher incidence of ONJ [68].
The primary objective of this study was to determine the QoL impact of bisphosphonate-associated ONJ in cancer patients. Our multidisciplinary team (oncology, oral medicine and surgery, QoL research, and psychiatry) developed a telephone survey to test whether oral health–specific and preference-based instruments capture the QoL effects of oral health complications of cancer therapy and whether the QoL impact of ONJ increases with ONJ disease severity.
Methods
Participant Recruitment and Eligibility
With Institutional Review Board approval, a cohort of cancer patients with ONJ was identified at two institutions by oral medicine (S.W. and N.T.) and oral/maxillofacial surgery (T.D. and M.A.) collaborators. Medical charts were reviewed to determine eligibility: (1) cancer diagnosis; (2) ONJ diagnosis [69]; (3) bisphosphonate exposure; (4) no radiation to head or neck; and (5) ability to complete English-language telephone survey.
Unless the primary oncologist declined participation on behalf of their patient, potential participants were mailed an introductory letter, information about declining participation, and a paper copy of the telephone survey questions. One week later, participants were called to request a telephone survey interview. Consent was implied by scheduling the interview.
Participants were encouraged to review the paper copy before the telephone interview. The telephone survey was administered by a single researcher (O.A.) using a standardized script and Microsoft Visual Basic cues. Health state descriptions were verbally reviewed prior to relevant survey sections. Participant responses were recorded in an Excel database by the interviewer. At survey completion, participants were offered a $20 gift card. The survey was not changed based on pilot results and pilot participants (n = 5) were included in final analysis [70].
In addition to collecting demographic and clinical information, the survey consisted of four instruments: Oral Health Impact Profile and three widely used preference-based QoL methods adapted for the study [71]: Visual Analogue Scale, [71–73], Time Trade-Off [74–77], and EQ-5D [72, 78–80].
Oral Health Impact Profile
A validated psychometric instrument with face, criterion, convergent, and construct validity that assesses seven oral health–specific QoL dimensions, the Oral Health Impact Profile 14 (OHIP-14) was used to assess participant oral health QoL [81–86]. Participants were asked to recall a typical week before and after they developed ONJ (any stage) to rank on a five-point Likert scale the average number of days per week each OHIP item occurred. Pre- and post-ONJ results were summed for the OHIP-Additive Score (ADD) [81–83]. Published item weights were used to calculate the OHIP-Weighted Score (WS) [81–83].
Preference-Based QoL Assessment
To compare each ONJ stage with other health states, participants evaluated four standardized ONJ health states with three preference-based instruments that quantitatively measure QoL: VAS, TTO, and EQ-5D. These QoL values are reported on a 0 (death) to 1 (perfect health) scale and can be used as utilities to produce quality-adjusted life years (QALYs) estimates, as is commonly done in the literature [71, 73, 79, 87, 88]. Because utilities allow accurate QoL comparisons between individuals and across diseases, they are necessary to assess the comparative value of health care interventions [89, 90].
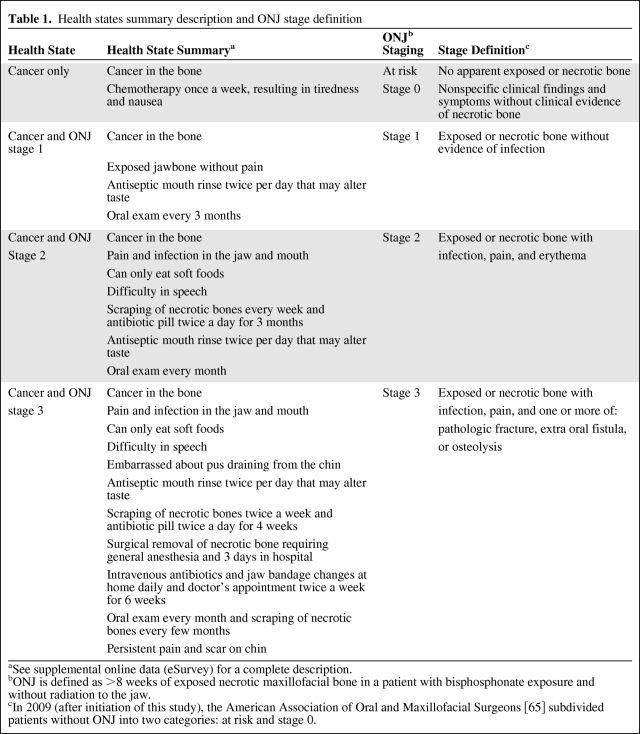
Standardized health states were developed to minimize bias from variations in participant experience (not all patients have all ONJ stages) and to ensure validity of comparisons. Iteratively developed following published recommendations [89], health state descriptions were drawn from ONJ diagnostic criteria [69], literature review/case analysis [91], and multidisciplinary expertise. On the basis of testing with clinical and nonclinical colleagues, written descriptions were used instead of pictures. Each health state depicted the same hypothetical, gender-neutral patient with an unnamed cancer with bone involvement (“cancer in the bone”) and one ONJ health state: no ONJ, ONJ stage 1, ONJ stage 2, or ONJ stage 3 (Table 1 and supplemental online Appendix pages 5–8).
Table 1.
Health states summary description and ONJ stage definition
aSee supplemental online data (eSurvey) for a complete description.
bONJ is defined as >8 weeks of exposed necrotic maxillofacial bone in a patient with bisphosphonate exposure and without radiation to the jaw.
cIn 2009 (after initiation of this study), the American Association of Oral and Maxillofacial Surgeons [65] subdivided patients without ONJ into two categories: at risk and stage 0.
Visual Analogue Scale (VAS)
The VAS familiarizes participants with the task of scoring and ordering standardized health states according to perceived QoL [72]. On the paper copy of the telephone survey, the VAS was represented by a vertical line marked “100 (Perfect Health)” at the top and “0 (Death)” at the bottom (adapted from the EQ-5D [72], supplemental online Appendix page 9). Color-coded health state summaries and removable, self-adhesive arrows were provided to assist ranking during the telephone interview. Final VAS scores for each standardized health state were recorded during the interview and rescaled to the 0 to 1 QoL scale by dividing by 100.
Time Trade-Off (TTO)
Generally accepted as an alternative to the standard gamble, the TTO method identifies the indifference point (X) at which the respondent believes that a longer amount of time in a less desirable health state is equivalent to a shorter amount of time in a more desirable health state such as perfect health [74, 76]. A two-stage approach was used to isolate the QoL impact of ONJ (supplemental online Fig. 1 and Appendix pages 10 and 11) [75, 77].
Participants initially compared a set life span (48 months) in the standardized cancer in the bone without ONJ health state to varying amounts of time in perfect health (QoL = 1). For example, if 48 months of perfect health was preferred over 48 months with cancer, the scenario was varied to the opposite extreme: 1 month in perfect health versus 48 months with cancer. If both choices were equivalent, the indifference point (X) was 1 month. Otherwise, the time in perfect health was varied until the participant considered X time in perfect health equivalent to 48 months with the standardized cancer without ONJ health state. This indifference point value (X) was divided by 48 months to obtain the QoL of cancer without ONJ on a zero-to-one scale.
With use of a similar pattern of questions, participants then compared a set life span (48 months) with cancer in the bone with ONJ to varying amounts of time with cancer in the bone without ONJ. The participant's indifference point was divided by 48 months and converted from a death-to-cancer scale to a 0 (death) to 1 (perfect heath) QoL scale by re-scaling proportionally with the QoL value of cancer without ONJ reported in the first TTO exercise. The resulting value is the QoL of the standardized patient with cancer and ONJ.
To identify the key clinical components affecting the QoL impact of ONJ, each ONJ stage was evaluated separately. A hypothetical life span of 48 months was chosen as the average survival for most cancers expected in the cohort [92, 93].
EQ-5D
A well-validated preference-based QoL instrument, the EQ-5D evaluates five QoL domains: mobility, self-care, activities, pain/discomfort, and anxiety/depression [72]. Participants rated the level of domain dysfunction (no problems, some problems, or extreme problems) for each standardized ONJ description (supplemental online Appendix page 12). Responses were converted to a 0 to 1 QoL scale using published U.S. societal weights [79, 80].
QoL Decrement
The amount of QoL lost because of ONJ was calculated using the mean across respondents. For each ONJ stage and method, the mean QoL value was divided by the mean QoL value of cancer without ONJ and the result subtracted from 1 [94].
Emotional Discomfort
Participant emotional discomfort was formally evaluated by the interviewer using a post-survey emotional assessment approach inspired by prior studies [95]. Participants indicating discomfort were immediately offered psychiatry referral (G.M.). Emotional discomfort results were reviewed (R.M.) after the pilot study (n = 5) and then after every 10 interviews to assess whether an early stopping rule was met: ≥50% of participants indicating greater than or equal to moderate emotional upset.
Statistical Analysis
The participants' own pre- and post-ONJ (any stage) scores for each OHIP item and the composite total score were compared across respondents using the nonparametric Wilcoxon signed rank test for paired data. To account for multiple comparisons, the p-value threshold was modified with Bonferroni adjustment (α = 0.05/14 = 0.0035). For overall QoL measurements, the significance of differences between ONJ stages and between instruments was tested with repeated measure analysis of variance.
All statistical analyses were performed using SAS [96]. Strengthening the Reporting of Observational Studies in Epidemiology guidelines were followed [97].
Results
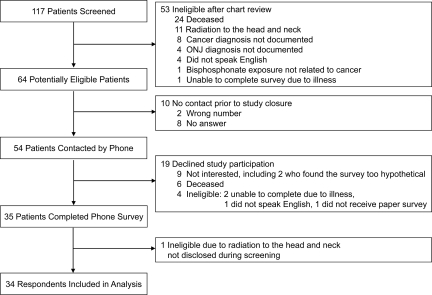
The medical records of 117 patients were reviewed from October 15, 2007, to May 2, 2008, and 64 were eligible. No patients were excluded by the primary oncologist. Telephone contact was established with 84.4% (54 of 64) of potential participants, and 35 (64.8%) completed the survey (overall response rate, 54.7%). Contact was not achieved for 10, largely because of no answer to the telephone call (8). The primary reasons for nonparticipation were not interested (47%) and deceased (32%). One respondent was ineligible because of undisclosed radiation. Data for 34 participants were included in the final analysis (Fig. 1).
Figure 1.
Enrollment of patients.
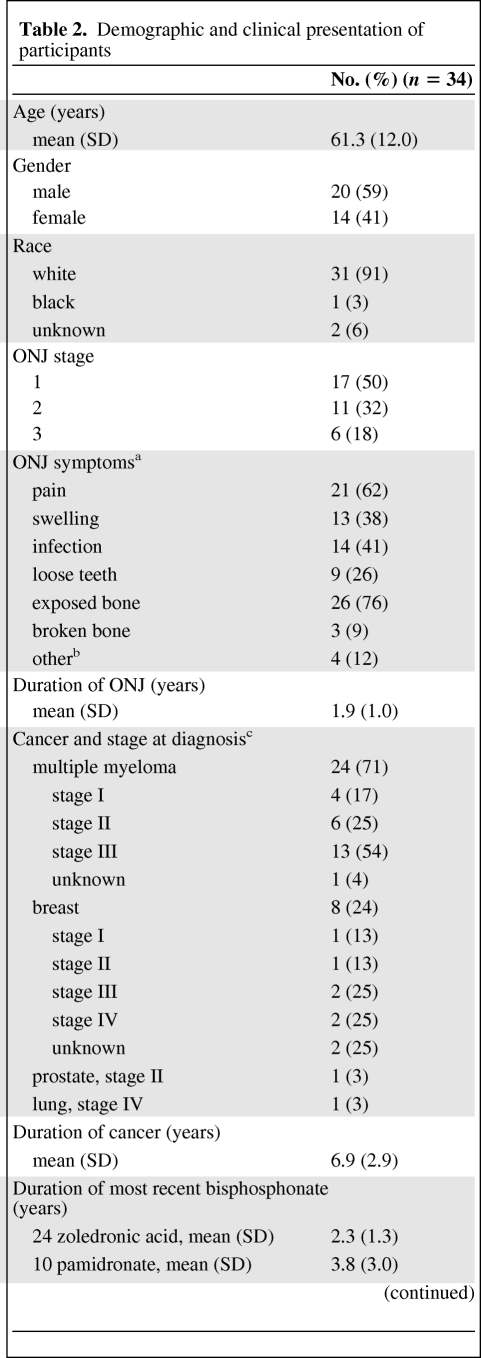
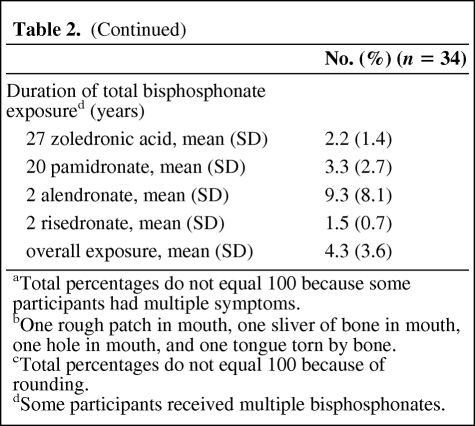
Mean participant age was 61.3 years and cancer diagnoses were multiple myeloma [24], breast cancer (8), prostate cancer (1), and lung cancer (1) (Table 2). There were slightly more men (59%) and the majority of subjects were white (91%). The most common bisphosphonate received immediately prior to ONJ diagnosis was zoledronic acid (71%) (mean exposure, 2.3 years). With overall mean bisphosphonate exposure of 4.3 years, participants had received both intravenous (zoledronic acid [79%] and pamidronate [59%]) and oral (alendronate [6%] and risedronate [6%]) bisphosphonates. At the time of study, participants had been diagnosed with ONJ for a mean of 1.9 years (0.3–3.9 years), and most had stage 1 (50%) or stage 2 (32%) ONJ. The most common self-reported ONJ symptoms were exposed bone (76%), pain (62%), and infection (41%).
Table 2.
Demographic and clinical presentation of participants
Table 2.
(Continued)
aTotal percentages do not equal 100 because some participants had multiple symptoms.
bOne rough patch in mouth, one sliver of bone in mouth, one hole in mouth, and one tongue torn by bone.
cTotal percentages do not equal 100 because of rounding.
dSome participants received multiple bisphosphonates.
OHIP
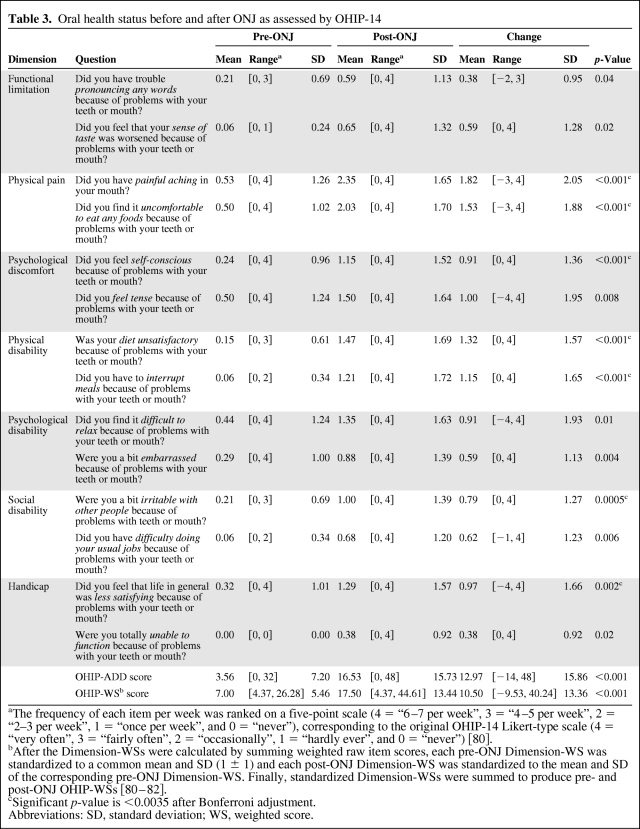
In retrospective assessment of participant oral health, the mean frequency of all OHIP items increased after ONJ diagnosis, indicating worse QoL. This change was statistically significant after Bonferroni adjustment (p < .0035) for seven items: painful aching (+1.82), discomfort eating (+1.53), self-consciousness (+0.91), unsatisfactory diet (+1.32), interrupted meals (+1.15), irritability (+0.79), and decreased life satisfaction (+0.97) (Table 3). Composite OHIP scores significantly increased (p < .001) after ONJ (from 3.56 to 16.53, OHIP-ADD; from 7.00 to 17.50, OHIP-WS).
Table 3.
Oral health status before and after ONJ as assessed by OHIP-14
aThe frequency of each item per week was ranked on a five-point scale (4 = “6–7 per week”, 3 = “4–5 per week”, 2 = “2–3 per week”, 1 = “once per week”, and 0 = “never”), corresponding to the original OHIP-14 Likert-type scale (4 = “very often”, 3 = “fairly often”, 2 = “occasionally”, 1 = “hardly ever”, and 0 = “never”) [80].
bAfter the Dimension-WSs were calculated by summing weighted raw item scores, each pre-ONJ Dimension-WS was standardized to a common mean and SD (1 ± 1) and each post-ONJ Dimension-WS was standardized to the mean and SD of the corresponding pre-ONJ Dimension-WS. Finally, standardized Dimension-WSs were summed to produce pre- and post-ONJ OHIP-WSs [80–82].
cSignificant p-value is <0.0035 after Bonferroni adjustment.
Abbreviations: SD, standard deviation; WS, weighted score.
Preference-Based QoL
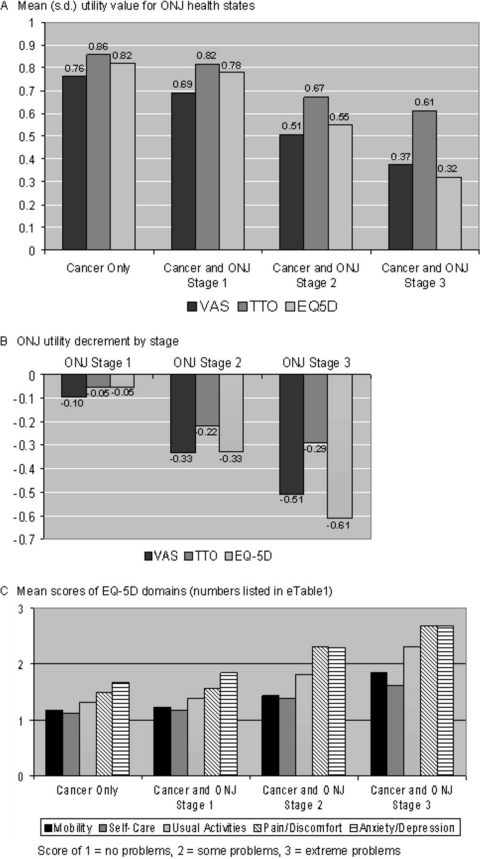
The mean QoL across respondents significantly decreased as ONJ severity increased (p < .001, for each evaluation method) (Fig. 2A). On the 0 (death) to 1 (perfect heath) QoL scale, mean QoL was highest (VAS, TTO, and EQ-5D, respectively) for cancer without ONJ (0.76, 0.86, 0.82) and lowest for ONJ stage 3 (0.37, 0.61, 0.32). The largest QoL change (VAS, EQ-5D, and TTO, respectively) occurred between ONJ stage 1 (0.69, 0.82, 0.78) and ONJ stage 2 (0.51, 0.67, 0.55).
Figure 2.
Preference-based QoL assessment. (A): Mean (SD) utility value for ONJ health states. (B): ONJ utility decrement by stage. (C): Mean scores of EQ-5D domains (numbers listed in supplemental online Table 1). Abbreviations: ONJ, osteonecrosis of the jaw; QoL, quality of life; TTO, Time Trade-off; VAS, Visual Analogue Scale.
On the 0 to 1 scale, mean QoL lost because of ONJ stage 3 was 0.51, 0.29, and 0.61 (VAS, TTO, and EQ-5D, respectively). The mean of these values (0.47) was similar to the QoL decrement from hip and vertebral fractures in women with osteoporosis (0.53) [98] (Fig. 2B). The mean QoL lost because of ONJ stage 2 was 0.33, 0.22, 0.33 (VAS, TTO, and EQ-5D, respectively). The mean of these values (0.29) was similar to the QoL decrement from urinary incontinence and bowel problems in prostate cancer patients (0.30) [99].
Each EQ-5D domain score significantly increased (lower QoL) as ONJ severity increased (p < .001, Fig. 2C). Domains that contributed most to declining QoL were pain/discomfort and anxiety/depression.
Consistent with published literature [78], VAS values were generally lower than TTO values, and differences among the three instruments was significant by health state (p < .005). However, contrast (pairwise) comparisons showed that TTO and EQ-5D QoL values were not significantly different for cancer without ONJ and with ONJ stage 1, whereas VAS and EQ-5D were not significantly different for cancer with ONJ stages 2 and 3.
Emotional Discomfort
One patient (3%) found the survey “moderately” upsetting and 9 (26%) were “a little” upset. However, after survey completion only 6 (18%) still felt “a little” emotionally upset. All declined psychiatry referral. Early stopping rules were not met.
Discussion
This study provides the first empirical evidence that bisphosphonate-associated ONJ significantly impairs QoL, the effect increasing with ONJ stage severity. ONJ adversely affects a wide range of oral health–specific and overall QoL domains, as supported by the concordance of results for three preference-based QoL methods and psychometric oral health–specific data. The magnitude of the negative QoL effects of ONJ stages 2 and 3 are equivalent to other cancer treatment side effects that influence treatment decisions [99–101]. Similarly, ONJ QoL may be an important factor for patients and their physicians considering bisphosphonate therapy.
To our knowledge, this is the first evaluation of the QoL effects of long-term oral health complications of cancer treatments using methods suitable for comprehensive economic evaluation. All QoL instruments were sensitive to QoL differences by ONJ stage, and the ordinal relationship between QoL values elicited by each instrument followed published patterns [71, 76, 78]. The QoL values also corresponded with psychometric (OHIP) and EQ-5D domain results. Finally, the response rate was consistent with prior studies and respondent fatigue was minimal. These findings of sensitivity, practicality, face, construct, and convergent validity [102] support the use of preference-based QoL instruments to assess oral health complications.
The QoL implications of ONJ may be increasingly important if oral bisphosphonates also cause ONJ and recent studies expand the indication for intravenous bisphosphonates to patients with early breast cancer and other conditions [40–42, 103, 104]. Because ONJ is difficult to cure, otherwise healthy osteoporosis patients and cancer survivors may suffer long-term reduced QoL from ONJ. QoL information may, therefore, improve bisphosphonate treatment decision-making.
The potential mechanisms by which bisphosphonates induce ONJ remain unclear, hampering treatment and prevention [105–107]. In addition, the bone half-life of bisphosphonates may be as long as 10 years [108], the ONJ incidence is unpredictable [9, 12–24, 109, 110], and bisphosphonate “drug holidays” may not be medically appropriate for some cancer patients [9, 12–24, 109, 110]. Preventative dentistry may decrease ONJ incidence, but it unfortunately does not eliminate the risk [111, 112]. Our findings support early detection and treatment approaches that reduce ONJ severity to improve QoL and potentially allow continued bisphosphonate therapy.
The results of our study must be considered in the context of the limitations. For resource allocation decisions, some economists prefer societal preferences. However, feedback during survey development demonstrated that visual depictions necessary to educate nonpatients were considered graphic and biasing. For “at risk” patients, ethical concerns were raised about disclosing potential complications outside of a patient-clinician relationship. Moreover, patients experienced with at least one ONJ stage may be better able to distinguish QoL differences between standardized descriptions of all ONJ stages. Finally, prior work suggests cancer patients and volunteers give similar QoL values and any discrepancies favor conservative allocation decisions [113]. Therefore, we believe the patient cohort knowledge increases accuracy of stage-specific QoL values, minimizes ethical concerns, and avoids biases from visual depictions. This choice is supported by consistency between our EQ-5D societal preference results and other study findings.
Logistical considerations (short life expectancy of target population and relative patient scarcity) constrained the sample size, prevented testing/retesting, and may limit generalizability. However, our cohort was drawn from two institutions, the overall response rate (54.7%) was consistent with other telephone surveys [114], and time to ONJ development was similar to other studies [115]. Finally, study findings are statistically significant after correction for multiple comparisons and are clinically compelling.
The survey was designed to minimize cognitive burden: personal experience was assessed first, and standardized health states were presented with the most familiar state (cancer in the bone without ONJ) first. To familiarize participants with rank ordering, health states were first evaluated with VAS. The most complex task (TTO) was placed mid-survey to minimize fatigue. Although ordering effects were not directly tested, ordering did not appear to affect results because (1) baseline state (cancer in the bone without ONJ) results were similar to the literature [116–118], (2) data were well distributed (no floor/ceiling effect), and (3) VAS, TTO, and EQ-5D results were similar.
In summary, our findings suggest ONJ significantly affects the QoL of patients with cancer and may be an important consideration for intravenous and oral bisphosphonate treatment decisions by patients, clinicians, and policy makers. With the pending introduction of denosumab into routine clinical practice and the increasing longevity of cancer survivors, ONJ and its impact on QoL may become more frequently encountered issues. As the first assessment of long-term oral health complications of cancer therapy with findings validated by three QoL instruments and oral health–specific psychometric data, this work also serves as a benchmark. Additional studies are needed to further elucidate the relationship of ONJ pathophysiology and QoL and to establish the comparative value of bisphosphonates and other drugs, such as denosumab, used to prevent SREs.
Supplementary Material
Acknowledgments
R. Miksad was supported by the following grants during the conduct of this research: American Society of Clinical Oncology Young Investigator Award, Dana-Farber Harvard Cancer Center Timely Opportunity Award, the Program in Cancer Outcomes Research Training (5R25CA 092203-04) and the Department of Medicine, Beth Israel Deaconess Medical Center, and the Clinical Investigator Training Program: Beth Israel Deaconess Medical Center–Harvard/MIT Health Sciences and Technology, in collaboration with Pfizer Inc. and Merck & Co. Funding sources had no role in study design, data collection, data analysis, data interpretation, writing of this manuscript, or the decision to submit for publication.
Pilot study results were presented at the Annual International Meeting of the International Society for Pharmacoeconomics, Toronto, May 2008, and the American Society of Clinical Oncology Annual Meeting, Chicago, June 2008.
R. Miksad, K.C. Lai, and J.S. Swan had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions
Conception/Design: Rebecca Anne Miksad, Thomas Benton Dodson, Sook-Bin Woo, Nathaniel Simon Treister, Omosalewa Akinyemi, Marian Bihrle, Guy Maytal, Meredith August, G. Scott Gazelle, J. Shannon Swan
Financial support: Rebecca Anne Miksad, G. Scott Gazelle, J. Shannon Swan
Provision of study material or patients: Thomas Benton Dodson, Sook-Bin Woo, Nathaniel Simon Treister, Meredith August
Collection and/or assembly of data: Rebecca Anne Miksad, Omosalewa Akinyemi, Marian Bihrle
Data analysis and interpretation: Rebecca Anne Miksad, Kuan-Chi Lai, G. Scott Gazelle, J. Shannon Swan
Manuscript writing: Rebecca Anne Miksad, Kuan-Chi Lai, J. Shannon Swan
Final approval of manuscript: Rebecca Anne Miksad, Kuan-Chi Lai, Thomas Benton Dodson, Sook-Bin Woo, Nathaniel Simon Treister, Omosalewa Akinyemi, Marian Bihrle, Guy Maytal, Meredith August, G. Scott Gazelle, J. Shannon Swan
References
- 1.Gutta R, Louis PJ. Bisphosphonates and osteonecrosis of the jaws: science and rationale. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(2):186–193. doi: 10.1016/j.tripleo.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Hewitt C, Farah CS. Bisphosphonate-related osteonecrosis of the jaws: a comprehensive review. J Oral Pathol Med. 2007;36(6):319–328. doi: 10.1111/j.1600-0714.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- 3.Lam DK, Sandor GK, Holmes HI, et al. A review of bisphosphonate-associated osteonecrosis of the jaws and its management. J Can Dent Assoc. 2007;73(5):417–422. [PubMed] [Google Scholar]
- 4.Mariotti A. Bisphosphonates and osteonecrosis of the jaws. J Dent Educ. 2008;72(8):919–929. [PubMed] [Google Scholar]
- 5.Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol. 2006;7(6):508–514. doi: 10.1016/S1470-2045(06)70726-4. [DOI] [PubMed] [Google Scholar]
- 6.Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaws. Compend Contin Educ Dent. 2008;29(2):96, 98, 100–102, 104–105. [PubMed] [Google Scholar]
- 7.Ruggiero SL, Fantasia J, Carlson E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(4):433–441. doi: 10.1016/j.tripleo.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144(10):753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson GS, Kuo YF, Freeman JL, et al. Intravenous bisphosphonate therapy and inflammatory conditions or surgery of the jaw: a population-based analysis. J Natl Cancer Inst. 2007;99(13):1016–1024. doi: 10.1093/jnci/djm025. [DOI] [PubMed] [Google Scholar]
- 10. In re Fosamax Products Liability Litigation (No. 1:06-MD-1789, S.D.N.Y.)
- 11. In re Aredia and Zometa Products Liability Litigation (No. 3:06-MD-7160, M.D. Tenn.)
- 12.Barker K, Lowe D, Olujohungbe A, et al. Survey of members of myeloma U.K. on biphosphonates-associated jaw osteonecrosis. Br J Haematol. 2007;139(4):626–628. doi: 10.1111/j.1365-2141.2007.06730.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23(6):826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang EP, Kaban LB, Strewler GJ, et al. Incidence of osteonecrosis of the jaw in patients with multiple myeloma and breast or prostate cancer on intravenous bisphosphonate therapy. J Oral Maxillofac Surg. 2007;65(7):1328–1331. doi: 10.1016/j.joms.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353(1):99–102. doi: 10.1056/NEJM200507073530120. discussion 199–102. [DOI] [PubMed] [Google Scholar]
- 16.Zervas K, Verrou E, Teleioudis Z, et al. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single-centre experience in 303 patients. Br J Haematol. 2006;134(6):620–623. doi: 10.1111/j.1365-2141.2006.06230.x. [DOI] [PubMed] [Google Scholar]
- 17.Boonyapakorn T, Schirmer I, Reichart PA, et al. Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol. 2008;44(9):857–869. doi: 10.1016/j.oraloncology.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Ortega C, Montemurro F, Faggiuolo R, et al. Osteonecrosis of the jaw in prostate cancer patients with bone metastases treated with zoledronate: a retrospective analysis. Acta Oncol. 2007;46(5):664–668. doi: 10.1080/02841860601185917. [DOI] [PubMed] [Google Scholar]
- 19.Fehm T, Beck V, Banys M, et al. Bisphosphonate-induced osteonecrosis of the jaw (ONJ): Incidence and risk factors in patients with breast cancer and gynecological malignancies. Gynecol Oncol. 2009;112(3):605–609. doi: 10.1016/j.ygyno.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Jadu F, Lee L, Pharoah M, et al. A retrospective study assessing the incidence, risk factors and comorbidities of pamidronate-related necrosis of the jaws in multiple myeloma patients. Ann Oncol. 2007;18(12):2015–2019. doi: 10.1093/annonc/mdm370. [DOI] [PubMed] [Google Scholar]
- 21.Walter C, Grotz KA, Kunkel M, et al. Prevalence of bisphosphonate associated osteonecrosis of the jaw within the field of osteonecrosis. Support Care Cancer. 2007;15(2):197–202. doi: 10.1007/s00520-006-0120-z. [DOI] [PubMed] [Google Scholar]
- 22.Mavrokokki T, Cheng A, Stein B, et al. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007;65(3):415–423. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim T, Barbanti F, Giorgio-Marrano G, et al. Osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: a retrospective study. The Oncologist. 2008;13(3):330–336. doi: 10.1634/theoncologist.2007-0159. [DOI] [PubMed] [Google Scholar]
- 24.Murad OM, Arora S, Farag AF, et al. Bisphosphonates and osteonecrosis of the jaw: a retrospective study. Endocr Pract. 2007;13(3):232–238. doi: 10.4158/EP.13.3.232. [DOI] [PubMed] [Google Scholar]
- 25.Dodson TB. Intravenous bisphosphonate therapy and bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(5 Suppl):44–52. doi: 10.1016/j.joms.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Tosi P, Zamagni E, Cangini D, et al. Osteonecrosis of the jaws in newly diagnosed multiple myeloma patients treated with zoledronic acid and thalidomide-dexamethasone. Blood. 2006;108(12):3951–3952. doi: 10.1182/blood-2006-07-033571. [DOI] [PubMed] [Google Scholar]
- 27.Barasch A. Clinical, Pharmacological, and Biological Issues in ONJ. Paper presented at: American Association for Dental Research 38th Annual Meeting; March 3–6, 2010; Washington, DC. [Google Scholar]
- 28.Curro FA. Findings of the CONDOR Case-Control Study of ONJ. Paper presented at: American Association for Dental Research 38th Annual Meeting; March 3–6, 2010; Washington, DC. [Google Scholar]
- 29.Favia G, Pilolli GP, Maiorano E. Osteonecrosis of the jaw correlated to bisphosphonate therapy in non-oncologic patients: clinicopathological features of 24 patients. J Rheumatol. 2009;36(12):2780–2787. doi: 10.3899/jrheum.090455. [DOI] [PubMed] [Google Scholar]
- 30.FDA. Actonel Label Prescribing Information. 2009 [Google Scholar]
- 31.FDA. Boniva Label Prescribing Information. 2010 [Google Scholar]
- 32.FDA. Fosamax Label Prescribing Information. 2010 [Google Scholar]
- 33.Fellows JL, Rindal DB, Barasch A, et al. PS1–31: osteonecrosis of the jaw in two dental PBRN health plans. Clin Med Res. 2010;8:42. [Google Scholar]
- 34.Hujoel P. Design of the CONDOR Case-Control Study of ONJ. Paper presented at: American Association for Dental Research 38th Annual Meeting; March 3–6, 2010; Washington, DC. [Google Scholar]
- 35.Lo JC, O'Ryan FS, Gordon NP, et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg. 2010;68(2):243–253. doi: 10.1016/j.joms.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.FDA. Prescription Drug User Fee Act (PDUFA) IV Drug Safety Five-Year Plan 2008–2012. [Accessed May 17, 2010]. Available at http://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM119244.pdf.
- 37.Giordano SH, Fang S, Duan Z, et al. Use of intravenous bisphosphonates in older women with breast cancer. The Oncologist. 2008;13(5):494–502. doi: 10.1634/theoncologist.2007-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novartis. Product Sales. [Accessed May 17, 2010]. Available at http://www.novartis.com/investors/sales-results/product-sales.shtml.
- 39.Phase III Randomized Study of Adjuvant Zoledronate Versus Clodronate Versus Ibandronate in Women With Resected Primary Stage I-III Adenocarcinoma of the Breast. [Accessed January 10, 2010]. Available at http://www.cancer.gov/clinicaltrials/SWOG-S0307.
- 40.Brufsky A, Bundred N, Coleman R, et al. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. The Oncologist. 2008;13(5):503–514. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- 41.Safra T, Bernstein R, Stephansky I, et al. Zolandronic acid protective effect on bone loss in postmenopausal women switched from tamoxifen to letrozole in the treatment of early breast cancer [abstract 1153]. Paper presented at: 31st Annual San Antonio Breast Cancer Symposium; December 10–14, 2008; San Antonio, TX. [Google Scholar]
- 42.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360(7):679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 43.Hershman DL, McMahon DJ, Crew KD, et al. Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2008;26(29):4739–4745. doi: 10.1200/JCO.2008.16.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wardley A, Davidson N, Barrett-Lee P, et al. Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration. Br J Cancer. 2005;92(10):1869–1876. doi: 10.1038/sj.bjc.6602551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuleihan Gel H, Salamoun M, Mourad YA, et al. Pamidronate in the prevention of chemotherapy-induced bone loss in premenopausal women with breast cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2005;90(6):3209–3214. doi: 10.1210/jc.2004-1444. [DOI] [PubMed] [Google Scholar]
- 46.Saarto T, Blomqvist C, Valimaki M, et al. Clodronate improves bone mineral density in post-menopausal breast cancer patients treated with adjuvant antioestrogens. Br J Cancer. 1997;75(4):602–605. doi: 10.1038/bjc.1997.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen LS, Gordon D, Tchekmedyian S, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial–the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol. 2003;21(16):3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 48.Ficarra G, Beninati F, Rubino I, et al. Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatment. J Clin Periodontol. 2005;32(11):1123–1128. doi: 10.1111/j.1600-051X.2005.00842.x. [DOI] [PubMed] [Google Scholar]
- 49.Merigo E, Manfredi M, Meleti M, et al. Jaw bone necrosis without previous dental extractions associated with the use of bisphosphonates (pamidronate and zoledronate): a four-case report. J Oral Pathol Med. 2005;34(10):613–617. doi: 10.1111/j.1600-0714.2005.00351.x. [DOI] [PubMed] [Google Scholar]
- 50.Mignogna MD, Fedele S, Lo Russo L, et al. Case 2. Osteonecrosis of the jaws associated with bisphosphonate therapy. J Clin Oncol. 2006;24(9):1475–1477. doi: 10.1200/JCO.2005.01.7756. [DOI] [PubMed] [Google Scholar]
- 51.Vannucchi AM, Ficarra G, Antonioli E, et al. Osteonecrosis of the jaw associated with zoledronate therapy in a patient with multiple myeloma. Br J Haematol. 2005;128(6):738. doi: 10.1111/j.1365-2141.2005.05382.x. [DOI] [PubMed] [Google Scholar]
- 52.Atchison KA, Gironda MW, Black EE, et al. Baseline characteristics and treatment preferences of oral surgery patients. J Oral Maxillofac Surg. 2007;65(12):2430–2437. doi: 10.1016/j.joms.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thierer T, Friedman B. Preferences for oral health states in a US community-dwelling functionally impaired older adult population: 2000–2001. J Public Health Dent. 2006;66(4):248–254. doi: 10.1111/j.1752-7325.2006.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 54.Nassani MZ, Devlin H, McCord JF, et al. The shortened dental arch–an assessment of patients' dental health state utility values. Int Dent J. 2005;55(5):307–312. doi: 10.1111/j.1875-595x.2005.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 55.Mileman PA, van den Hout WB. Preferences for oral health states: effect on prescribing periapical radiographs. Dentomaxillofac Radiol. 2003;32(6):401–407. doi: 10.1259/dmfr/15454473. [DOI] [PubMed] [Google Scholar]
- 56.Brickley M, Armstrong R, Shepherd J, et al. The relevance of health state utilities to lower third molar surgery. Int Dent J. 1995;45(2):124–128. [PubMed] [Google Scholar]
- 57.Rogers SN, Miller RD, Ali K, et al. Patients' perceived health status following primary surgery for oral and oropharyngeal cancer. Int J Oral Maxillofac Surg. 2006;35(10):913–919. doi: 10.1016/j.ijom.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 58.Ackerman SJ, Sullivan EM, Beusterien KM, et al. Cost effectiveness of recombinant human insulin-like growth factor I therapy in patients with ALS. Pharmacoeconomics. 1999;15(2):179–195. doi: 10.2165/00019053-199915020-00006. [DOI] [PubMed] [Google Scholar]
- 59.Brown B, Diamantopoulos A, Bernier J, et al. An economic evaluation of cetuximab combined with radiotherapy for patients with locally advanced head and neck cancer in Belgium, France, Italy, Switzerland, and the United Kingdom. Value Health. 2008;11(5):791–799. doi: 10.1111/j.1524-4733.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 60.Center for the Evaluation of Value and Risk in Health. The Cost-Effectiveness Analysis Registry. IfCRaHPS, Tufts Medical Center. [Accessed November 14, 2009]. Available at www.cearegistry.org.
- 61.Lloyd A, Nafees B, Narewska J, et al. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–690. doi: 10.1038/sj.bjc.6603326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berenson JR, Hillner BE, Kyle RA, et al. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2002;20(17):3719–3736. doi: 10.1200/JCO.2002.06.037. [DOI] [PubMed] [Google Scholar]
- 63.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21(21):4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 64.Khan AA, Sandor GK, Dore E, et al. Canadian consensus practice guidelines for bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2008;35(7):1391–1397. [PubMed] [Google Scholar]
- 65.Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws–2009 update. J Oral Maxillofac Surg. 2009;67(5 Suppl):2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 66.Botteman M, Barghout V, Stephens J, et al. Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann Oncol. 2006;17(7):1072–1082. doi: 10.1093/annonc/mdl093. [DOI] [PubMed] [Google Scholar]
- 67.Hillner BE, Weeks JC, Desch CE, et al. Pamidronate in prevention of bone complications in metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol. 2000;18(1):72–79. doi: 10.1200/JCO.2000.18.1.72. [DOI] [PubMed] [Google Scholar]
- 68.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 69.American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65(3):369–376. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Miksad RA, Woo S, Dodson T, et al. Quality of life implications of osteonecrosis of the jaw in cancer patients: a pilot study. J Clin Oncol. 2008;26(15 suppl) Abstract 17557. [Google Scholar]
- 71.Hunink M, Glasziou P, Siegel J, et al. Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge, U.K.: Cambridge University Press; 2001. Valuing outcomes: Relationships among techniques for valuing outcomes; pp. 106–107. [Google Scholar]
- 72.Kind P. The EuroQol Instrument: An Index of Health Related Quality of Life. In: Spilker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. Second Edition. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 191–201. [Google Scholar]
- 73.Torrance GW, Feeny D, Furlong W. Visual analog scales: do they have a role in the measurement of preferences for health states? Med Decis Making. 2001;21(4):329–334. doi: 10.1177/0272989X0102100408. [DOI] [PubMed] [Google Scholar]
- 74.Torrance GW, Thomas WH, Sackett DL. A utility maximization model for evaluation of health care programs. Health Serv Res. 1972;7(2):118–133. [PMC free article] [PubMed] [Google Scholar]
- 75.Furlong W, Feeny D, Torrance GW, et al. Guide to Design and Development of Health-State Utility Instrumentation. McMaster University CHEPA Working Paper Series. 1990 Paper 90-9. Cited with permission. [Google Scholar]
- 76.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes. Third Edition. New York: Oxford University Press; 2005. Cost-utility analysis: Measuring preferences; pp. 147–154. [Google Scholar]
- 77.Froberg DG, Kane RL. Methodology for measuring health-state preferences–II: Scaling methods. J Clin Epidemiol. 1989;42(5):459–471. doi: 10.1016/0895-4356(89)90136-4. [DOI] [PubMed] [Google Scholar]
- 78.Krabbe PF, Essink-Bot ML, Bonsel GJ. The comparability and reliability of five health-state valuation methods. Soc Sci Med. 1997;45(11):1641–1652. doi: 10.1016/s0277-9536(97)00099-3. [DOI] [PubMed] [Google Scholar]
- 79.Calculating the U.S. Population-based EQ-5D Index Score. Rockville, MD: Agency for Healthcare Research and Quality; 2005. Aug, [Accessed November 13, 2008]. Available at http://www.ahrq.gov/rice/EQ5Dscore.htm. [Google Scholar]
- 80.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Slade GD. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol. 1997;25(4):284–290. doi: 10.1111/j.1600-0528.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 82.Robinson PG, Gibson B, Khan FA, et al. Validity of two oral health-related quality of life measures. Community Dent Oral Epidemiol. 2003;31(2):90–99. doi: 10.1034/j.1600-0528.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 83.Allen PF, Locker D. Do item weights matter? An assessment using the oral health impact profile. Community Dent Health. 1997;14(3):133–138. [PubMed] [Google Scholar]
- 84.Soe KK, Gelbier S, Robinson PG. Reliability and validity of two oral health related quality of life measures in Myanmar adolescents. Community Dent Health. 2004;21(4):306–311. [PubMed] [Google Scholar]
- 85.Locker D. Measuring oral health: a conceptual framework. Community Dent Health. 1988;5(1):3–18. [PubMed] [Google Scholar]
- 86.Baker SR, Pankhurst CL, Robinson PG. Utility of two oral health-related quality-of-life measures in patients with xerostomia. Community Dent Oral Epidemiol. 2006;34(5):351–362. doi: 10.1111/j.1600-0528.2006.00285.x. [DOI] [PubMed] [Google Scholar]
- 87.Gold MR, Patrick DL, Torrance GW, et al. Identifying and Valuing Outcomes. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost Effectiveness in Health and Medicine. Oxford, U.K.: Oxford University Press; 1996. pp. 82–134. [Google Scholar]
- 88.Stein K, Fry A, Round A, et al. What value health?: A review of health state values used in early technology assessments for NICE. Appl Health Econ Health Policy. 2005;4(4):219–228. doi: 10.2165/00148365-200504040-00004. [DOI] [PubMed] [Google Scholar]
- 89.Bennett KJ, Torrance GW. Measuring Health State Preferences and Utilities: Rating Scale, Time Trade-Off, and Standard Gamble Techniques. In: Spilker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. Second Edition. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 253–265. [Google Scholar]
- 90.Green C, Brazier J, Deverill M. Valuing health-related quality of life. A review of health state valuation techniques. Pharmacoeconomics. 2000;17(2):151–165. doi: 10.2165/00019053-200017020-00004. [DOI] [PubMed] [Google Scholar]
- 91.Akbari M, Miksad RA, Weinstein MC. The Role of Bisphosphonate Therapy in Multiple Myeloma Patients: A perspective on Osteonecrosis of the Jaw and Quality of Life. Paper presented at: 30th Annual Meeting of the Society for Medical Decision Making.2008. [Google Scholar]
- 92.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 93.Woodward WA, Strom EA, Tucker SL, et al. Changes in the 2003 American Joint Committee on Cancer staging for breast cancer dramatically affect stage-specific survival. J Clin Oncol. 2003;21(17):3244–3248. doi: 10.1200/JCO.2003.03.052. [DOI] [PubMed] [Google Scholar]
- 94.Fryback DG, Lawrence WF., Jr Dollars may not buy as many QALYs as we think: a problem with defining quality-of-life adjustments. Med Decis Making. 1997;17(3):276–284. doi: 10.1177/0272989X9701700303. [DOI] [PubMed] [Google Scholar]
- 95.Galea S, Nandi A, Stuber J, et al. Participant reactions to survey research in the general population after terrorist attacks. J Trauma Stress. 2005;18(5):461–465. doi: 10.1002/jts.20053. [DOI] [PubMed] [Google Scholar]
- 96.SAS software, Version 9.1 of the SAS System for Windows. Cary, NC USA: Copyright 2002–2003 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. [Google Scholar]
- 97.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 98.Tosteson AN, Gabriel SE, Grove MR, et al. Impact of hip and vertebral fractures on quality-adjusted life years. Osteoporos Int. 2001;12(12):1042–1049. doi: 10.1007/s001980170015. [DOI] [PubMed] [Google Scholar]
- 99.Stewart ST, Lenert L, Bhatnagar V, et al. Utilities for prostate cancer health states in men aged 60 and older. Med Care. 2005;43(4):347–355. doi: 10.1097/01.mlr.0000156862.33341.45. [DOI] [PubMed] [Google Scholar]
- 100.Loeb S, Roehl KA, Helfand BT, et al. Complications of open radical retropubic prostatectomy in potential candidates for active monitoring. Urology. 2008;72(4):887–891. doi: 10.1016/j.urology.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 101.Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92(19):1582–1592. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 102.The Standards for Educational and Psychological Testing. Second Edition. Washington, DC: American Educational Research Association; 1999. [Google Scholar]
- 103.Solomayer E, Gebauer G, Hirnle P, et al. Influence of zoledronic acid on disseminated tumor cells (DTC) in primary breast cancer patients [abstract 2048]. Paper presented at: 31st Annual San Antonio Breast Cancer Symposium; December 10–14, 2008; San Antonio, TX. [Google Scholar]
- 104.Winter M, Thorpe H, Burkinshaw R, et al. The addition of zoledronic acid to neoadjuvant chemotherapy may influence pathological response exploratory evidence for direct anti-tumor activity in breast cancer [abstract 5101]. Paper presented at: 31st Annual San Antonio Breast Cancer Symposium; December 10–14, 2008; San Antonio, TX. [Google Scholar]
- 105.Edwards BJ, Hellstein JW, Jacobsen PL, et al. Updated recommendations for managing the care of patients receiving oral bisphosphonate therapy: an advisory statement from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2008;139(12):1674–1677. doi: 10.14219/jada.archive.2008.0110. [DOI] [PubMed] [Google Scholar]
- 106.Comparison of Two Schedules of Zoledronic Acid in Treating Patients With Breast Cancer That Has Spread to the Bone. [Accessed January 10, 2010]. Available at http://www.clinicaltrials.gov/ct2/show/NCT00458796?term=NCT00458796&rank=1.
- 107.Wessel JH, Dodson TB, Zavras AI. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: a case-control study. J Oral Maxillofac Surg. 2008;66(4):625–631. doi: 10.1016/j.joms.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18(2):75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 109.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol. 2005;23(34):8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 110.Dimopoulos MA, Kastritis E, Anagnostopoulos A, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91(7):968–971. [PubMed] [Google Scholar]
- 111.Dimopoulos MA, Kastritis E, Bamia C, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20(1):117–120. doi: 10.1093/annonc/mdn554. [DOI] [PubMed] [Google Scholar]
- 112.Ripamonti CI, Maniezzo M, Campa T, et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 2009;20(1):137–145. doi: 10.1093/annonc/mdn526. [DOI] [PubMed] [Google Scholar]
- 113.Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216–220. doi: 10.1016/j.ygyno.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bartoces MG, Severson RK, Rusin BA, et al. Quality of life and self-esteem of long-term survivors of invasive and noninvasive cervical cancer. J Womens Health (Larchmt) 2009;18(5):655–661. doi: 10.1089/jwh.2008.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Palaska PK, Cartsos V, Zavras AI. Bisphosphonates and time to osteonecrosis development. The Oncologist. 2009;14(11):1154–1166. doi: 10.1634/theoncologist.2009-0115. [DOI] [PubMed] [Google Scholar]
- 116.Earle CC, Chapman RH, Baker CS, et al. Systematic overview of cost-utility assessments in oncology. J Clin Oncol. 2000;18(18):3302–3317. doi: 10.1200/JCO.2000.18.18.3302. [DOI] [PubMed] [Google Scholar]
- 117.Lidgren M, Wilking N, Jonsson B, et al. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16(6):1073–1081. doi: 10.1007/s11136-007-9202-8. [DOI] [PubMed] [Google Scholar]
- 118.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.