Definitions of time to deterioration in quality of life scores were examined as a modality of longitudinal quality of life assessment in breast cancer patients undergoing different surgical procedures, according to different cutoffs of the minimal clinically important difference.
Keywords: Quality of life, Longitudinal analysis, Methodology, Breast cancer
Abstract
Purpose.
This prospective multicenter study explored different definitions of time to deterioration (TTD) in quality of life (QoL) scores, according to different cutoffs of the minimal clinically important difference (MCID) as a modality for longitudinal QoL assessment in breast cancer patients.
Methods.
QoL was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 and BR-23 before surgery, after surgery, and 6 and 12 months later. The global health score, arm symptoms score (BRAS), and breast symptoms score were analyzed. For a given baseline score, QoL was considered to have deteriorated if this score decreased by ≥5 points at any time point after baseline. Analyses were repeated using an MCID of 10 points and taking the score after surgery as the reference score (to explore the occurrence of response shift). TTD was calculated using the Kaplan–Meier method and Cox regression was used to identify independent factors associated with TTD.
Results.
Two hundred thirty-five patients underwent axillary lymph node dissection (ALND), 222 underwent sentinel lymph node biopsy (SLNB), and 61 underwent SLNB plus ALND. Patients who underwent SLNB had a significantly longer TTD for the BRAS dimension than those who underwent ALND. Cox multivariate analyses showed that treatment using SLNB and age >59 years were independently associated with longer TTD for the BRAS, whereas surgery elsewhere than at the Centre Georges François Leclerc was associated with a shorter TTD.
Conclusion.
Exploration of different definitions of TTD in QoL provides meaningful longitudinal QoL results for clinicians.
Introduction
In cancer research, the number of studies that incorporate quality of life (QoL) has been growing over the last decade [1]. Although the number of cancer clinical trials that include QoL assessment is increasing, there is also evidence that analysis of QoL presents methodological and statistical difficulties because of the type of data generated and the multidimensional nature of the instruments. One of the major concerns has been missing data [2]. Indeed, in longitudinal studies, observations of patients can be missed at certain time points because they miss visits or do not fill in certain questionnaires. In these cases, the interpretation of results for QoL can be seriously hampered by these missing data. Many publications have proposed methods for the collection, analysis, and interpretation of health-related quality of life (QoL) data [3–7]. Although these are useful to researchers and clinical trial investigators devoted to QoL research, some methods present statistical analyses and results that are difficult for physicians to use and interpret. Thus, there is a need to define a standard analysis of QoL data and presentation of results in ways that are clinically meaningful and deal with missing data. Indeed, QoL results must help clinicians in decision making while keeping the strength of the analyses [8].
The aim of this study was to explore definitions of time to deterioration (TTD) in QoL scores as a modality of longitudinal QoL assessment in breast cancer (BC) patients undergoing different surgical procedures, according to different cutoffs of the minimal clinically important difference (MCID).
Patients and Methods
Patients
The design of this study has been described elsewhere [9]. Briefly, it was a multicenter prospective cohort study including all women operated on for BC as the primary treatment in five hospitals of the Côte d'Or and Saône-et-Loire. Patients underwent axillary lymph node dissection (ALND) or sentinel lymph node biopsy (SLNB) according to the usual practice of the surgeon. A third group was defined for patients who underwent SLNB with additional lymphadenectomy. Cases were registered from January 2005 to January 2006.
All patients signed a written informed consent form and the protocol of the study was approved by the regional ethics committee.
QoL
QoL was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30 and the EORTC QLQ-BR23 [10] before surgery, just after surgery, and 6 and 12 months later. QoL was assessed at 12 months because the purpose of this study was to assess the early impact of surgical modality on QoL. We assessed QoL at these specific times based on clinical meaning in order to focus on the impact of surgery at 1 year with a feasible pragmatic collection of QoL data.
The global health score (GHS), arm symptoms score (BRAS), and breast symptoms score (BRBS) were targeted for analyses. The QLQ-C30 is a cancer-specific tool composed of 30 items that generate 15 scores: five scores of functional parameters, a financial difficulties scale, and eight scores for symptoms. The BC module is comprised of 23 questions assessing disease symptoms and side effects of treatment.
These scores vary from 0 (worst) to 100 (best) for the GHS and from 0 (best) to 100 (worst) for symptom parameters.
Statistical Methods
Continuous and qualitative variables are described using means, standard deviations, medians, and percentages. Patient characteristics are described according to the baseline completion of the questionnaire in order to determine nonrandom missing patient profiles. We also reported baseline QoL scores according to type of surgery. Then, analyses were done in patients with QoL completion at baseline.
TTD was defined as the time from inclusion in the study to deterioration in the following scores: GHS, BRAS, and BRBS. Patients were considered to have deteriorated for a given dimension if a decrease ≥5 points (5% of the theoretical score range) at any time point after baseline [5] was observed. Patients were censored at the time of the last QoL assessment completed if they had not deteriorated before that. All patients who had a baseline and at least one follow-up QoL assessment were included in the TTD analysis. Analyses were also performed for events defined as the first 5-point decrease in at least one of the following scores: GHS, BRAS, or BRBS.
Sensitivity analyses were performed to assess different definitions of TTD. Analyses were repeated for two other models: (a) considering patients to have deteriorated if the change in their score from baseline was ≥10 points [5] and (b) considering the score just after surgery as the reference score to integrate the occurrence of a response shift [11]. Analyses were also performed in which missing values were considered events.
TTD was calculated using the Kaplan–Meier method and compared using the log-rank test according to type of surgery. TTD is described using medians with 95% confidence intervals (CIs). The univariate Cox model was used to calculate the hazard ratio (HR) with 95% CI. Multivariate Cox regression, with type of surgery and other covariates, was applied to identify independent factors associated with TTD for each symptom. All variables with a univariate p-value ≤ .20 from the Cox univariate analyses were eligible for multivariate analyses. Correlations were tested for eligible variables. To prevent collinearity, when two variables were significantly correlated, one variable was retained according to its clinical relevance or to the value of the likelihood ratio. The type of surgery and the hospital of treatment were forced into the multivariate analyses. All tests were two sided, and analyses were performed with Stata, version 11 software (StataCorp LP, College Station, TX).
Results
Patients
Between January 1, 2005 and January 1, 2006, 518 BC patients were included. Two hundred thirty-five patients underwent ALND, 222 patients underwent SLNB, and 61 patients had SLNB with complementary ALND. Patient characteristics according to surgery group are detailed elsewhere [9].
QoL Scores at Baseline and Compliance
Three hundred eleven patients (60%) completed the QoL questionnaire at baseline, 354 (68%), 428 (83%), and 412 (80%) completed the QoL questionnaire after surgery, 6 months, and 12 months later, respectively. Patient clinical and pathologic characteristics, according to QoL completion at baseline, are presented in Table 1. At baseline, patients who completed the QoL questionnaires and those who did not were similar for most clinical characteristics except for hospital: hormonal status (p = .44), hormonal replacement therapy (p = .14), histoprognostic Scarff–Bloom–Richardson grade (p = .31), c-erb-2 status (p = .89), hormone receptor status (p = .1), tumor histology (p = .97), hospital (p < .0001). In fact, the proportion of patients who did not complete a QoL questionnaire at baseline was higher in women treated in Châlon sur Saône Hospital and the Centre Georges François Leclerc (CGFL). Despite this unique difference, the mean QoL scores at baseline were similar in all domains (Table 2).
Table 1.
Characteristics of patients according to the completion of a quality of life questionnaire at baseline
Abbreviations: HRT, hormone replacement therapy; SD, standard deviation.
Table 2.
Quality of life at baseline according to type of node dissection
Abbreviations: ALND, axillary lymph node dissection; QLQ, quality of life questionnaire; SD, standard deviation; SLNB, sentinel lymph node biopsy.
QoL TTD Analyses
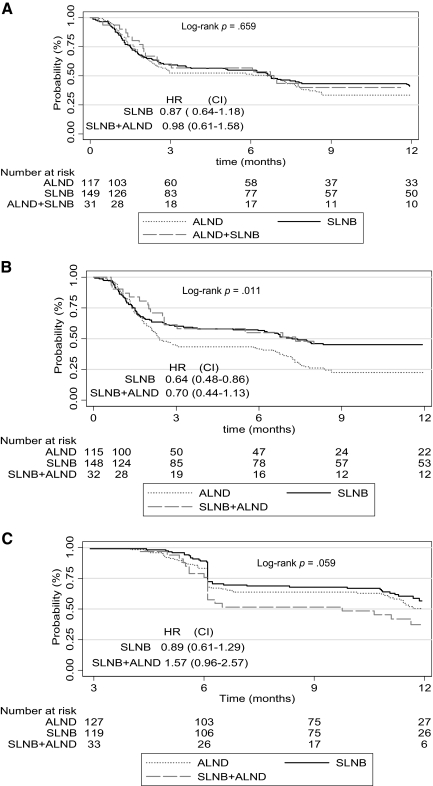
The results in Table 3 show that a TTD ≥5 points in QoL score differed according to type of surgery only for the BRAS (Fig. 1). In fact, 92 patients in the ALND group, 86 patients in the SLNB group, and 21 patients in the SLNB plus ALND group experienced a TTD ≥5 points. The median TTD in the BRAS was 2.4 months (95% CI, 2.0–6.1 months) in the ALND group, 7.2 months (95% CI, 3.7–12.9 months) in the SLNB group, and 7.3 months (95% CI, 2.5–13.6 months) in the SLNB plus ALND group (log-rank p = .011). Compared with the ALND group, the univariate hazard ratios (HRs) were 0.64 (95% CI, 0.48–0.86) and 0.70 (95% CI, 0.44–1.13) for the SLNB and SLNB plus ALND groups, respectively.
Table 3.
Time to deterioration ≥5 points in quality of life score according to type of node dissection
Abbreviations: ALND, axillary lymph node dissection; CI, confidence interval; HR, hazard ratio; SLNB, sentinel lymph node biopsy.
Figure 1.
Time to a five-point deterioration in quality of life score. (A): Global health. (B): Arm symptoms. (C): Breast symptoms.
Abbreviations: ALND, axillary lymph node dissection; CI, confidence interval; HR, hazard ratio; SLNB, sentinel lymph node biopsy.
For the GHS, 81 patients in the ALND group, 90 patients in the SLNB group, and 22 patients in the SLNB plus ALND group experienced a deterioration ≥5 points. The median TTD ≥5 points in the GHS was 6.6 months (95% CI, 2.4–7.7 months) for patients who underwent ALND, 6.7 months (95% CI, 3.26–11.9 months) for patients in the SLNB group, and 6.8 months (95% CI, 2.0–13.0 months) for patients in the SLNB plus ALND group (log-rank p-value = .66). Compared with the ALND group, the univariate HRs were 0.87 (95% CI, 0.64–1.18) and 0.98 (95% CI, 0.61–1.58) for the SLNB and SLNB plus ALND groups, respectively.
For the BRBS, 59 patients in the ALND group, 53 patients in the SLNB group, and 22 patients in the SLNB plus ALND group experienced a deterioration ≥5 points. The median TTD in the BRBS was 12.2 months (95% CI, 11.0 to not reached [NR]), 12.2 months (95% CI, 11.7 to NR), and 9.7 months (95% CI, 6.1–12.2 months) for the ALND, SLNB, and SLNB plus ALND groups, respectively (log-rank p = .059). Compared with the ALND group, the univariate HRs were 0.89 (95% CI, 0.61–1.29) and 1.57 (95% CI, 0.96–2.57) for the SLNB and SLNB plus ALND groups, respectively.
Cox multivariate analyses (Table 4) showed that treatment with SLNB (HR, 0.42; 95% CI, 0.19–0.95) and age >59 years (HR, 0.63; 95% CI, 0.45–0.87) were independently associated with a longer TTD for the BRAS, whereas, surgery elsewhere than at the CGFL (HR, 1.48; 95% CI, 1.006–2.18) was associated with a shorter TTD.
Table 4.
Multivariate Cox analysis for a 5- and 10-point deterioration
Table 4a.
(continued)
Abbreviations: ALND, axillary lymph node dissection; CGFL, Centre Georges François Leclerc; CI, confidence interval; HR, hazard ratio; SLNB, sentinel lymph node biopsy.
When considering a deterioration (5 or 10 points as the MCID) of at least one score as an event, the TTD did not differ significantly according to type of treatment (supplemental online Fig. 1). The number of patients who experienced a deterioration ≥5 points in one of the three scores was 125 in the ALND group, 125 in the SLNB group, and 36 in the SLNB plus ALND group, with median TTD of 2.86 months (2.26–5.06 months), 3.56 months (2.03–6.1 months), and 5 months (2.0–6.0 months) for the ALND, SLNB, and ALND plus SLNB groups, respectively (log-rank p = .45). Multivariate Cox analysis showed that only treatment elsewhere than at the CGFL (HR, 1.47; 95% CI, 1.11–1.94) was independently associated with a shorter TTD for a 5-point decrease. For a 10-point decrease, treatment elsewhere than at the CGFL (HR, 1.44; 95% CI, 1.08–1.92) was independently associated with a shorter TTD, whereas age >59 years (HR, 0.76; 95% CI, 0.60–0.98) was independently associated with a longer TTD.
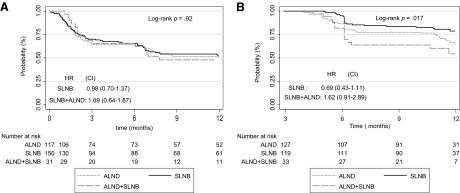
The Kaplan–Meier probability curves for TTD ≥10 points in QoL scores are shown in Figure 2. For the GHS, the TTD was not significantly different according to the type of surgery—HRs of 0.98 (95% CI, 0.70–1.37) and 1.09 (95% CI, 0.60–1.87) for the SLNB and SLNB plus ALND groups, respectively, compared with the ALND group. For the BRBS, the TTD was not significantly different according to type of surgery either—HRs of 0.69 (95% CI, 0.43–1.11) and 1.62 (95% CI, 0.91–2.89) for the SLNB and SLNB plus ALND groups, respectively, compared with the ALND group. However, the TTD was significantly shorter in the SLNB plus ALND group (log-rank p = .017) than in the SLNB group. The results were the same as those for a 5-point deterioration in the BRAS.
Figure 2.
Time to 10-point deterioration in quality of life score. (A): Global health. (B): Breast symptoms.
Abbreviations: ALND, axillary lymph node dissection; CI, confidence interval; HR, hazard ratio; SLNB, sentinel lymph node biopsy.
When the score after surgery was used as the reference, the TTD did not differ significantly according to type of surgery (log-rank p = .751 and 0.128 for the GHS and BRAS, respectively) (supplemental online Fig. 2).
When missing values were considered events for censored patients, the overall results did not differ significantly. Cox univariate analysis (supplemental online Fig. 3b) shows that SLNB was associated with a longer TTD than with ALND (HR, 0.72; 95% CI, 0.55–0.95) for arm symptoms. For the GHS (p = .859, supplemental online Fig. 3a) and BRBS (p = .081, supplemental online Fig. 3c), the TTD did not differ significantly according to treatment protocol.
Discussion
In this study, we examined TTD as a method for longitudinal analysis of QoL in BC studies. We evaluated this method on data from a prospective multicenter study comparing the impact of three different surgical procedures on health-related QoL in patients with BC: SLNB, ALND, and SLNB plus ALND. QoL was assessed just after surgery and 6 and 12 months later, based on clinical meaning and on a pragmatic approach for this study. In fact, we believe that, at 6 or 12 months after surgery, patients could have recovered their baseline QoL level or shown a trend for QoL deterioration. Our study shows that, for the BRAS, women who underwent SLNB experienced a longer TTD than women who underwent ALND. Cox regression analyses showed that SLNB and age >59 years were independently associated with a longer TTD for the BRAS, whereas surgery elsewhere than at the CGFL was associated with a shorter TTD. This difference in the TTD for patients treated at CGFL compared with those treated at the other participating centers was probably a result of the fact that the CGFL is a comprehensive cancer care center with a high quality of care and because of the greater number of patients treated at this institution. In addition, when the study was performed, the learning curve for the SLNB surgical technique was complete at CGFL but it was ongoing at the other participating centers.
Our results are in agreement with those obtained with a mixed model analysis of variance for repeated measures. In every instance, the beneficial effect of SLNB on BC patient QoL, compared with ALND, was observed [9].
Previous nonrandomized and randomized [12–14] studies have already reported that SLNB is associated with less arm and shoulder morbidity than with ALND. Moreover, our results show that, when considering the first five-point decrease in one of the three scores as an event, the TTD did not differ significantly according to type of treatment. This is explained by the difficulty in creating a single measure that aggregates the multiple dimensions of QoL and that is valid in all contexts. There is also potential for a particular intervention to produce benefits in one dimension and deficits in another that cancel each other out [10].
To assess how missing data could affect QoL results, in sensitivity analyses, patients were considered to have deteriorated when they did not have an assessment at a time point. This assumed that the reason for the missing data was the worsening of the patient's QoL. The results were only slightly different. In particular, for the BRAS, the TTD was still significantly shorter in patients who underwent ALND than in those who underwent SLNB.
In this study, the results for TTD were similar for a deterioration of five points and for a deterioration of 10 points. The small differences in the numbers of patients who deteriorated with a threshold MCID of five points compared with those experiencing deterioration with a threshold of 10 points indicate that, even with a five-point difference in QoL score, we could capture the information related to QoL deterioration. Based on our results, we suggest that a threshold MCID of five points is a clinically meaningful cutoff for event definition. Nonetheless, it is also important to underline that major differences in QoL deterioration could not be captured using a five-point difference in the QoL score. For example, our results showed a significant difference between the SLNB plus ALND group (log-rank p = .017) and the SLNB group for the BRBS QoL only when the deterioration threshold was 10 points, suggesting differences for major QoL deterioration only.
One of the limits of our study is that a response-shift assessment had not been integrated in the design of the study. In fact, as reported by Bernard et al. [15], a response shift may attenuate estimates of treatment effects because of adaptation of the patient to the treatment or the disease. In this study, therefore, the occurrence of a response shift was assessed by changing the QoL reference score. The score just after surgery was used as the reference score rather than the inclusion QoL. The results showed no effect of treatment on TTD in all three QoL dimensions. As mentioned in the literature, changes in patients' internal standards, values, and the disease trajectory may distort the comparability of longitudinal assessments [15]. Although using the score just after surgery as the reference score could only be considered a surrogate method to assess response shift, we expected to have an overview of how QoL scores could vary over time when the reference is modified. The same issue exists with the Response Evaluation Criteria in Solid Tumors regarding the choice of reference for tumor measurement to capture progression or response (i.e., tumor measurement before treatment or nadir as the reference) [16]. However, another study to assess the impact of response shift on longitudinal QoL in patients with primary BC using the dedicated then-test method as the reference [17] is in progress.
Here, we used TTD in QoL as a conservative approach that took into account nonignorable missing data in cancer clinical trials [18]. Indeed, as can be expected in cancer clinical trials, a substantial proportion of patients often progress or deteriorate and withdraw from the study. The usual high attrition rate can be related to the treatment received and is a complicating factor in repeated-measures analysis of variance and interpretation of QoL data. The TTD approach is similar to other time-to-event analyses, such as time to progression, and has already been used in the analysis of QoL in other cancer locations [19–22]. TTD is less affected by missing data than is a classical analysis of variance. Patients can be kept in the analysis even if some of their questionnaires are missing as long as they have assessable questionnaires afterward. Moreover, because this is not an absorbing state, that is, it is not a time to definitive QoL deterioration, these results could help clinicians to determine time frames for QoL deterioration and then to adapt the therapeutic strategy to improve QoL. One of the benefits of the TTD approach is that it provides results that are readily meaningful to clinicians and are more likely to influence clinical decision making. Other definitions are to be studied, including those that take into account the phenomenon of response shift or use appropriate methods to take account of intermittent missing data.
In conclusion, our study showed that, for the BRAS, women who underwent SLNB experienced a longer TTD than did women who underwent ALND. Furthermore, our results showed that for the GHS and BRAS QoL, there was no significant longitudinal difference between BC patients who underwent SLNB followed by a complementary ALND and those who underwent ALND alone. However, the TTD was significantly shorter in the SLNB plus ALND group than in the SLNB group for the BRBS dimension. One explanation could be that these patients underwent two operations and therefore had more postoperative side effects. In clinical practice, therefore, clinicians should be aware that the selection of patients for SLNB is critical for patient QoL. Although SLNB could improve QoL, complementary ALND could have a deleterious effect. Patients should thus be clearly identified to maintain the potential beneficial effect of this strategy.
Supplementary Material
Acknowledgments
We thank Melanie Gauthier (Unité de Biostatistique et d'Épidémiologie, DIM, CGFL) for statistical assistance and Philip Bastable for correcting the manuscript.
Funded by a grant from Ligue Regionale Contre le Cancer (Côte d'Or Committee) and by Fondation de France.
Author Contributions
Conception/Design: Mariette Mercier, Franck Bonnetain
Provision of study material or patients: Jean Fraisse, Sylvain Causeret, Hervé Tixier, Marie-Martine Padeano, Catherine Loustalot, Jean Cuisenier, Jean-Marc Sauzedde, M. Smail, Jean-Philibert Combier, Patrick Chevillote, Christian Rosburger, Patrick Arveux
Collection and/or assembly of data: Jean Fraisse, Sylvain Causeret, Hervé Tixier, Marie-Martine Padeano, Catherine Loustalot, Jean Cuisenier, Jean-Marc Sauzedde, M. Smail, Jean-Philibert Combier, Patrick Chevillote, Christian Rosburger, Patrick Arveux
Data analysis and interpretation: Zeinab Hamidou, Tienhan S. Dabakuyo, Franck Bonnetain
Manuscript writing: Zeinab Hamidou, Tienhan S. Dabakuyo, Franck Bonnetain
Final approval of manuscript: Zeinab Hamidou, Tienhan S. Dabakuyo, Mariette Mercier, Jean Fraisse, Sylvain Causeret, Hervé Tixier, Marie-Martine Padeano, Catherine Loustalot, Jean Cuisenier, Jean-Marc Sauzedde, M. Smail, Jean-Philibert Combier, Patrick Chevillote, Christian Rosburger, Patrick Arveux, Franck Bonnetain
References
- 1.Sanders C, Egger M, Donovan J, et al. Reporting on quality of life in randomised controlled trials: Bibliographic study. BMJ. 1998;317:1191–1194. doi: 10.1136/bmj.317.7167.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Post WJ, Buijs C, Stolk RP, et al. The analysis of longitudinal quality of life measures with informative drop-out: A pattern mixture approach. Qual Life Res. 2010;19:137–148. doi: 10.1007/s11136-009-9564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staquet M, Berzon R, Osoba D, et al. Guidelines for reporting results of quality of life assessments in clinical trials. Qual Life Res. 1996;5:496–502. doi: 10.1007/BF00540022. [DOI] [PubMed] [Google Scholar]
- 4.Osoba D, Zee B. Completion rates in health-related quality-of-life assessment: Approach of the National Cancer Institute of Canada Clinical Trials Group. Stat Med. 1998;17:603–612. doi: 10.1002/(sici)1097-0258(19980315/15)17:5/7<603::aid-sim807>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 6.Fairclough DL. Design and Analysis of Quality of Life Studies in Clinical Trials. Boca Raton, FL: Chapman and Hall, 2002:1–307. [Google Scholar]
- 7.Sprangers MA, Moinpour CM, Moynihan TJ, et al. Assessing meaningful change in quality of life over time: A users' guide for clinicians. Mayo Clin Proc. 2002;77:561–571. doi: 10.4065/77.6.561. [DOI] [PubMed] [Google Scholar]
- 8.Lipscomb J, Donaldson MS, Arora NK, et al. Cancer outcomes research. J Natl Cancer Inst Monogr. 2004;(33):178–197. doi: 10.1093/jncimonographs/lgh039. [DOI] [PubMed] [Google Scholar]
- 9.Dabakuyo TS, Fraisse J, Causeret S, et al. A multicenter cohort study to compare quality of life in breast cancer patients according to sentinel lymph node biopsy or axillary lymph node dissection. Ann Oncol. 2009;20:1352–1361. doi: 10.1093/annonc/mdp016. [DOI] [PubMed] [Google Scholar]
- 10.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 11.Sprangers MA, Schwartz CE. The challenge of response shift for quality-of-life-based clinical oncology research. Ann Oncol. 1999;10:747–749. doi: 10.1023/a:1008305523548. [DOI] [PubMed] [Google Scholar]
- 12.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 13.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 14.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: Results from a randomized controlled trial. J Clin Oncol. 2005;23:4312–4321. doi: 10.1200/JCO.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 15.Bernhard J, Hr̈ny C, Maibach R, et al. Quality of life as subjective experience: Reframing of perception in patients with colon cancer undergoing radical resection with or without adjuvant chemotherapy. Swiss Group for Clinical Cancer Research (SAKK) Ann Oncol. 1999;10:775–782. doi: 10.1023/a:1008311918967. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Bonnetain F, Conroy T, Velten M, et al. Impact of response shift (RS) in longitudinal post-operative quality of life (QoL) analysis among breast cancer (BC) patients: A randomized multicenter cohort study. Presented at the annual meeting of the International Society for Quality of Life Research (ISOQOL) in London; October 28–30, 2010; England. [Google Scholar]
- 18.Donaldson GW, Moinpour CM. Learning to live with missing quality-of-life data in advanced-stage disease trials. J Clin Oncol. 2005;23:7380–7384. doi: 10.1200/JCO.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Bonnetain F, Dahan L, Maillard E, et al. Time until definitive quality of life score deterioration as a means of longitudinal analysis for treatment trials in patients with metastatic pancreatic adenocarcinoma. Eur J Cancer. 2010;46:2753–2762. doi: 10.1016/j.ejca.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Bezjak A, Tu D, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: Quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2006;24:3831–3837. doi: 10.1200/JCO.2006.05.8073. [DOI] [PubMed] [Google Scholar]
- 21.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: The V-325 Study Group. J Clin Oncol. 2007;25:3210–3216. doi: 10.1200/JCO.2006.08.3956. [DOI] [PubMed] [Google Scholar]
- 22.Kabbinavar FF, Wallace JF, Holmgren E, et al. Health-related quality of life impact of bevacizumab when combined with irinotecan, 5-fluorouracil, and leucovorin or 5-fluorouracil and leucovorin for metastatic colorectal cancer. The Oncologist. 2008;13:1021–1029. doi: 10.1634/theoncologist.2008-0003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.