The epidemiological data linking a hyperandrogen state to a higher risk for ovarian cancer are reviewed, in vitro studies of the role of androgens in influencing the growth of epithelial ovarian cancer are described, and completed clinical trials with compounds that exploit the androgen axis in patients with ovarian cancer are reviewed.
Keywords: Androgen receptor, Ovarian cancer, Endocrine treatment, Abiraterone, Consolidation treatment
Learning Objectives
After completing this course, the reader will be able to:
Explain the role of the androgen axis in the development of ovarian cancer.
Discuss the potential compounds with anti-androgen activity that can be assessed for the treatment of patients with ovarian cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Androgen receptors are frequently expressed in epithelial ovarian cancer (EOC). Their role in the development of EOC is not fully understood. In the present review we first discuss the epidemiological data linking a hyperandrogen state to a higher risk for ovarian cancer, second describe in vitro studies of the role of androgens in influencing the growth of EOC, and finally review the completed clinical trials with compounds that exploit the androgen axis in patients with ovarian cancer. The therapeutic approaches that inhibit androgen signaling have so far produced only modest response rates. In the light of new data regarding the role of androgen stimulation in the evolution of EOC and the emergence of new compounds used for the treatment of other hormone-driven malignancies, such as prostate and breast cancer, we provide suggestions for new studies of antiandrogen therapeutics in the treatment of EOC. A specific example is the new agent abiraterone. In addition, we propose a panel of molecules that could be assessed as potential biomarkers that may aid patient selection for this approach in the future.
Introduction
The androgen receptor (AR) is a nuclear receptor that functions as an androgen-dependent transcriptional regulator [1, 2]. In its basal state, AR is inactive and is bound to heatshock proteins and other cellular chaperones. Upon activation by androgen hormones, it undergoes a series of events, including dissociation from the heatshock proteins, dimerization, and nuclear translocation. It then directly binds to specific hormone response elements in the promoter regions of target genes, resulting in upregulation of these genes [3].
In epithelial ovarian cancer (EOC), AR is expressed more often than estrogen receptor (ER) and has been reported to be detectable in up to 90% of cases using immunohistochemistry (IHC) [4]. In this review we aim to (a) summarize the evidence from epidemiological, clinical, and in vitro studies on the importance of androgens and the AR in relation to EOC, (b) give an overview of completed clinical trials that target the androgren axis, and (c) suggest ways to optimize the exploitation of the AR pathway in future clinical trials.
Epidemiological Data on Androgens and the Risk for EOC
In women, androgens are mainly synthesized in the adrenal glands, the ovaries, and adipose tissue, and they have an important physiological significance for bone and muscle growth and maintenance as well as cognitive function [1, 5]. There is a growing body of evidence supporting the notion that androgens influence proliferation of the normal ovarian epithelium and are a risk factor for EOC [6].
Epidemiological studies have attempted to correlate conditions that are associated with high circulating androgen levels in women with the risk for developing EOC. Such conditions include polycystic ovary syndrome (PCOS) and obesity.
PCOS affects up to one in five women of reproductive age and is characterized by polycystic ovaries, chronic anovulation, and ovarian hyperandrogenism [7]. Schildkraut and colleagues observed a 2.5-fold higher risk for EOC among women with PCOS in a retrospective study (seven cases with PCOS and 24 controls), although other studies did not report any association between PCOS and a higher risk for EOC [8, 9].
Obesity is another condition that affects the levels of circulating sex hormones. High body mass index (BMI) is well proven to be a risk factor for the development of endocrine-related cancers such as breast cancer (1.5× higher risk per 10 units of BMI) and endometrial cancer (3–4× higher risk per 10 units of BMI) [10, 11]. For EOC, however, the association with BMI is less pronounced, showing only a modestly higher risk of 1.1× per 10 units of BMI in some studies [12, 13], whereas other studies failed to detect any significant association [14–16]. Recently, a large epidemiological study, including 226,798 women from across Europe, showed a clear association between a high BMI and the risk for EOC (1.3× higher risk for obese women than for nonobese women) [17]. Overall, there is a moderate but robust association between high BMI and EOC.
Some retrospective case–control studies and case reports suggest that exogenous androgen intake raises the risk for EOC [18]. In a larger cohort analysis, 15 women reporting the use of testosterone supplementation had a 3.7-fold higher risk for developing EOC than the control group [9].
The Role of Genetic Polymorphisms of Androgen Pathways in EOC
We are now beginning to develop a better understanding of the role of androgens and their interaction with the AR in the progression and proliferation of ovarian neoplasms. The fact that the majority of EOCs maintain expression of the AR to a higher degree than estrogen or progesterone receptors suggests that the AR is involved in molecular signaling in EOC. For prostate cancer, which is well known to express AR and depend on AR signaling, there is evidence that, in addition to androgen levels, AR signaling can be dependent on polymorphisms of the receptor [19]. Exon 1 of the AR gene contains a polymorphic trinucleotide repeat, CAG, which varies normally in the range of about 8–31 repeats [19]. Interestingly, it has been shown in vitro that the number of the trinucleotide units correlates inversely with the transcriptional activity of the AR [20–22]. Moreover, epidemiological cohort studies have shown that a shorter CAG repeat length is associated with a greater risk for prostate cancer as well as earlier diagnosis [19], although recent reports in larger populations have failed to confirm these findings [23, 24]. Rodriguez-Gonzalez and colleagues showed an association between a shorter CAG repeat length and the aggressiveness of prostate cancer, which is characterized by greater prostate-specific antigen staining and a higher Gleason score [25]. These findings support the hypothesis that fewer CAG repeats are associated with greater AR signaling in prostate cancer, and with more aggressive forms of the disease. Based on these findings, several studies have determined CAG repeat length in cohort studies of healthy women and correlated the findings with the risk for developing EOC. Ludwig and colleagues reported a significant inverse correlation of a short CAG repeat length and a higher risk for EOC [26], although a study by Terry and colleagues provides contradictory results [27].
Among patients with EOC, a short CAG repeat length in the AR gene at diagnosis was associated with a significantly shorter overall survival time than in patients with more CAG repeats [28]. More specifically, the overall survival time of patients with <19 CAG repeats (n = 9) was 5.5 months, versus 32.6 months for patients with >20 CAG repeats (n = 68). The combination of a high BMI and a short CAG repeat length was an even stronger prognostic marker for survival, suggesting that greater AR signaling promotes a more aggressive phenotype in EOC [29].
Polymorphism of the gene encoding cytochrome P450 17A1 (CYP17A1), the 7α-hydroxylase/17,20 lyase enzyme that is involved in the synthesis of androgens, namely, polymorphism A2, has been linked to susceptibility for developing EOC. Polymorphism A2 leads to the substitution of C for T in the promoter region of the gene [30], whereas the variant of this polymorphism has been hypothesized to alter promoter activity, increasing CYP17 transcription and, subsequently, estrogen and androgen production [31]. This hypothesis was confirmed by a large study analyzing polymorphism A2 of CYP17A1 in blood samples of a cohort of 225 women 1 year prior to diagnosis of EOC, compared with a matched control cohort [30]. Polymorphism A2, either homozygous or heterozygous, was found to be more frequent in women who developed EOC (69%) than in women in the control group (54%), and its presence was associated with a 1.86-fold greater risk for EOC [30]. Among women with EOC, this polymorphism was further associated with a worse prognosis than in patients with wild-type alleles [32]. However, the biological importance of the CYP17 polymorphism remains unclear, because its presence is not always associated with higher levels of circulating androgens [33].
AR Signaling in EOC
The transactivational potential of AR is coregulated by AR-associated proteins that can enhance the potential of AR signaling [34]. Amplified in breast 1 (AIB1) and AR-associated protein 70 (ARA70), both AR-associated proteins, are overexpressed and/or amplified in EOC [34]. ARA70 is overexpressed in the majority of EOCs when compared with normal ovarian surface epithelium [35], whereas AIB1 is amplified in 25% of EOCs and is associated with ER positivity [36]. As is the case with the AR gene itself, AIB1 harbors polymorphic trinucleotide repeats (CAG) [34], with short repeat lengths associated with aggressive EOC [37].
A well-studied pathway by which AR might influence the growth of EOC is through the modulation of the sensitivity of transforming growth factor (TGF)-β, which is a potent inhibitor of epithelial cells [38]. In primary EOC cell cultures derived from patients' ascites, TGF-β exhibited a growth inhibitory effect [39]. This finding was confirmed by a study in malignant and nonmalignant ovarian epithelial cells in which TGF-β induced growth inhibition [38]. Furthermore, Evangelou and colleagues observed that treatment with dihydrotestosterone reversed the growth inhibitory effect by downregulation of TGF-β receptors I and II [38, 40]. Recently, it was further shown that the expression of several TGF-β pathway proteins (mothers against decapentaplegic family member 3 [Smad3], plasminogen activator inhibitor type 1, transcriptional co-activator with PDZ-binding motif) is associated with a response to cisplatin-based chemotherapy in patients with serous EOC [41] and that the TGF-β–Smad3 pathway might play an important role in mediating ovarian oncogenesis by enhancing metastatic potential [42]. However, the specific molecular mechanism and interactions between AR and the TGF-β pathway still need to be elucidated for EOC.
A second AR-associated pathway is the epithelial growth factor receptor (EGFR) signaling cascade. EGFR is a tyrosine kinase receptor and a therapeutic target for several human tumors [43]. Ligand binding to EGFR promotes EGFR homo- and heterodimerization with related ErbB family members such as the human epidermal growth factor receptor 2, activation of the catalytic intracellular tyrosine kinase domain, and phosphorylation of specific tyrosine residues of the receptor cytoplasmic domain. The latter leads to the stimulation of numerous downstream signaling cascades associated with cell growth and survival, angiogenesis, and tumor metastasis. In EOC, EGFR expression has been reported to be in the range of 10%–70% (with an average of 50%) [44] and EGFR amplification has been reported to be in the range of 1%–6% of cases [45]. Indeed, studies on prostate cancer cells suggest that there is crosstalk between AR and EGFR [46]. In EOC, higher EGFR expression levels were found in AR-expressing tumors than in tumors not expressing AR [47]. In addition, in ovarian cell lines greater AR expression was correlated with greater inhibition of phosphorylated EGFR and phosphorylated mitogen-activated protein kinase (MAPK) upon androgen treatment [48]. Taken together, these data suggest AR may interact with EGFR signaling by inhibiting downstream targets such as the phosphoinositide 3-kinase–AKT and MAPK/extracellular signal–related kinase kinase–MAPK pathways.
Microarray-based gene expression studies on EOC cell lines before and after androgen treatment were successful in identifying 121 genes that are significantly upregulated [49]. Among these genes, most of which regulate transcription, proliferation, and G-protein signaling, eight G-protein genes were validated using quantitative reverse transcription-polymerase chain reaction. The GTPase Rab35, which is involved in vesicle trafficking, was the most upregulated gene following androgen stimulation. Using immunohistochemistry, Rab35 was expressed in the majority of ovarian tumors (95%) and its expression levels were correlated with those of AR, suggesting that Rab35 might be useful as a biomarker of AR function [49].
Androgens might also increase the activity of telomerase through transcriptional (mRNA and protein levels) as well as through post-translational (phosphorylation) modulation, introducing another possible mechanism for androgen-related ovarian carcinogenesis [50].
Clinical Trials with Compounds Affecting the Androgen Axis
Only a limited number of clinical trials have assessed the efficacy of androgen manipulation in EOC. Most of these trials involved patients with recurrent EOC, with only one trial assessing antiandrogens as consolidation treatment. The compounds studied in the trials were either gonadotropin-releasing hormone (GnRH) analogs such as goserelin, triptorelin, and leuprolide or receptor antagonists that bind to the AR and prevent its activation, such as bicalutamide and flutamide.
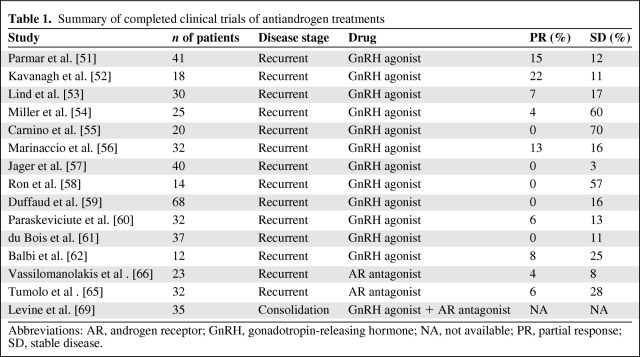
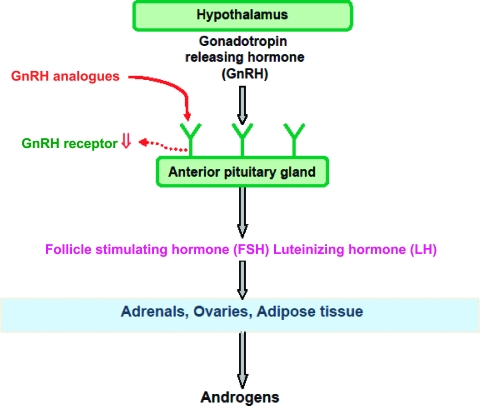
Our review of the published literature indicates that 12 clinical trials, totaling 369 patients, have been performed in recurrent EOC patients with GnRH analogs (Table 1) [51–62]. The proposed mechanism of action of GnRH analogs in EOC is the desensitization or downregulation of GnRH receptors in the pituitary, resulting in a decline in gonadotrophin secretion and a subsequent reduction in gonadal steroids, including androgens, which act as tumor growth factors [63] (Fig. 1). Interestingly, recent data suggest that follicle-stimulating hormone (FSH) may be involved in angiogenesis, raising the possibility that GnRH analogs may impact ovarian cancer growth by additional mechanisms [64]. Combining the data from all 12 trials, a total of 21 (5.7%) patients achieved an objective response and 77 patients (21%) had stable disease (SD). It is difficult to interpret the high SD rate in the absence of randomized trials, and it could relate to the slow growth of a percentage of the tumors treated. Interestingly, in two studies, responses were observed in patients with well-differentiated tumors [51, 52]. However, it is not yet clear whether patients with low-grade ovarian tumors do represent a group more likely to benefit from treatments affecting the GnRH axis.
Table 1.
Summary of completed clinical trials of antiandrogen treatments
Abbreviations: AR, androgen receptor; GnRH, gonadotropin-releasing hormone; NA, not available; PR, partial response; SD, stable disease.
Figure 1.
Gonadotropin-releasing hormone (GnRH)–gonadotropin axis. The proposed mechanism of GnRH analogs (goserelin, triptorelin) in epithelial ovarian cancer is thought to be desensitization or downregulation of GnRH receptors in the pituitary, resulting in a decline in gonadotropin secretion and subsequent reduction in gonadal steroids including androgens.
The AR inhibitor flutamide was assessed in two, nonrandomized phase II trials [65, 66]. Tumolo and colleagues reported a response rate (RR) of 6.3% (n = 2) and SD rate of 28% (n = 9) with a median SD duration of 24 weeks [65]. All patients were heavily pretreated (median of two chemotherapy lines) and had documented disease progression on screening. In a second trial by Vasillomanolakis and colleagues, 24 patients who progressed on chemotherapy received a low dose (100 mg three times a day) of flutamide [66]. One patient responded to the treatment (4.3%) and two patients had SD for ≥7 months.
In addition to the above, combination treatment with tamoxifen and GnRH analogs was evaluated in two phase II, single-arm clinical trials in patients with recurrent, chemotherapy-resistant EOC. The combination of tamoxifen and goserelin was assessed by Hasan and colleagues [67]. Those authors reported an RR of 11.8% (three of 26) and SD for >6 months in 10 patients (38.5%) with a combined clinical benefit rate (RR + SD rate) of 50% (13 of 26). The median progression-free interval was 4 months (95% confidence interval [CI], 2.4–9.6 months), whereas the median overall survival duration was 13.6 months (95% CI, 5.5–30.6 months). Treatment-limiting toxicity was not seen in any patient in the study population. There was no correlation between luteinizing hormone or FSH suppression and tumor response with the biomarkers assessed. Likewise, no relationship was observed between inhibin A or B and pro-α C levels and tumor response. In a similar trial, the same combination of tamoxifen and goserelin produced a high SD rate of 40% (10 of 25 patients) [68]. Interestingly, in both trials high SD rates were observed, which is disproportionate to the low RR reported. The high SD rate and the minimal toxicity make the combination of hormonal treatments an attractive option for future clinical trials, including those assessing consolidation strategies.
In this context, Levine and colleagues evaluated the combination of goserelin and bicalutamide in 35 patients with EOC in second or higher clinical disease remission [69]. The intervention produced a longer progression-free survival interval than in historical controls—11.4 months (95% CI, 10.2–12.6 months) and 10.7 months (95% CI, 9.3–12.2 months), respectively—but it did not reach the predetermined, clinically meaningful, endpoint set by the investigators (16.5 months). The proportions of patients remaining in remission at 6, 12, 18, and 24 months were 100%, 47%, 22%, and 13%, respectively. These data provide a useful benchmark for future trials, and the absence of significant toxicity confirms that effective hormonal therapy using a consolidation approach would be an attractive option.
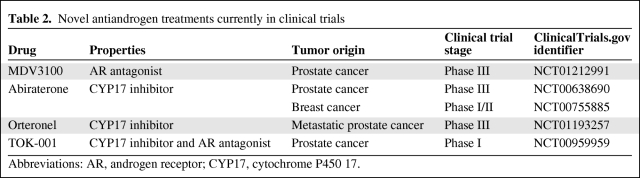
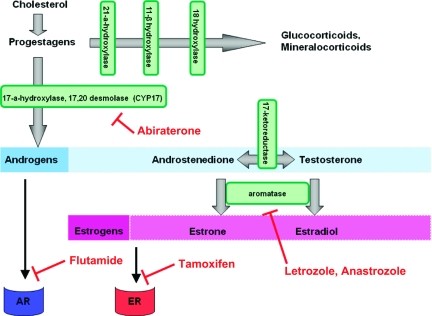
In conclusion, the above data demonstrate that existing antiandrogen treatments have only a modest effect with potential benefit for only a minority of patients. Therefore, newer drugs that act on the androgen axis are needed in addition to better patient selection (Table 2). A drug that could be evaluated in clinical trials is abiraterone, which is a novel CYP17 inhibitor that irreversibly inhibits the generation of adrenal steroids downstream of CYP17 by blocking the conversion of pregnenolone to dehydroepiandrostenedione and progesterone to androstenedione (Fig. 2) [70]. Downstream of this reaction, it further suppresses the generation of estrogens and androgens [71]. The drug is well tolerated and it has been shown to have significant activity in patients with castrate-resistant prostate cancer, leading to a recently reported randomized phase III trial that provided positive evidence of the drug's efficacy in this disease [72]. Because the steroid hormones upstream of aromatase and downstream of CYP17 could be important in the activation of ARs and possibly other nuclear steroid hormone receptors in EOC growth, abiraterone may impact EOC cell proliferation and survival by its androgen-suppressing activity. In addition, suppression of estrogens that might contribute to mitogenesis may add to the anti-tumor effect of abiraterone. Indeed, in a recent report of a phase I trial of abiraterone in women with hormone-resistant breast cancer, it was at a dose of 2,000 mg that all five patients had maximal suppression of estradiol and androgenic steroids. Two of 25 patients remained on treatment for >1 year, one with a confirmed partial response. The drug was well tolerated and a phase II trial is planned to start in 2011 [73]. We believe that the inhibition of the synthesis of both estrogens and androgens will have an additive effect. In addition, the low rate of side effects will allow patients to receive the drug for a longer time and derive the maximum possible benefit from it.
Table 2.
Novel antiandrogen treatments currently in clinical trials
Abbreviations: AR, androgen receptor; CYP17, cytochrome P450 17.
Figure 2.
Steroid synthesis pathway and aromatization. Abiraterone is a novel cytochrome P450 (CYP)17 inhibitor that irreversibly inhibits the generation of adrenal steroids downstream of CYP17. It suppresses the generation of both androgens and estrogens. Flutamide competes with testosterone for androgen receptors (ARs), preventing their activation. Tamoxifen is an antagonist of the estrogen receptor (ER), blocking its downstream signaling. Anastrozole and letrozole block the production of estrogens by inhibiting the enzyme aromatase.
Discussion
EOC represents the most lethal gynecological cancer. Optimal surgical debulking followed by adjuvant chemotherapy has been the mainstay of primary treatment for many years. Unfortunately, the majority of women relapse and, despite the high RR achieved using chemotherapy for recurrent EOC, the prognosis remains poor. Newer treatments, such as bevacizumab or poly(ADP-ribose) polymerase (PARP) inhibitors for BRCA mutation carriers, were recently shown to improve upon the beneficial effects of chemotherapy and provide new therapeutic options [74, 75]. Hormone therapy that targets the estrogen pathway has also been assessed in patients with recurrent EOC [76–80]. The reported RR varies in the range of 0%–28% and the SD rate varied in the range of 20%–75% [79]. For example, letrozole was evaluated as therapy for biochemically only (cancer antigen [CA]125) relapsing patients following primary chemotherapy. It was demonstrated that patients with tumors expressing high levels of ER had a better response to letrozole, suggesting that the efficacy of hormonal treatment is dependent not only on the presence of relevant receptors but also on their quantity [78]. Another target for endocrine therapy is the AR. The rationale for further evaluation of antiandrogen treatment in patients with EOC includes the following.
First, most clinical trials evaluating the use of androgen blockade for the treatment of EOC have been small, nonrandomized studies involving patients with platinum-resistant disease. In this difficult-to-treat patient population, high rates of long-term SD have been reported and these results need to be validated in large, randomized clinical trials.
Second, those agents that have been tested for AR blockade in EOC, for example, flutamide, are known to be weak AR antagonists. Newer promising treatments that are more potent suppressors of the AR axis or that inhibit androgen and estrogen synthesis should be evaluated in clinical trials for EOC. For example, abiraterone has been proven to be beneficial in prostate cancer patients who have progressed on previous treatment with GnRH analogs [81].
Third, most clinical trials assessing anti-AR strategies in EOC have not measured AR expression. The trials that did measure it used IHC, the results of which may not always reflect AR activity in EOC. We suggest the use of fluorescence in situ hybridization (FISH) to measure AR amplification in future trials of antiandrogen compounds in EOC.
The efficacy of antiandrogens in EOC patients in trials reported to date has been modest. All patients taking part in these trials had chemotherapy-resistant EOC and therefore represent a group with a poor prognosis. Similar results were reported in breast cancer patients with visceral metastases who received anastrazole (RR, 7%–14%) or exemestane (RR, 13.5%–25%) [82–85]. Endocrine treatment in breast cancer is generally more beneficial for patients with soft tissue metastases or low-volume disease. One way, therefore, of assessing the efficacy of abiraterone in ovarian cancer is to do randomized trials in asymptomatic patients with biochemically relapsing disease only (rising CA125).
Finally, patient selection is another important element that needs to be carefully addressed for a well-designed trial. Greater understanding of the contribution of androgens to the biology of EOC will assist with the selection of patients most likely to benefit from such treatments. Toward that end, we suggest as possible biomarkers: (a) the gene polymorphisms that correlate with AR activity, such as CAG repeats in exon 1 of the AR-encoding gene; (b) polymorphism A2 of CYP17A1; (c) measurement of AR amplification using FISH and its correlation with response to treatment; and (d) expression of AR pathway–associated genes such as TGF-β, ARA70, AIB1, EGFR, and Rab35. IHC quantification of Rab35 expression could be an initial starting point in this context. As suggested in the study by Smyth and colleagues, selection of patients according to percentage of tumor cells expressing hormone receptors might be more informative on the efficacy of a trial drug than patient selection based only on the presence or absence of the receptor [78]. We, therefore, suggest that correlation of the response to treatment with percentage of AR-expressing tumor cells also be assessed in future trials.
Conclusions
Antiandrogen compounds have been shown to have modest activity in patients with EOC, but data supporting their further evaluation are emerging. Newer, more potent compounds that block the synthesis of androgens and estrogens at the level of CYP17, such as abiraterone, could prove to be a useful addition to existing treatments. We suggest that the evaluation of abiraterone and other compounds with similar effects is worthwhile and should be undertaken within carefully planned clinical trials. In the light of evidence suggesting that ARs contribute to the mechanisms of EOC pathogenesis, patient selection should be aided by assessing potential biomarkers specific for that pathway.
Acknowledgments
K.J.D. was supported by a grant from the Swiss National Science Foundation (SNF). SBK and JdeB were supported by grants from Cancer Research UK, The Experimental Cancer Medicine Centres (ECMC) and the National Institute for Health Research (NIHR) to the Institute of Cancer Research, and the Royal Marsden Hospital NHS Foundation Trust.
Author Contributions
Conception/Design: Stanley B. Kaye, Johann S. de Bono, Dionysis Papadatos-Pastos
Provision of study material or patients: Dionysis Papadatos-Pastos
Collection and/or assembly of data: Konstantin J. Dedes, Dionysis Papadatos-Pastos
Data analysis and interpretation: Stanley B. Kaye, Konstantin J. Dedes, Johann S. de Bono, Dionysis Papadatos-Pastos
Manuscript writing: Stanley B. Kaye, Konstantin J. Dedes, Johann S. de Bono, Dionysis Papadatos-Pastos
Final approval of manuscript: Stanley B. Kaye, Konstantin J. Dedes, Johann S. de Bono, Dionysis Papadatos-Pastos
References
- 1.Walters KA, Allan CM, Handelsman DJ. Androgen actions and the ovary. Biol Reprod. 2008;78:380–389. doi: 10.1095/biolreprod.107.064089. [DOI] [PubMed] [Google Scholar]
- 2.Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: A hard habit to break? Cancer Cell. 2009;16:458–462. doi: 10.1016/j.ccr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Bennett NC, Gardiner RA, Hooper JD, et al. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2010;42:813–827. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 4.van Doorn HC, Burger CW, van der Valk P, et al. Oestrogen, progesterone, and androgen receptors in ovarian neoplasia: Correlation between immunohistochemical and biochemical receptor analyses. J Clin Pathol. 2000;53:201–205. doi: 10.1136/jcp.53.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger HG. Androgen production in women. Fertil Steril. 2002;77(suppl 4):S3–S5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- 6.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90:1774–1786. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 7.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 8.Schildkraut JM, Schwingl PJ, Bastos E, et al. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol. 1996;88:554–559. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]
- 9.Olsen CM, Green AC, Nagle CM, et al. Epithelial ovarian cancer: Testing the ‘androgens hypothesis’. Endocr Relat Cancer. 2008;15:1061–1068. doi: 10.1677/ERC-08-0075. [DOI] [PubMed] [Google Scholar]
- 10.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: The Iowa Women's Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 11.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–1411. [PubMed] [Google Scholar]
- 12.Olsen CM, Green AC, Whiteman DC, et al. Obesity and the risk of epithelial ovarian cancer: A systematic review and meta-analysis. Eur J Cancer. 2007;43:690–709. doi: 10.1016/j.ejca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 14.Engeland A, Tretli S, Bjørge T. Height, body mass index, and ovarian cancer: A follow-up of 1.1 million Norwegian women. J Natl Cancer Inst. 2003;95:1244–1248. doi: 10.1093/jnci/djg010. [DOI] [PubMed] [Google Scholar]
- 15.Pan SY, Johnson KC, Ugnat AM, et al. Association of obesity and cancer risk in Canada. Am J Epidemiol. 2004;159:259–268. doi: 10.1093/aje/kwh041. [DOI] [PubMed] [Google Scholar]
- 16.Wolk A, Gridley G, Svensson M, et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 17.Lahmann PH, Cust AE, Friedenreich CM, et al. Anthropometric measures and epithelial ovarian cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;126:2404–2415. doi: 10.1002/ijc.24952. [DOI] [PubMed] [Google Scholar]
- 18.Cottreau CM, Ness RB, Modugno F, et al. Endometriosis and its treatment with danazol or lupron in relation to ovarian cancer. Clin Cancer Res. 2003;9:5142–5144. [PubMed] [Google Scholar]
- 19.Giovannucci E, Stampfer MJ, Krithivas K, et al. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci U S A. 1997;94:3320–3323. doi: 10.1073/pnas.94.7.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22:3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazemi-Esfarjani P, Trifiro MA, Pinsky L. Evidence for a repressive function of the long polyglutamine tract in the human androgen receptor: Possible pathogenetic relevance for the (CAG)n-expanded neuronopathies. Hum Mol Genet. 1995;4:523–527. doi: 10.1093/hmg/4.4.523. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan G, Yang M, Cheong A, et al. Structural and functional consequences of glutamine tract variation in the androgen receptor. Hum Mol Genet. 2004;13:1677–1692. doi: 10.1093/hmg/ddh181. [DOI] [PubMed] [Google Scholar]
- 23.Lindström S, Ma J, Altshuler D, et al. A large study of androgen receptor germline variants and their relation to sex hormone levels and prostate cancer risk. Results from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. J Clin Endocrinol Metab. 2010;95:E121–E127. doi: 10.1210/jc.2009-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price DK, Chau CH, Till C, et al. Androgen receptor CAG repeat length and association with prostate cancer risk: Results from the Prostate Cancer Prevention Trial. J Urol. 2010;184:2297–2302. doi: 10.1016/j.juro.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Gonzalez G, Cabrera S, Ramirez-Moreno R, et al. Short alleles of both GGN and CAG repeats at the exon-1 of the androgen receptor gene are associated to increased PSA staining and a higher Gleason score in human prostatic cancer. J Steroid Biochem Mol Biol. 2009;113:85–91. doi: 10.1016/j.jsbmb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig AH, Murawska M, Panek G, et al. Androgen, progesterone, and FSH receptor polymorphisms in ovarian cancer risk and outcome. Endocr Relat Cancer. 2009;16:1005–1016. doi: 10.1677/ERC-08-0135. [DOI] [PubMed] [Google Scholar]
- 27.Terry KL, De Vivo I, Titus-Ernstoff L, et al. Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res. 2005;65:5974–5981. doi: 10.1158/0008-5472.CAN-04-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li AJ, Baldwin RL, Karlan BY. Short androgen receptor allele length is a poor prognostic factor in epithelial ovarian carcinoma. Clin Cancer Res. 2003;9:3667–3673. [PubMed] [Google Scholar]
- 29.Li AJ, Elmore RG, Pavelka JC, et al. Hyperandrogenism, mediated by obesity and receptor polymorphisms, promotes aggressive epithelial ovarian cancer biology. Gynecol Oncol. 2007;107:420–423. doi: 10.1016/j.ygyno.2007.07.059. [DOI] [PubMed] [Google Scholar]
- 30.Garner EI, Stokes EE, Berkowitz RS, et al. Polymorphisms of the estrogen-metabolizing genes CYP17 and catechol-O-methyltransferase and risk of epithelial ovarian cancer. Cancer Res. 2002;62:3058–3062. [PubMed] [Google Scholar]
- 31.Haiman CA, Hankinson SE, Spiegelman D, et al. The relationship between a polymorphism in CYP17 with plasma hormone levels and breast cancer. Cancer Res. 1999;59:1015–1020. [PubMed] [Google Scholar]
- 32.Nagle CM, Chenevix-Trench G, Webb PM, et al. Ovarian cancer survival and polymorphisms in hormone and DNA repair pathway genes. Cancer Lett. 2007;251:96–104. doi: 10.1016/j.canlet.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Dunning AM, Dowsett M, Healey CS, et al. Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J Natl Cancer Inst. 2004;96:936–945. doi: 10.1093/jnci/djh167. [DOI] [PubMed] [Google Scholar]
- 34.Anzick SL, Kononen J, Walker RL, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 35.Shaw PA, Rittenberg PV, Brown TJ. Activation of androgen receptor-associated protein 70 (ARA70) mRNA expression in ovarian cancer. Gynecol Oncol. 2001;80:132–138. doi: 10.1006/gyno.2000.6068. [DOI] [PubMed] [Google Scholar]
- 36.Tanner MM, Grenman S, Koul A, et al. Frequent amplification of chromosomal region 20q12–q13 in ovarian cancer. Clin Cancer Res. 2000;6:1833–1839. [PubMed] [Google Scholar]
- 37.Li AJ, Lerner DL, Gapuzan ME, et al. AIB1 polymorphisms predict aggressive ovarian cancer phenotype. Cancer Epidemiol Biomarkers Prev. 2005;14:2919–2922. doi: 10.1158/1055-9965.EPI-05-0540. [DOI] [PubMed] [Google Scholar]
- 38.Evangelou A, Jindal SK, Brown TJ, et al. Down-regulation of transforming growth factor beta receptors by androgen in ovarian cancer cells. Cancer Res. 2000;60:929–935. [PubMed] [Google Scholar]
- 39.Havrilesky LJ, Hurteau JA, Whitaker RS, et al. Regulation of apoptosis in normal and malignant ovarian epithelial cells by transforming growth factor beta. Cancer Res. 1995;55:944–948. [PubMed] [Google Scholar]
- 40.Evangelou A, Letarte M, Jurisica I, et al. Loss of coordinated androgen regulation in nonmalignant ovarian epithelial cells with BRCA1/2 mutations and ovarian cancer cells. Cancer Res. 2003;63:2416–2424. [PubMed] [Google Scholar]
- 41.Carey MS, Agarwal R, Gilks B, et al. Functional proteomic analysis of advanced serous ovarian cancer using reverse phase protein array: TGF-beta pathway signaling indicates response to primary chemotherapy. Clin Cancer Res. 2010;16:2852–2860. doi: 10.1158/1078-0432.CCR-09-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Do TV, Kubba LA, Du H, et al. Transforming growth factor-β1, transforming growth factor-β2, and transforming growth factor-β3 enhance ovarian cancer metastatic potential by inducing a Smad3-dependent epithelial-to-mesenchymal transition. Mol Cancer Res. 2008;6:695–705. doi: 10.1158/1541-7786.MCR-07-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto I. Epidermal growth factor receptor in relation to tumor development: EGFR-targeted anticancer therapy. FEBS J. 2010;277:309–315. doi: 10.1111/j.1742-4658.2009.07449.x. [DOI] [PubMed] [Google Scholar]
- 44.Hudson LG, Moss NM, Stack MS. EGF-receptor regulation of matrix metalloproteinases in epithelial ovarian carcinoma. Future Oncol. 2009;5:323–338. doi: 10.2217/FON.09.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stadlmann S, Gueth U, Reiser U, et al. Epithelial growth factor receptor status in primary and recurrent ovarian cancer. Mod Pathol. 2006;19:607–610. doi: 10.1038/modpathol.3800575. [DOI] [PubMed] [Google Scholar]
- 46.Traish AM, Morgentaler A. Epidermal growth factor receptor expression escapes androgen regulation in prostate cancer: A potential molecular switch for tumour growth. Br J Cancer. 2009;101:1949–1956. doi: 10.1038/sj.bjc.6605376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ilekis JV, Connor JP, Prins GS, et al. Expression of epidermal growth factor and androgen receptors in ovarian cancer. Gynecol Oncol. 1997;66:250–254. doi: 10.1006/gyno.1997.4764. [DOI] [PubMed] [Google Scholar]
- 48.Li AJ, Scoles DR, Armstrong KU, et al. Androgen receptor cytosine-adenine-guanine repeat polymorphisms modulate EGFR signaling in epithelial ovarian carcinomas. Gynecol Oncol. 2008;109:220–225. doi: 10.1016/j.ygyno.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Sheach LA, Adeney EM, Kucukmetin A, et al. Androgen-related expression of G-proteins in ovarian cancer. Br J Cancer. 2009;101:498–503. doi: 10.1038/sj.bjc.6605153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nourbakhsh M, Golestani A, Zahrai M, et al. Androgens stimulate telomerase expression, activity and phosphorylation in ovarian adenocarcinoma cells. Mol Cell Endocrinol. 2010;330:10–16. doi: 10.1016/j.mce.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 51.Parmar H, Phillips RH, Rustin G, et al. Therapy of advanced ovarian cancer with D-Trp-6-LH-RH (decapeptyl) microcapsules. Biomed Pharmacother. 1988;42:531–538. [PubMed] [Google Scholar]
- 52.Kavanagh JJ, Roberts W, Townsend P, et al. Leuprolide acetate in the treatment of refractory or persistent epithelial ovarian cancer. J Clin Oncol. 1989;7:115–118. doi: 10.1200/JCO.1989.7.1.115. [DOI] [PubMed] [Google Scholar]
- 53.Lind MJ, Cantwell BM, Millward MJ, et al. A phase II trial of goserelin (Zoladex) in relapsed epithelial ovarian cancer. Br J Cancer. 1992;65:621–623. doi: 10.1038/bjc.1992.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller DS, Brady MF, Barrett RJ. A phase II trial of leuprolide acetate in patients with advanced epithelial ovarian carcinoma. A Gynecologic Oncology Group study. Am J Clin Oncol. 1992;15:125–128. doi: 10.1097/00000421-199204000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Carnino F, Iskra L, Fuda G, et al. The treatment of progressive ovarian carcinoma with D-Trp-LHRH (Decapeptyl). Gruppo Oncologico Nord Ovest (GONO) Eur J Cancer. 1994;30A:1903–1904. doi: 10.1016/0959-8049(94)00272-7. [DOI] [PubMed] [Google Scholar]
- 56.Marinaccio M, D'Addario V, Serrati A, et al. Leuprolide acetate as a salvage-therapy in relapsed epithelial ovarian cancer. Eur J Gynaecol Oncol. 1996;17:286–288. [PubMed] [Google Scholar]
- 57.Jager W, Sauerbrei W, Beck E, et al. A randomized comparison of triptorelin and tamoxifen as treatment of progressive ovarian cancer. Anticancer Res. 1995;15:2639–2642. [PubMed] [Google Scholar]
- 58.Ron IG, Wigler N, Merimsky O, et al. A phase II trial of D-Trp-6-LHRH (decapeptyl) in pretreated patients with advanced epithelial ovarian cancer. Cancer Invest. 1995;13:272–275. doi: 10.3109/07357909509094461. [DOI] [PubMed] [Google Scholar]
- 59.Duffaud F, van der Burg ME, Namer M, et al. D-TRP-6-LHRH (Triptorelin) is not effective in ovarian carcinoma: An EORTC Gynaecological Cancer Co-operative Group study. Anticancer Drugs. 2001;12:159–162. doi: 10.1097/00001813-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Paskeviciute L, Roed H, Engelholm S. No rules without exception: Long-term complete remission observed in a study using a LH-RH agonist in platinum-refractory ovarian cancer. Gynecol Oncol. 2002;86:297–301. doi: 10.1006/gyno.2002.6778. [DOI] [PubMed] [Google Scholar]
- 61.du Bois A, Meier W, Lück HJ, et al. Chemotherapy versus hormonal treatment in platinum- and paclitaxel-refractory ovarian cancer: A randomised trial of the German Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) Study Group Ovarian Cancer. Ann Oncol. 2002;13:251–257. doi: 10.1093/annonc/mdf038. [DOI] [PubMed] [Google Scholar]
- 62.Balbi G, Piano LD, Cardone A, et al. Second-line therapy of advanced ovarian cancer with GnRH analogs. Int J Gynecol Cancer. 2004;14:799–803. doi: 10.1111/j.1048-891X.2004.014511.x. [DOI] [PubMed] [Google Scholar]
- 63.Kang SK, Cheng KW, Nathwani PS, et al. Autocrine role of gonadotropin-releasing hormone and its receptor in ovarian cancer cell growth. Endocrine. 2000;13:297–304. doi: 10.1385/ENDO:13:3:297. [DOI] [PubMed] [Google Scholar]
- 64.Radu A, Pichon C, Camparo P, et al. Expression of follicle-stimulating hormone receptor in tumour blood vessels. N Engl J Med. 2010;363:1621–1630. doi: 10.1056/NEJMoa1001283. [DOI] [PubMed] [Google Scholar]
- 65.Tumolo S, Rao BR, van der Burg ME, et al. Phase II trial of flutamide in advanced ovarian cancer: An EORTC Gynaecological Cancer Cooperative Group study. Eur J Cancer. 1994;30A:911–914. doi: 10.1016/0959-8049(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 66.Vassilomanolakis M, Koumakis G, Barbounis V, et al. A phase II study of flutamide in ovarian cancer. Oncology. 1997;54:199–202. doi: 10.1159/000227688. [DOI] [PubMed] [Google Scholar]
- 67.Hasan J, Ton N, Mullamitha S, et al. Phase II trial of tamoxifen and goserelin in recurrent epithelial ovarian cancer. Br J Cancer. 2005;93:647–651. doi: 10.1038/sj.bjc.6602752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hofstra LS, Mourits MJ, de Vries EG, et al. Combined treatment with goserelin and tamoxifen in patients with advanced chemotherapy resistant ovarian cancer. Anticancer Res. 1999;19:3627–3630. [PubMed] [Google Scholar]
- 69.Levine D, Park K, Juretzka M, et al. A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission. Cancer. 2007;110:2448–2456. doi: 10.1002/cncr.23072. [DOI] [PubMed] [Google Scholar]
- 70.Barrie SE, Potter GA, Goddard PM, et al. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17–20 lyase) J Steroid Biochem Mol Biol. 1994;50:267–273. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 71.Ang JE, Olmos D, de Bono JS. CYP17 blockade by abiraterone: Further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br J Cancer. 2009;100:671–675. doi: 10.1038/sj.bjc.6604904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;21:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Basu B, Ang J, Blanco M, et al. Preliminary report of efficacy of abiraterone acetate in patients with estrogen (ER) or androgen receptor (AR) positive, advanced breast carcinoma resistant to standard endocrine therapies. Presented at the 2010 European Organization for Research and Treatment of Cancer–National Cancer Institute–American Association for Cancer Research Symposium; November 16–19, 2010; Berlin, Germany. [Google Scholar]
- 74.Banerjee S, Gore M. The future of targeted therapies in ovarian cancer. The Oncologist. 2009;14:706–716. doi: 10.1634/theoncologist.2009-0013. [DOI] [PubMed] [Google Scholar]
- 75.Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 76.Bowman A, Gabra H, Langdon SP, et al. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: Identification of an endocrine-sensitive subgroup. Clin Cancer Res. 2002;8:2233–2239. [PubMed] [Google Scholar]
- 77.Papadimitriou CA, Markaki S, Siapkaras J, et al. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer: Long-term results of a phase II study. Oncology. 2004;66:112–117. doi: 10.1159/000077436. [DOI] [PubMed] [Google Scholar]
- 78.Smyth JF, Gourley C, Walker G, et al. Antiestrogen therapy is active in selected ovarian cancer cases: The use of letrozole in estrogen receptor-positive patients. Clin Cancer Res. 2007;13:3617–3622. doi: 10.1158/1078-0432.CCR-06-2878. [DOI] [PubMed] [Google Scholar]
- 79.del Carmen MG, Fuller AF, Matulonis U, et al. Phase II trial of anastrozole in women with asymptomatic mullerian cancer. Gynecol Oncol. 2003;91:596–602. doi: 10.1016/j.ygyno.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 80.Makar AP. Hormone therapy in epithelial ovarian cancer. Endocr Relat Cancer. 2000;7:85–93. doi: 10.1677/erc.0.0070085. [DOI] [PubMed] [Google Scholar]
- 81.Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jonat W, Howell A, Blomqvist C, et al. A randomised trial comparing two doses of the new selective aromatase inhibitor anastrozole (Arimidex) with megestrol acetate in postmenopausal patients with advanced breast cancer. Eur J Cancer. 1996;32A:404–412. doi: 10.1016/0959-8049(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 83.Kaufmann M, Bajetta E, Dirix LY, et al. Exemestane is superior to megestrol acetate after tamoxifen failure in postmenopausal women with advanced breast cancer: Results of a phase III randomized double-blind trial. The Exemestane Study Group. J Clin Oncol. 2000;18:1399–1411. doi: 10.1200/JCO.2000.18.7.1399. [DOI] [PubMed] [Google Scholar]
- 84.Kvinnsland S, Anker G, Dirix LY, et al. High activity and tolerability demonstrated for exemestane in postmenopausal women with metastatic breast cancer who had previously failed on tamoxifen treatment. Eur J Cancer. 2000;36:976–982. doi: 10.1016/s0959-8049(00)00041-1. [DOI] [PubMed] [Google Scholar]
- 85.Mauriac L, Pippen JE, Quaresma Albano J, et al. Fulvestrant (Faslodex) versus anastrozole for the second-line treatment of advanced breast cancer in subgroups of postmenopausal women with visceral and non-visceral metastases: Combined results from two multicentre trials. Eur J Cancer. 2003;39:1228–1233. doi: 10.1016/s0959-8049(03)00199-0. [DOI] [PubMed] [Google Scholar]