The case of a woman undergoing fertility preservation treatment who showed a severely compromised ovarian response to gonadotropin stimulation while on imatinib with a normal ovarian response after stopping this medication is reported.
Keywords: Ovary, Growth factor, Fertility, Leukemia
Abstract
Imatinib mesylate is the first in a family of highly effective, minimally toxic, targeted agents used widely to treat Philadelphia-positive leukemias and selected other cancers, leading to a steady rise in the prevalence of patients using such therapy. Because failure of therapy would require conventional gonadotoxic chemotherapeutics, many female patients using imatinib may choose to preserve fertility. Herein, we provide evidence of a potential negative effect of imatinib on ovarian function by reporting the first case of a woman who showed a severely compromised ovarian response to gonadotropin stimulation while on imatinib, with a normal ovarian response after stopping this medication.
Introduction
A 17-year-old Asian female was referred to us, desiring fertility preservation prior to undergoing chemotherapy for her chronic myelogenous leukemia (CML). She was diagnosed at age 15 years, 4 months after presenting with persistent vague abdominal discomfort. Initial findings included a leukocyte count of 64,000/μL with a left shift, a platelet count of 755,000/μL, and bone marrow biopsy consistent with chronic phase CML. Identification of Philadelphia (Ph) chromosome presence (fluorescence in situ hybridization, 99.5% positive for Bcr-Abl) supported the use of monotherapy with imatinib at a dose of 400 mg daily.
The initial response was favorable, with hematologic remission after 2 weeks, complete cytogenetic remission after 6 months, and major molecular response (≥3 log reduction in Bcr-Abl transcripts measured by quantitative polymerase chain reaction) after 9 months. Imatinib toxicity was limited to early transient neutropenia requiring a 4-week period of reduced dose, gastritis requiring omeprazole therapy, and subjective fatigue. After 20 months of therapy, minimal progression was noted (loss of major molecular response), and the imatinib dose was increased to 400 mg twice daily. Worsening fatigue and the return of abdominal pain were noted but a rapid response was observed, with Bcr-Abl transcripts falling to undetectable levels (complete molecular remission) after 3 months of imatinib at 400 mg twice daily.
At the time of minimal progression, stem cell transplantation was planned. Prior to the use of conditioning chemotherapy (busulfan, fludarabine, and rabbit antithymocyte globulin), a regimen known to be ovotoxic, the patient's mother requested that the patient be seen to discuss fertility preservation options.
Ultrasound evaluation at the patient's initial visit showed an age-appropriate ovarian reserve with an antral follicle count of 26 and ovarian volumes of 7.0 cc for the right ovary and 8.8 cc for the left ovary with an emerging dominant follicle of 13 mm noted on the left. She received extensive counseling regarding the likely impact of the planned chemotherapy regimen on her future ovarian function and likely need for hormone replacement and possible fertility preservation options, including oocyte cryopreservation, embryo cryopreservation, and ovary tissue cryopreservation. After counseling with the patient and her mother, the patient chose ovarian stimulation with oocyte cryopreservation.
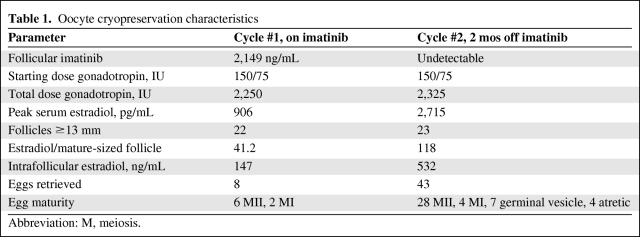
Her ovarian stimulation involved pituitary suppression with a gonadotropin-releasing hormone (GnRH) antagonist and daily ovarian stimulation with 150 IU follicle-stimulating hormone (FSH) and 75 IU Menopur (a mixture of FSH and luteinizing hormone [LH]) (Table 1). During ovarian stimulation, her serum estradiol was noted to be lower than anticipated given the number of developing follicles, and on the day of human chorionic gonadotropin (hCG) administration her serum estradiol was 906 pg/mL, with 22 follicles measuring ≥13 mm for an average of 41 pg/mL per mature-sized follicle. A typical value for this ratio is 150–200 pg/mL per mature follicle. Additionally, on the day of the planned hCG administration, her follicles were noted to have a somewhat hazy appearance consistent with blood or debris within the follicles. An ovum retrieval was attempted on that day in case the patient had spontaneously begun to ovulate despite pituitary suppression. Despite aspiration of multiple follicles, no eggs were retrieved. hCG was given that evening as planned. The next day, albeit low, her estradiol level continued to rise, which was inconsistent with spontaneous premature ovulation. At 36 hours after hCG administration, only eight eggs were obtained, of which six were mature meiosis II (MII) oocytes.
Table 1.
Oocyte cryopreservation characteristics
Abbreviation: M, meiosis.
A discussion was held with the patient, her mother, and the patient's hematologist regarding the unusual ovarian stimulation findings in the context of CML and imatinib use. Based on her impending stem cell transplant, the improved quality of her remission after imatinib dose escalation, and the question of an imatinib effect on her ovarian response, her hematologist felt it reasonable to interrupt imatinib for repeat stimulation.
The patient was off imatinib for 2 months prior to the next ovarian stimulation. The stimulation protocol was identical, with the use of a GnRH antagonist for pituitary suppression and the same dose of gonadotropin. The patient's estradiol followed the expected trajectory, with an appropriate level given the number of developing follicles. On the day of hCG administration, her serum estradiol was 2,715 pg/mL with 23 follicles ≥13 mm. Additionally, follicular fluid (FF) was obtained from a single dominant follicle as per our institutional review board (IRB) protocol in order to evaluate the intrafollicular biochemical milieu in comparison with the FF obtained from the similarly timed (prior to hCG) FF collection when the patient was taking imatinib. Thirty-six hours after hCG, 43 oocytes were retrieved, of which 28 were mature MII oocytes, four were MI oocytes, seven were germinal vesicle immature oocytes, and four were atretic. The patient had no symptoms of ovarian hyperstimulation syndrome.
Materials and Methods
FF Collection
FF was collected from individual follicles according to a standard in vitro fertilization technique. The FF was clarified by centrifugation at room temperature for 10 minutes at 1,500 × g, aliquoted, snap-frozen, and stored at −80°C for later analysis. All biological samples were obtained from patients who received informed consent under an IRB approved protocol.
FF Analysis
Concentrations of imatinib and its active N-desmethyl metabolite (CGP 74588) were determined by a previously described, validated liquid chromatographic–mass spectrometic assay [1]. FF and granulosa cell culture media hormone concentrations were quantified in duplicate using commercially available automated chemiluminescent immunoassays on the DPC Immulite 2000 analyzer (Siemens Healthcare Diagnostics; Deerfield, IL) as previously described [2].
Granulosa Cell Culture
Pooled FF was obtained from two consenting patients (one a healthy ovum donor and the other a 39-year-old female). Luteinized mural granulosa cells were isolated by centrifugation at 1,500 × g for 15 minutes over a PureSperm 40TM (Nidacon; Healdsburg, CA) gradient and resuspended in M-199 media supplemented with 10% (heat inactivated) fetal bovine serum. Cells were grown at 37°C with 5% CO2 for 48 hours. FSH and hCG were used at a final concentration of 10 mIU/mL where indicated. Testosterone was added to the media at 30 ng/mL to serve as a substrate for conversion to estrogen. Protein concentrations were determined by the BCA Protein Assay (Pierce; Rockford, IL).
Results
Cycle-specific characteristics and hormonal evaluation are shown in Table 1.
The intrafollicular imatinib concentration in the first FF sample was 2,149 ng/mL, and the concentration of its active N-desmethyl metabolite was 506 ng/mL, consistent with equilibrium between the plasma and follicular compartments. There was no imatinib in the second FF samples, showing that the drug had been completely cleared from the patient's system at the time of the second oocyte retrieval.
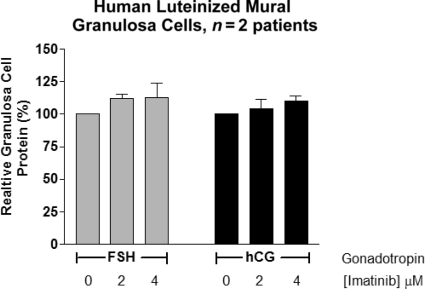
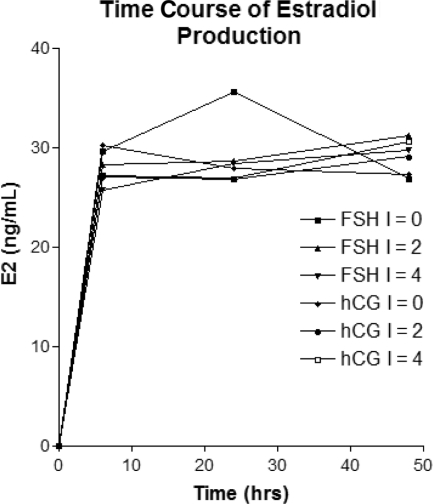
In order to determine whether imatinib was cytotoxic to human granulosa cells, we treated primary human granulosa cell cultures with varying concentrations of imatinib (approximating treatment level serum concentrations ranging up to 4 μM) and found no significant difference in cell survival as assessed by granulosa cell protein content from a similarly plated number of granulosa cells treated with the gonadotropins FSH and hCG (Fig. 1). We used this in vitro culture system to further examine the classical granulosa cell steroidogenic function of aromatization of androgens using testosterone as a substrate. No significant differences were seen in the ability of granulosa cells to produce estradiol from testosterone when stimulated with gonadotropins with imatinib levels up to 4 μM (Fig. 2).
Figure 1.
Effect of imatinib on human luteinized mural granulosa cell viability.
Abbreviations: FSH, follicle-stimulating hormone; hCG, human chorionic gonadotropin.
Figure 2.
Effect of various concentrations of imatinib (0, 2, 4 μM) on in vitro estradiol (E2) production by human luteinized mural granulosa cells.
Abbreviations: FSH, follicle-stimulating hormone; hCG, human chorionic gonadotropin.
Discussion
We present a unique case of a patient who underwent two consecutive ovarian stimulations for oocyte cryopreservation, with and without imatinib. Our data suggest that imatinib use in humans has an adverse effect on ovarian function. As usage increases, more women taking imatinib will choose to use assisted reproductive technologies and this unexpected negative finding is important for counseling.
Previous studies have suggested that imatinib can have potential reproductive effects in both males and females. Animal studies have shown that imatinib can potentially inhibit postnatal testicular development and sperm capacitation [3, 4]. There are two reported cases of impaired spermatogenesis potentially related to imatinib use, one in a young adult [5] and one wherein imatinib was used long term prior to puberty [6]. However, there have been multiple reports of men on imatinib conceiving with no significantly higher risk for anomalies in the offspring [7]. The use of imatinib by women has potentially greater implications for pregnancy and offspring. Clearly, women taking imatinib retain at least some level of fertility, as evidenced by the numerous case reports of women conceiving while on imatinib therapy. The largest case series assessed 180 pregnancies, and of the pregnancies with known outcomes, there were serious fetal malformations in 9.6%, with approximately half of the pregnancies resulting in a healthy infant [8]. There has been one prior controversial report of an association between imatinib and premature ovarian failure [9, 10], but gonadal toxicity has not been specifically studied in either standard or high-dose imatinib trials, and this remains an area ripe for exploration.
The most commonly employed methods of female fertility preservation involve ovarian stimulation to increase the number of mature oocytes beyond what is achieved in a natural cycle. At the onset of a regular menstrual cycle, an initial rise in FSH secreted from the pituitary selects a follicle for development. Once a follicle is selected, estradiol is produced, which creates a negative feedback loop on the pituitary and subsequently suppresses FSH such that only a single mature follicle develops. Toward the end of follicular development, a positive feedback loop is established whereby the maturing follicle produces sufficient estrogen to sensitize the pituitary and result in a burst of gonadotropin secretion (termed the LH surge) to induce the final stages of oocyte maturation and initiate the ovulatory process. With ovarian stimulation, one main goal is to override this natural feedback mechanism by administering exogenous gonadotropins and a GnRH receptor blocker to cause development of multiple mature follicles while preventing the endogenous LH surge [11]. Physiologically, GnRH secreted by the hypothalamus acts on the pituitary to cause both FSH and LH secretion. Prevention of the endogenous LH surge is accomplished either by long-term (multiple weeks) GnRH agonist administration to downregulate pituitary GnRH receptors or through the short-term use of GnRH antagonists during the latter part of the ovarian stimulation treatment.
For patients using imatinib, the use of gonadotropin stimulation to recover multiple oocytes is required either for classical fertility preservation, involving oocyte or embryo cryopreservation (when gonadotoxic chemotherapy is anticipated), or for use with gestational surrogacy because teratogenicity in animal studies and limited human data make maternal use of imatinib during pregnancy contraindicated [12]. We were fortunate to have a patient who consented to analysis of her intraovarian follicular response and to have pre-hCG FF from each of her ovarian stimulation cycles. Analysis of the FF showed that imatinib and its active metabolite were present in concentrations suggesting equilibrium between the plasma and ovarian follicle compartments. Given the molecular weight of imatinib (589.7) and the permeability of the follicular basement membrane, this is not necessarily unexpected [13]. In the presence of imatinib, ovarian hormone production and oocyte recovery were compromised despite a comparable number of developing ovarian follicles.
The apparent difference in intrafollicular estradiol concentrations, despite identical gonadotropin stimulation, suggests that imatinib adversely affects steroidogenesis, possibly through an inhibitory effect on either the theca or granulosa cells. The lower number of oocytes retrieved while on imatinib is more difficult to explain and potentially more problematic clinically. We hypothesized that the granulosa cells underwent apoptosis leading to oocyte atresia and lower egg recovery [14, 15]. We therefore assessed the effect of imatinib on human luteinized mural granulosa cells in primary culture for any global effects on cell growth and survival by measuring cellular protein levels. As shown in Figure 1, we did not see any significant effect of imatinib on luteinized granulosa cells under these conditions. With treatment using a pharmacologically relevant range of imatinib concentrations, we also did not see a negative effect of increasing concentrations of imatinib on steroidogenesis in our luteinized granulosa cell culture system (Fig. 2). Based on these results, imatinib may be having an effect on either theca cells or granulosa cell proliferation during the follicular phase. Additional studies involving theca cells and nonluteinized granulosa cells could be performed to measure steroidogenic activity and levels of apoptosis with imatinib treatment to elucidate potential mechanisms for the effects we observed.
Imatinib mesylate (Gleevec®; Novartis Pharmaceuticals Corporation, East Hanover, NJ) is the first in a family of orally available, rationally designed specific kinase inhibitors, highly effective in CML and other select diseases. Nilotinib (Tasigna®; Novartis) and dasatinib (Sprycel®; Bristol-Myers Squibb, Princeton, NJ) have been developed and approved for use in imatinib-resistant and imatinib-intolerant patients; both have published data from the frontline setting and nilotinib is now approved by the U.S. Food and Drug Administration for use in newly diagnosed patients [16, 17]. Imatinib, the prototype compound, is a phenylaminopyridine derivative that inhibits constitutively activated Bcr-Abl tyrosine kinase by binding to the active site ATP-binding cleft [18].
CML, at presentation and in the setting of imatinib intolerance or resistance (over time, ∼30% of de novo imatinib-treated patients), is felt to be reliant on Bcr-Abl activation, and Bcr-Abl–specific inhibitors are the mainstay of treatment of an increasing population of patients with Ph+ leukemia. In addition to Bcr-Abl+ leukemias, imatinib has been approved for the treatment of metastatic gastrointestinal stromal tumors (GISTs) [19]. Although not yet in clinical use, several trials have evaluated the use of imatinib in combination with other chemotherapeutic agents for more prevalent cancers, such as prostate, breast, and ovarian cancer [20–23].
More recent studies have shown that imatinib is not completely specific for its intended target and that it inhibits several other kinases to varying degrees. Imatinib is known to bind with high affinity to Kit and platelet-derived growth factor receptor (PDGFR)-α and PDGFR-β, with the former being the rationale for using imatinib to treat c-Kit+ GISTs. Kinase inhibitors are typically selective but not absolutely specific for their intended targets. A recent analysis of the interactions of 38 kinase inhibitors (including imatinib) for a representative subset of the human kinome using 317 kinases showed that, in addition to Abl, Kit, PDGFR-α, and PDGFR-β, imatinib also bound to 25 other kinases with varying affinities [24]. Other Bcr-Abl inhibitors used with increasing frequency may have narrower (nilotinib) or broader (dasatinib) target profiles, and “off-target” (e.g., non-Bcr-Abl) effects continue to be explored.
The targets of imatinib—Abl, c-Kit, PDGFR-α, and PDGFR-β—were found to be expressed at relatively uniform levels in 10 normal human premenopausal ovaries and at variable levels in granulosa tumor–derived cell lines [25]. That publication further characterized the effect of imatinib on granulosa tumor cell line proliferation, showing dose-dependent decreases in cell proliferation and viability and increased apoptosis, with an EC50 (half maximal effective concentration) that implicated off-target effects of imatinib on granulosa cells. This is consistent with the effects seen in our patient. Although there is considerable interpatient variability in imatinib pharmacokinetics, typical adult steady-state peak blood concentrations are in the range of 2,000–4,000 ng/mL [26, 27]. Interestingly, the distribution coefficients for many of the putative interactions of imatinib with other kinases were within these steady-state plasma concentrations, raising the possibility of clinically relevant off-target effects. It would be very interesting to perform microarray analyses of the gene-expression profiles of granulosa cells (mural and cumulus) exposed to imatinib, compared with those from unexposed patients, to understand the global effects imatinib could have on the somatic compartment of the developing follicle. Analyses of this type may also improve out understanding of which signaling pathways, if any, are altered in the ovarian follicle by imatinib treatment.
Recent studies in rodents have shown that imatinib targets are present in the murine ovary [28]. Interestingly, the consequence of imatinib use in these animal studies was protection of early postnatal murine ovaries from cisplatin-induced loss of follicular reserve of primary and primordial follicles. The mechanism for this phenomenon appears to be that blocking c-Abl tyrosine kinase activity prevents phosphorylation of p63, a key component for activation of proapoptotic genes within this system. Therefore, imatinib has been proposed as a unique method for preserving ovarian reserve during the administration of ovotoxic chemotherapy. However, this proposal should proceed with caution. Clearly, there are other effects on the ovary, and it is possible that by inhibiting ovarian responsiveness and function, imatinib may provide protection by creating a quiescent ovarian state, favorable under the threat of cytotoxic agents but paradoxically detrimental for fertility under similar conditions. Given an elimination half-life of ∼18 hours for the active drug, and up to 40 hours for active N-desmethylated piperazine metabolites (http://www.micromedex.com), it would appear reasonable to stop imatinib for at least 2 weeks prior to attempting ovarian stimulation. Given that the time to recruit a primary follicle into the gonadotropin-responsive pool is on the order of a few months [14], our data show no evidence that very early follicle recruitment or development (prior to the antral stage) is compromised, because the oocytes obtained from our patient's second ovarian stimulation had already begun their early initial development while our patient was still on imatinib.
The field of oncology is transitioning from an era of empirically based cancer therapy to one based on a precise understanding of the molecular defects in cancer. The success of imatinib has made it the prototype for such targeted therapies. We report a human case in which the use of imatinib during gonadotropin stimulation of the ovary was associated with an aberrant response, including lower ovarian steroidogenesis and lower oocyte recovery. Therefore, the expanding use of imatinib may come at the price of abnormal ovarian function. Based on the increasing prevalence of patients living with CML on imatinib therapy potentially desiring fertility via assisted reproductive technologies, and the potential incorporation of second-generation kinase inhibitors into frontline use for CML patients, further study of this phenomenon is warranted.
Acknowledgments
Merrill J. Egorin is deceased.
AMZ was supported by grant K12 HD001262-12 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development sponsored Women's Reproductive Health Research Career Development Program.
Author Contributions
Conception/Design: Mitchell P. Rosen, Brian J. Druker
Provision of study material or patients: Mitchell P. Rosen, Alberuni M. Zamah, Marcelle I. Cedars
Collection and/or assembly of data: Alberuni M. Zamah, Merrill J. Egorin
Data analysis and interpretation: Mitchell P. Rosen, Alberuni M. Zamah, Michael Mauro, Brian J. Druker, Kutluk Oktay, Merrill J. Egorin
Manuscript writing: Mitchell P. Rosen, Alberuni M. Zamah, Michael Mauro, Brian J. Druker, Kutluk Oktay
Final approval of manuscript: Mitchell P. Rosen, Alberuni M. Zamah, Michael Mauro, Brian J. Druker, Kutluk Oktay, Merrill J. Egorin, Marcelle I. Cedars
References
- 1.Parise RA, Ramanathan RK, Hayes MJ, et al. Liquid chromatographic-mass spectrometric assay for quantitation of imatinib and its main metabolite (CGP 74588) in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:39–44. doi: 10.1016/s1570-0232(03)00206-x. [DOI] [PubMed] [Google Scholar]
- 2.Rosen MP, Zamah AM, Shen S, et al. The effect of follicular fluid hormones on oocyte recovery after ovarian stimulation: FSH level predicts oocyte recovery. Reprod Biol Endocrinol. 2009;7:35. doi: 10.1186/1477-7827-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker MA, Hetherington L, Curry B, et al. Phosphorylation and consequent stimulation of the tyrosine kinase c-Abl by PKA in mouse spermatozoa; its implications during capacitation. Dev Biol. 2009;333:57–66. doi: 10.1016/j.ydbio.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Nurmio M, Toppari J, Zaman F, et al. Inhibition of tyrosine kinases PDGFR and C-Kit by imatinib mesylate interferes with postnatal testicular development in the rat. Int J Androl. 2007;30:366–376. doi: 10.1111/j.1365-2605.2007.00755.x. discussion 376. [DOI] [PubMed] [Google Scholar]
- 5.Seshadri T, Seymour JF, McArthur GA. Oligospermia in a patient receiving imatinib therapy for the hypereosinophilic syndrome. N Engl J Med. 2004;351:2134–2135. doi: 10.1056/NEJM200411113512024. [DOI] [PubMed] [Google Scholar]
- 6.Mariani S, Basciani S, Fabbri A, et al. Severe oligozoospermia in a young man with chronic myeloid leukemia on long-term treatment with imatinib started before puberty. Fertil Steril. 2011;95:1120.e15–e17. doi: 10.1016/j.fertnstert.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 7.Apperley J. Issues of imatinib and pregnancy outcome. J Natl Compr Canc Netw. 2009;7:1050–1058. doi: 10.6004/jnccn.2009.0069. [DOI] [PubMed] [Google Scholar]
- 8.Pye SM, Cortes J, Ault P, et al. The effects of imatinib on pregnancy outcome. Blood. 2008;111:5505–5508. doi: 10.1182/blood-2007-10-114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christopoulos C, Dimakopoulou V, Rotas E. Primary ovarian insufficiency associated with imatinib therapy. N Engl J Med. 2008;358:1079–1080. doi: 10.1056/NEJMc0707841. [DOI] [PubMed] [Google Scholar]
- 10.Malozowski S, Nelson L, Calis KA. More on ovarian insufficiency with imatinib. N Engl J Med. 2008;358:2648. doi: 10.1056/NEJMc080707. author reply 2648–2649. [DOI] [PubMed] [Google Scholar]
- 11.Macklon NS, Stouffer RL, Giudice LC, et al. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27:170–207. doi: 10.1210/er.2005-0015. [DOI] [PubMed] [Google Scholar]
- 12.Ault P, Kantarjian H, O'Brien S, et al. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2006;24:1204–1208. doi: 10.1200/JCO.2005.04.6557. [DOI] [PubMed] [Google Scholar]
- 13.Clarke HG, Hope SA, Byers S, et al. Formation of ovarian follicular fluid may be due to the osmotic potential of large glycosaminoglycans and proteoglycans. Reproduction. 2006;132:119–131. doi: 10.1530/rep.1.00960. [DOI] [PubMed] [Google Scholar]
- 14.Gougeon A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 15.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 17.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 18.Druker BJ, Lydon NB. Lessons learned from the development of an Abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest. 2000;105:3–7. doi: 10.1172/JCI9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagher R, Cohen M, Williams G, et al. Approval summary: Imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8:3034–3038. [PubMed] [Google Scholar]
- 20.Chew HK, Barlow WE, Albain K, et al. A phase II study of imatinib mesylate and capecitabine in metastatic breast cancer: Southwest Oncology Group Study 0338. Clin Breast Cancer. 2008;8:511–515. doi: 10.3816/CBC.2008.n.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matei D, Emerson RE, Schilder J, et al. Imatinib mesylate in combination with docetaxel for the treatment of patients with advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis: A Hoosier Oncology Group trial. Cancer. 2008;113:723–732. doi: 10.1002/cncr.23605. [DOI] [PubMed] [Google Scholar]
- 22.Mathew P, Thall PF, Bucana CD, et al. Platelet-derived growth factor receptor inhibition and chemotherapy for castration-resistant prostate cancer with bone metastases. Clin Cancer Res. 2007;13:5816–5824. doi: 10.1158/1078-0432.CCR-07-1269. [DOI] [PubMed] [Google Scholar]
- 23.Yardley DA, Burris HA, 3rd, Markus T, et al. Phase II trial of docetaxel plus imatinib mesylate in the treatment of patients with metastatic breast cancer. Clin Breast Cancer. 2009;9:237–242. doi: 10.3816/CBC.2009.n.040. [DOI] [PubMed] [Google Scholar]
- 24.Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 25.Chu S, Alexiadis M, Fuller PJ. Expression, mutational analysis and in vitro response of imatinib mesylate and nilotinib target genes in ovarian granulosa cell tumors. Gynecol Oncol. 2008;108:182–190. doi: 10.1016/j.ygyno.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Champagne MA, Capdeville R, Krailo M, et al. Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: Results from a Children's Oncology Group phase 1 study. Blood. 2004;104:2655–2660. doi: 10.1182/blood-2003-09-3032. [DOI] [PubMed] [Google Scholar]
- 27.Peng B, Hayes M, Resta D, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol. 2004;22:935–942. doi: 10.1200/JCO.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 28.Gonfloni S, Di Tella L, Caldarola S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15:1179–1185. doi: 10.1038/nm.2033. [DOI] [PubMed] [Google Scholar]