The evolution of the local therapy of breast cancer is reviewed with an emphasis on current areas of controversy.
Keywords: Breast surgery, Lumpectomy, Sentinel node, Partial breast irradiation
Learning Objectives
After completing this course, the reader will be able to:
Describe the influence of tumor biology when selecting options for local treatment of breast cancer.
Identify key areas of controversy relating to localized treatment of breast cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Breast-conserving therapy (BCT) and mastectomy have equal survival outcomes. Rates of local recurrence after BCT have declined steadily, largely as a result of the widespread use of systemic therapy. Sentinel node biopsy has replaced axillary dissection for staging the axilla, and in women undergoing BCT with whole-breast irradiation (WBI), axillary dissection is not needed for local control or survival in those with fewer than three involved sentinel nodes. Alternatives to 6 weeks of WBI have been shown to be safe and effective for subsets of breast cancer patients, and the use of preoperative chemotherapy allows BCT in some women who require mastectomy if surgery is the initial step in treatment. The combination of the smaller cancers detected with screening and the routine use of multimodality therapy has resulted in a decrease in the morbidity of local therapy and improved cancer treatment outcomes.
Introduction
The locoregional therapy of breast cancer has changed dramatically over the past 30 years, from the era of radical mastectomy for all to options including breast-conserving therapy (BCT) with whole-breast irradiation (WBI), mastectomy with immediate reconstruction, and mastectomy alone, with treatment selection based on a combination of extent of disease and patient preference. More recently, attempts have been made to further tailor the extent of therapy, with the omission of axillary dissection in some node-positive women and preservation of the nipple areolar complex (NAC) to facilitate reconstruction in others, and the use of hypofractionated WBI and accelerated partial breast irradiation (PBI) for selected patients.
At the same time, our thinking regarding the role of locoregional management has evolved. For many years, locoregional therapy was the only method of breast cancer treatment available. However, the observation that many patients with breast cancer were not cured by radical mastectomy [1] ultimately resulted in acceptance of the idea that treatment failure after breast cancer surgery is usually a result of disseminated tumor cells present at the time of surgery rather than an insufficiently radical operative procedure. This resulted in the belief that, although local therapy is important to reduce the likelihood of complications at the primary tumor site, variations in local therapy are unlikely to have a major impact on breast cancer survival—the “systemic disease” hypothesis promulgated by Dr. Bernard Fisher [2]. The 2005 publication from the Early Breast Trialists' Collaborative Group (EBCTCG) demonstrating that differences >10%–20% in local control between treatments at 5 years are associated with statistically significant differences in breast cancer survival at 15 years [3] has brought us full circle to again believe that adequate locoregional therapy is an important component of breast cancer survival. The purpose of this article is to review the evolution of the local therapy of breast cancer with an emphasis on current areas of controversy.
Catalysts for Change
In the 1970s, the major debate in the local therapy of breast cancer was the safety of the switch from radical mastectomy to modified radical mastectomy. In that environment, the National Surgical Adjuvant Breast and Bowel Project (NSABP) B04 trial asked a radical question: Did removal of the axillary nodes in clinically node-negative women contribute to survival? The study randomized clinically node-negative women to radical mastectomy, simple mastectomy with nodal irradiation, or simple mastectomy with delayed axillary dissection if clinical evidence of nodal recurrence occurred [4, 5]. Through 25 years of follow-up, that trial showed no difference in survival outcomes. In addition, although 40% of the women randomized to radical mastectomy had axillary nodal metastases, only 18.5% of those in the axillary observation arm required delayed dissection. Although the study did not result in the abandonment of axillary dissection, primarily because of the prognostic importance of nodal involvement and the use of nodal status to select patients for adjuvant therapy at that time, the results were a direct repudiation of the Halstedian concept of breast cancer biology and opened the door to the study of BCT as an alternative to mastectomy and to the use of immediate breast reconstruction.
Current Status of BCT
Six prospective randomized trials, some with follow-up of 20 years, have established that survival is equivalent after BCT and mastectomy [4–11]. Local recurrence after BCT has been a great source of concern for both patients and physicians. It is interesting to note that, in the NSABP B06 trial [4, 8], the only randomized study that required histologically negative margins, the 20-year rate of ipsilateral breast tumor recurrence (IBTR) after BCT did not differ significantly from the rate of local recurrence in the mastectomy group. Since the time that these initial trials were conducted, rates of IBTR have decreased steadily as a result of a combination of improvements in mammography, the routine inking of specimen margins, and the widespread use of adjuvant therapy in women with breast cancer.
The importance of both endocrine therapy and chemotherapy to maintaining local control is well documented in prospective randomized trials. The majority of women with invasive breast cancer now receive some form of adjuvant systemic therapy in addition to surgery and radiation therapy (RT). In the NSABP B14 trial, in which node-negative, estrogen receptor (ER)+ women were randomized to tamoxifen citrate or placebo, the 10-year rate of in-breast recurrence was lower in the tamoxifen group than in the placebo group (4.3% versus 14.7%) [12]. In the NSABP B13 trial, node-negative ER− women were randomized to chemotherapy or a no-treatment control group [13]. At 8 years, local recurrence was seen in only 2.6% of those receiving chemotherapy, compared with 13.4% of controls. In a report of 3,799 node-negative women participating in five NSABP trials of adjuvant systemic therapy, the cumulative incidence of in-breast recurrence at 12 years for those receiving adjuvant therapy was only 6.6% [14]. Since the time that those trials were conducted, more effective systemic therapy has become available. As survival has increased as a result of improved systemic therapy, a parallel decrease in local recurrence rates has been observed. For example, in the randomized trials that established the efficacy of adjuvant trastuzumab, the addition of trastuzumab to chemotherapy resulted in a 50% lower locoregional recurrence (LRR) rate than with treatment with chemotherapy alone [15]. Similar results were reported in ER+, node-negative patients when systemic treatment was selected on the basis of the Oncotype DX™ (Genomic Health, Inc., Redwood City, CA) score. Although this score was developed to predict the risk for systemic recurrence, Mamounas et al. [16] demonstrated that, in the absence of systemic treatment, patients with high-risk Oncotype DX™ scores had an 18.4% risk for LRR, compared with those with low-risk scores who had a 10.8% risk. The addition of tamoxifen, appropriate treatment for those with low-risk scores, led to a >50% lower incidence of LRR (4.3%, in the low-risk group). In contrast, a much more modest LRR difference, 15.8% with tamoxifen versus 18.4%, was seen in the high-risk group. However, when chemotherapy was added, the LRR rate in the high-risk group was only 7.8%.
The importance of biology and targeted therapy is further supported by the emerging literature on the impact of tumor subtype on local recurrence after BCT or mastectomy. Both Millar et al. [17] and Nguyen et al. [18] demonstrated that the rate of local recurrence after BCT varies among the intrinsic subtypes of breast cancer as approximated by the ER, progesterone receptor (PR), and human epidermal growth factor receptor (HER)-2 status. In both studies, the lowest rates of local recurrence at 5 years were seen among the ER+PR+HER-2− (luminal A-like) group, and the highest rates were seen among triple-negative (basal-like) and ER−HER-2+ patients in the absence of adjuvant trastuzumab. However, ER, PR, and HER-2 status are not selection factors for mastectomy or for more widely clear lumpectomy margins because the same pattern of a greater risk for chest-wall recurrence among ER− patients, regardless of HER-2 status, was observed in a retrospective analysis of the Danish Breast Cancer Group randomized trials of mastectomy with or without RT [19]. A similar impact of biology on LRR, independent of the use of BCT or mastectomy, was shown in the analysis by Mamounas et al. [16] of the impact of the Oncotype DX™ score on LRR. In a multivariate analysis, young patient age, high histologic grade, and high-risk Oncotype DX™ score, but not lumpectomy versus mastectomy, were predictors of a higher rate of LRR.
These data suggest that local control is the result of a complex interaction among tumor burden, the intrinsic biologic characteristics of the tumor, and the effectiveness of the systemic therapy that is available. If the tumor burden is too high, as evidenced by a positive lumpectomy margin [20], the rate of local recurrence is greater. Once the tumor burden is reduced to subclinical levels, as evidenced by negative margins and the absence of additional mammographic abnormalities, it is unclear whether further attempts to reduce the subclinical tumor burden will significantly improve local control. This raises interesting issues as increasingly sensitive imaging modalities become clinically available. It has been known for many years that cancers that appear to be clinically and mammographically unicentric are microscopically multifocal in as many as 60% of cases [21]. This was the argument initially used to suggest that all breast cancers should be treated with mastectomy, a contention disproven in clinical trials. As discussed above, rates of IBTR for patients selected for BCT with clinical exam and mammography are about 6% at 10 years, and lower in ER+ subsets. These clinical findings are difficult to reconcile with the observation that magnetic resonance imaging (MRI) shows additional cancer in 16% (95% confidence interval [CI], 6%–34%) of patients [22] and suggest that the majority of this subclinical disease is controlled with RT. This contention is supported by retrospective studies [23, 24] that do not demonstrate a lower rate of IBTR after BCT in women selected for the procedure using MRI, in spite of the fact that significantly more women end up undergoing a mastectomy based on disease found with MRI [25, 26]. As tracers that target abnormalities in cancer cells are used to further improve the sensitivity of imaging, an unwanted side effect may be the use of more extensive surgery unless we continue to re-examine our selection criteria for BCT in the context of newer diagnostic tools and the effectiveness of systemic therapy.
At present, BCT is a mature technique with well-defined selection criteria (Table 1). Population-based data indicate that ∼90% of women with stage 0, I, or II carcinoma in whom BCT is attempted have successful completion of the procedure [27]. Rates of IBTR have decreased considerably since the procedure was first developed, and eligibility has been expanded with the use of neoadjuvant chemotherapy to shrink operable cancers to avoid mastectomy.
Table 1.
Absolute contraindications to breast-conserving therapy
aWith radiation required during pregnancy.
Neoadjuvant Chemotherapy to Allow BCT
Because BCT does not result in a better survival outcome than with mastectomy, its primary benefit is cosmetic, and a large tumor relative to the size of the breast has been considered a contraindication to BCT [28]. Multiple prospective randomized trials have addressed the question of giving chemotherapy preoperatively in women with operable breast cancer, and a meta-analysis of these studies showed no survival disadvantage (or advantage) for the use of chemotherapy preoperatively. A higher rate of LRR was not observed after BCT or mastectomy, and the mastectomy rate was 16.6% lower (95% CI, 15.1%–18.1%) with the use of neoadjuvant therapy [29]. This is actually an underestimation of the rate of downstaging because many of the women in those studies were candidates for BCT without chemotherapy. In the two studies reporting BCT rates in women who would have required mastectomy if surgery was performed initially, the rates of BCT were 27% and 23% [30, 31]. The biggest barrier to the use of BCT after neoadjuvant therapy remains the inability to determine the extent of viable residual tumor preoperatively, particularly when cancer dies in a honeycomb or buckshot-type pattern. MRI appears to be the most reliable way to assess both the extent of residual disease and the pattern of response, but it may still overestimate the extent of residual disease or fail to identify microscopic islands of viable residual tumor [32, 33].
Newer Radiation Approaches for BCT
The initial trials of BCT all employed WBI using tangent fields. Subsequent studies have shown that an additional boost dose of RT results in better local control, although the magnitude of benefit varies with patient age [34]. However, the time and travel necessary for a 6-week course of RT are burdensome to some patients, whereas fear of the side effects of RT causes others to opt for mastectomy [35]. This has prompted a number of new approaches to RT after BCT that are designed to decrease the time of treatment, the toxicity of treatment, or both.
Hypofractionation
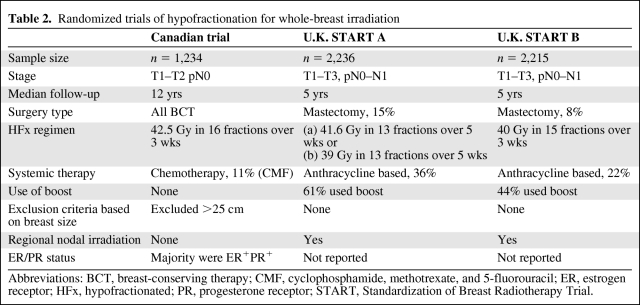
Hypofractionated (HFx) RT, defined as the delivery of larger-than-standard doses of radiation over a shorter period of time, has come full circle since its inception in the early 1960s–1970s, when it was associated with a substantially higher rate of late toxicity [36]. Ultimately, these poor results were attributed to the failure to decrease the total dose. Improved understanding of the radiobiological parameters that govern the response of breast tissue to fraction sizes has led to a resurgence of interest in HFx regimens for early-stage breast cancer. To date, three randomized trials comparing HFx with standard fractionated (SF) RT have been published, challenging previous assumptions regarding toxicity [37–39].
The first of those trials, conducted by the Ontario Clinical Oncology Group, established the efficacy of the “Canadian fractionation regimen,” an HFx schedule commonly used in the U.S. [39]. In that trial, 1,234 women with node-negative breast cancer undergoing wide local excision and axillary dissection were randomized to receive RT with either the SF (50 Gy in 25 fractions, over 35 days) or HFx (42.6 Gy in 16 fractions, over 22 days) regimen. Patients were stratified according to age, tumor size, the use of systemic therapy, and treatment center. With a median follow-up of 12 years, the risks for local recurrence at 10 years were equivalent in the two groups (6.7% for SF versus 6.2% for HFx), with no difference in cosmesis or late effects from RT.
These findings were corroborated by the results of two large, randomized trials performed in the U.K.: the Standardization of Breast Radiotherapy (START) A and START B trials, which compared various HFx regimens with SF RT (50 Gy in 25 fractions over 5 weeks) in patients with both node-negative and node-positive breast cancer after BCT or mastectomy. The START A trial examined two different HFx regimens, 41.6 and 39 Gy, delivered in 13 fractions over 5 weeks. After a median follow-up of 5.1 years, the local recurrence rate in the 41.6-Gy group was equivalent to that in the SF arm (3.5% versus 3.6%), whereas the local control rate in the 39-Gy arm was inferior (5.2%) [37]. In the START B trial, the HFx arm consisted of 40 Gy delivered in 15 fractions over 3 weeks. With a median follow-up of 6 years, the locoregional relapse rate was 3.3% in the SF arm and 2.2% in the HFx arm, with a trend for changes in breast appearance to be less in the HFx group (p = .06) [38].
Despite the uniformity of the clinical findings demonstrating equivalent local control and a low incidence of major toxicities, underrepresentation of certain subsets of patients has precluded the widespread adoption of HFx in the U.S. Key differences in baseline characteristics of patients enrolled in these trials are outlined in Table 2. The majority of the patients in the Canadian trial had low-risk features, including tumors that were primarily T1–T2, grade 1 or 2, and ER+. None of the patients received a boost dose of RT, a minority received chemotherapy, and women with large breast separations were excluded altogether. On subset analysis, a lower local control rate was observed in women with high-grade tumors in the HFx arm, raising the possibility that high-grade tumors may exhibit differential sensitivities to HFx radiation. In contrast, a meta-analysis of the U.K. START trials [40] did not substantiate this finding in a study population that included more patients with larger tumors and node-positive disease. Up to one-third of patients received chemotherapy, whereas some even received regional lymph node irradiation. The use of a boost was uncontrolled, there was no exclusion based on breast size, and information on ER status was unavailable.
Table 2.
Randomized trials of hypofractionation for whole-breast irradiation
Abbreviations: BCT, breast-conserving therapy; CMF, cyclophosphamide, methotrexate, and 5-fluorouracil; ER, estrogen receptor; HFx, hypofractionated; PR, progesterone receptor; START, Standardization of Breast Radiotherapy Trial.
The controversy underlying the optimal selection of patients for HFx is exemplified by the inability of an American Society for Radiation Oncology (ASTRO) panel to agree on the appropriateness of HFx for women who did not meet all the following criteria: ≥50 years old with pathologic T1–T2N0 disease treated with BCT, no chemotherapy, and a radiation plan demonstrating ≤7% dose inhomogeneity [41]. On the other hand, proponents of HFx are currently investigating even more aggressive HFx regimens that evaluate higher doses of radiation per fraction and integrate the tumor bed boost. Based on the promising results of the START A and START B trials, the FAST (Faster radiotherapy for breast cancer patients) trial will randomize women to SF (50 Gy in 25 fractions) or HFx (30 Gy in five fractions or 27.5 Gy in five fractions) over 5 weeks [42]. The Radiation Therapy Oncology Group (RTOG) has launched a phase III, noninferiority trial in BCT patients, comparing SF (50 Gy in 25 fractions) or HFx (42.7 Gy in 16 fractions) plus a sequential boost with an HFx regimen that includes a concomitant boost to the tumor bed (40–48 Gy in 15 fractions).
It is important to consider HFx RT in the context of the modern radiation techniques available today, such as prone positioning and intensity-modulated RT, which may facilitate the delivery of a uniform dose to the breast and mitigate concerns regarding toxicities of HFx RT, particularly in large-breasted women. However, until data from the aforementioned trials and confirmatory long-term results from the START trials become available, judicious use of HFx in clinical practice is advised, particularly in patients who do not meet the conservative criteria stipulated by the ASTRO task force.
Prone Technique
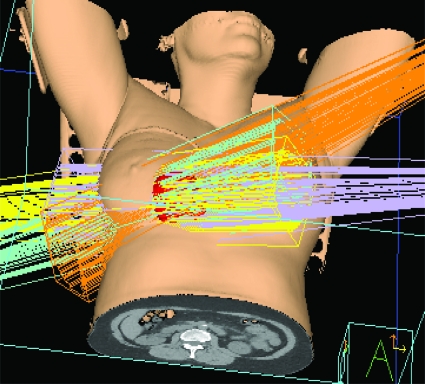
Standard tangential radiation was predominantly performed in the supine position in the landmark trials of both conventional and HFx RT [3, 37–39]. Prone positioning emerged as an alternative to supine positioning in the early 1990s for women with large, pendulous breasts, in an effort to decrease dose inhomogeneity and skin toxicity resulting from large breast separation and skin folds. The prone position requires patients to lie with the treated breast suspended through an aperture in the breast board into air, resulting in displacement of the breast away from the chest wall (Fig. 1). Although data are limited to small single-institution series, prone RT has been shown to deliver a lower radiation dose to the lung and, to a lesser extent, the heart, without compromising tumor control [43–45].
Figure 1.
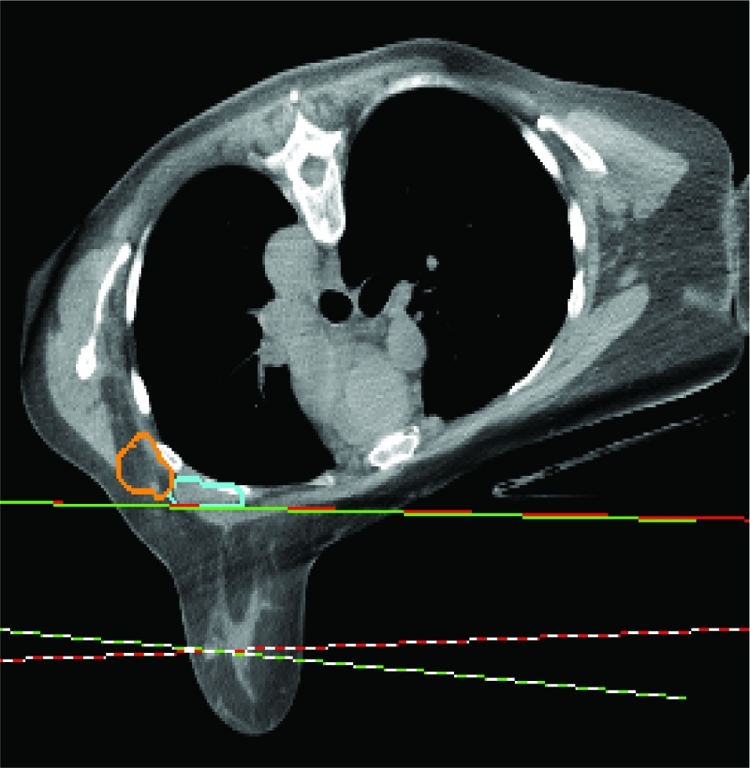
Prone breast radiation. The breast falls anteriorly toward the ground, with two tangential beams enveloping the whole breast. The axillary lymph nodes (contoured in orange and blue) are not included in the beams.
One trade-off of the prone technique is lesser coverage of level I and level II axillary lymph nodes [43], restricting its use in patients who require nodal irradiation. Other poor candidates for prone RT include elderly or morbidly obese patients who have difficulty tolerating the position and patients with deep-seated tumors near the chest wall, where the potential for suboptimal coverage by the radiation field exists. Because both supine and prone positioning have been shown to be safe and effective methods of delivering WBI, individual anatomy plays the largest role in determining the optimal setup.
PBI
The concept of PBI represents a substantial departure from the traditional treatment approach for early-stage breast cancer. WBI using SF RT has been the widely accepted standard for several decades based on its use in multiple randomized trials that proved the equivalence of BCT to mastectomy, as well as a meta-analysis that unequivocally demonstrated long-term survival benefits with this approach [3, 5–11]. PBI delivers larger-than-standard doses of daily radiation over 1–10 days to the resection cavity plus a 1- to 2-cm margin, permitting faster, more convenient treatment and sparing of the uninvolved portions of the breast and adjacent tissues from high doses of radiation (Fig. 2).
Figure 2.
A three-dimensional view of partial breast radiation with the external-beam technique, using four beams to target the partial breast cavity (red).
The rationale for PBI is based on both clinical and pathologic data, suggesting that the immediate vicinity of the index tumor is the area at greatest risk for local recurrence. Pathologic studies have demonstrated that tumor cells rarely extend beyond 4 cm from the index lesion in mastectomy specimens without an extensive intraductal component [21]. Clinical support for these pathologic studies stems from the observed patterns of failure within the breast in patients treated with BCT. Each of the three prospective randomized trials that compared wide excision alone with or without RT demonstrated that 80%–90% of recurrences were located at the site of the lumpectomy [8, 46, 47]. The rate of “elsewhere failures” at sites far removed from the tumor bed was ∼4%, which approaches the risk for developing contralateral breast cancer. Taken together, these data suggest that the benefit of WBI is derived mainly from the delivery of radiation to the region of the tumor bed.
PBI can be delivered with any one of the following four techniques: (a) multicatheter interstitial brachytherapy, (b) balloon-based brachytherapy such as MammoSite® (Hologic Inc., Bedford, MA), (c) external-beam 3-dimensional (3D) conformal RT, and (d) intraoperative RT (IORT) using electrons or orthovoltage photons. Distinct advantages, disadvantages, and technical requirements are associated with each method, underscoring the importance of individualizing the method to patient anatomy, preferences, and availability. Multicatheter brachytherapy has the most mature data and dosing flexibility, but its widespread use is limited by the invasive nature of the procedure and the expertise required. Balloon-based brachytherapy represents by far the most popular method of PBI in the U.S., as evidenced by the >50,000 women treated with this device to date [48]. The MammoSite® Registry Trial, a prospective study launched by the American Society of Breast Surgeons, reported the 5-year outcomes of 1,449 patients treated with this technique. Good to excellent cosmesis was achieved in 90.6% of the study population, and the actuarial rate of IBTR was 2.5% [49]. External-beam RT is a relative newcomer to PBI. Although appealing for its simplicity, noninvasiveness, and homogeneous dose distributions, follow-up with this approach is short, and the optimal dose and fractionation are undetermined. With IORT, radiation is administered to the operative bed plus a margin at the time of lumpectomy. Direct visualization of the cavity decreases the likelihood of marginal miss and allows for shielding of the surrounding skin. The ability to deliver all the treatment in a single fraction inside a standard operating room (in the case of orthovoltage photons) is hugely convenient. Potential disadvantages include the inability to confirm final margin and lymph node status prior to delivery of treatment, the lack of centers equipped to perform this procedure in the U.S., and the unknown late effects stemming from the delivery of a large, single dose of RT.
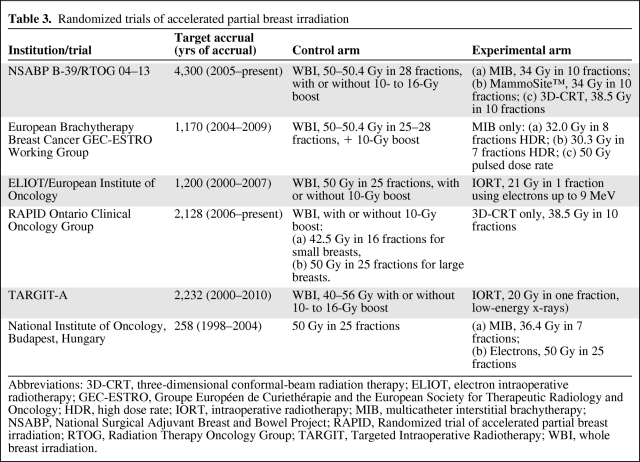
Presently, studies of PBI with ≥4 years of follow-up consist of small, single-institution cohort studies [50–53] that demonstrated low local recurrence rates that are similar to those seen in patients treated with contemporary WBI and adjuvant systemic therapy. Notably, the majority of the patients in those trials had low-risk prognostic features (age ≥50 years, tumors ≤2 cm, margins ≥2 mm, node negative), whereas the excellent local control rates seen with WBI were obtained in a much more diverse patient population. Randomized trials of WBI and PBI conducted before the advent of computed tomography–based planning and 3D conformal radiation are flawed by poor patient selection, pathologic evaluation, and inadequate technology that hindered the accurate localization and delivery of radiation, resulting in suboptimal local control rates [54–56]. Data from modern randomized trials comparing PBI with WBI have just emerged or are currently under way (Table 3). The largest of these trials, the NSABP B-39/RTOG 04–13 study, is aiming to accrue 4,300 patients, including patients with all grades of ductal carcinoma in situ and invasive cancers with one to three positive lymph nodes—a somewhat higher risk group than typically selected for PBI. External-beam, interstitial catheter, and MammoSite® brachytherapy are all methods of PBI permitted in the NSABP B-39 trial, which will provide important information on the efficacy and toxicities of these different techniques.
Table 3.
Randomized trials of accelerated partial breast irradiation
Abbreviations: 3D-CRT, three-dimensional conformal-beam radiation therapy; ELIOT, electron intraoperative radiotherapy; GEC-ESTRO, Groupe Européen de Curiethérapie and the European Society for Therapeutic Radiology and Oncology; HDR, high dose rate; IORT, intraoperative radiotherapy; MIB, multicatheter interstitial brachytherapy; NSABP, National Surgical Adjuvant Breast and Bowel Project; RAPID, Randomized trial of accelerated partial breast irradiation; RTOG, Radiation Therapy Oncology Group; TARGIT, Targeted Intraoperative Radiotherapy; WBI, whole breast irradiation.
Although IORT is less prevalent in North America, interest was recently heightened by the publication of the Targeted Intraoperative Radiotherapy (TARGIT)-A study, a multi-institutional noninferiority trial of 2,232 women randomized to receive IORT or WBI with or without a boost following BCT [57]. At 4 years of follow-up, the local recurrence rates were comparatively low (1.2% for IORT versus 0.95% in the WBI arm; 95% CI, 1.04%–1.54%; p = .41), as was the incidence of major toxicities (3.3% for WBI versus 3.9% for IORT; p = .44). Notably, only a minority (14%) of patients in the IORT arm received an additional external-beam RT boost when the final pathology was found to contain prespecified adverse features. Given the proven benefit of a boost on local control [34], critics have raised concerns regarding the therapeutic efficacy of the dose delivered in the TARGIT trial to the more distant margins of the target volume—an issue that time will resolve as longer-term data become available.
Despite the lack of data from randomized trials with durable follow-up, the practice of PBI outside a clinical trial has increased over the past decade, prompting an ASTRO task force to issue a consensus statement defining groups of patients who are “suitable,” “cautionary,” and “unsuitable” for PBI performed off protocol [41]. The categorization of patients into “cautionary” and “unsuitable” groups was based on the lack of mature data from modern studies to support treatment of these subsets, rather than disproven efficacy or concerns regarding toxicities.
In summary, PBI is a rapidly evolving technique that represents a significant paradigm shift in fundamental beliefs regarding local therapy. Although the favorable results from prospective single-arm trials and the TARGIT trial are reassuring, long-term data from large, phase III trials on efficacy and cosmesis are needed to validate PBI as an equivalent treatment to WBI for early-stage breast cancer patients. The refinement of techniques that improve target delineation and treatment accuracy are active topics of research that will contribute importantly to our ability to safely deliver PBI.
Advances in Mastectomy Technique: Skin Sparing and Nipple Sparing
Skin sparing mastectomy (SSM) has become standard practice in women undergoing immediate breast reconstruction, providing an envelope into which the reconstruction is created and minimizing surgical scars. A recent single-institution retrospective study comparing 799 patients undergoing SSM with 1,011 undergoing conventional mastectomy (CM) in 2000–2005 found no difference in local or regional recurrence rates between the procedures [58]. A meta-analysis of nine observational studies involving 3,739 patients also found no differences in the local recurrence rate between SSM (6.2%) and CM (4.0%) (odds ratio, 1.25; 95% CI, 0.81–1.94) [59].
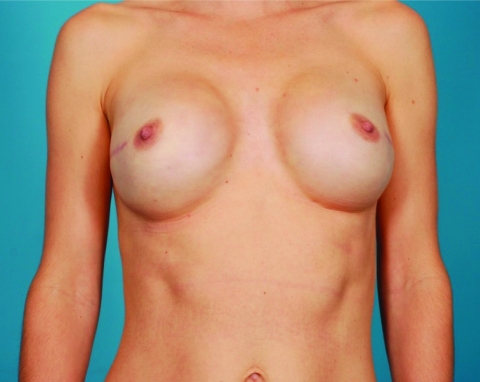
The use of nipple-sparing mastectomy (NSM) (Fig. 3) is more controversial. Unlike the skin overlying the breast, which is separated from the breast parenchyma by a layer of fat, there is no s.c. fat layer beneath the NAC. Preservation of the NAC means that breast tissue must be left behind in order to maintain a blood supply and that ductal tissue may also be left behind in the nipple itself, raising concerns about both the risk for cancer recurrence and the development of new cancers in the future. The reported incidence of nipple involvement in women with breast cancer in larger series is in the range of 6%–30% depending upon the patient population studied and the extent of pathologic evaluation [60, 61]. In most studies, the size of the primary cancer and the distance from nipple to tumor are predictive of nipple involvement [62, 63], suggesting that the best candidates for NSM are women with small peripheral tumors who could actually undergo BCT. Retrospective studies comparing total LRR after NSM with other types of mastectomy have not shown differences [64–66], and recurrences in the NAC itself are infrequent. However, these results are difficult to assess because of differences between patient groups. To address the oncologic concerns with NSM, the group at the European Institute of Oncology has added IORT of the NAC to the procedure. With this approach, in 800 patients, total necrosis of the NAC was seen in 3.5%, with NAC removal in 5% of cases [67]. With a mean follow-up of only 20 months, LRR was seen in 1.4% of cases, but none of the recurrences were in the NAC. Unfortunately, the availability of IORT is limited, so this approach is unlikely to become widespread. When discussing NSM with patients, it is important that they are aware that the preserved NAC differs from the NAC prior to surgery. Loss of pigmentation is frequent, and normal sensation is retained in one-third or fewer patients [68], with concomitant loss of erectile function. In addition, nipple loss in the postoperative period is reported in 0%–48% of cases, with most series reporting rates <10% [63].
Figure 3.
Bilateral nipple-sparing mastectomies with implant reconstruction performed through an incision lateral to the nipple areolar complex.
Management of the Axilla
In the past decade, dramatic changes in the approach to the axilla in women with clinically node-negative breast cancer have taken place. As previously discussed, the NSABP B04 trial demonstrated that, in women treated with mastectomy alone, axillary dissection did not contribute to survival [4], but it continues to be used for staging and local control. Sentinel node (SN) biopsy is based on the concept that there is a reproducibly identifiable node (or nodes) that drains the breast and predicts the status of the remaining axillary nodes. The SN hypothesis in breast cancer was originally validated by Giuliano et al. [69], and SN biopsy is now a mature technique. Two large, prospective multi-institutional studies, the American College of Surgeons Oncology Group (ACOSOG) Z10 trial and the NSABP B32 trial, demonstrated, in >10,000 patients, that an SN could be identified in 98% of cases [70, 71]. In the NSABP B32 study, wherein patients were randomized to completion axillary dissection or not after a negative SN biopsy, the false-negative rate was 9.8% [70]. However, in two studies in which patients were randomized to SN biopsy or axillary dissection, the rate of identification of nodal metastases did not differ, indicating that there is a false-negative rate for axillary dissection as well [72, 73]. Axillary recurrence rates <1% after a negative SN biopsy further support the safety of the procedure [74, 75]. At this point in time, SN biopsy should be considered the standard of care for staging the axilla in women with T1–T3, clinically node-negative breast cancer. Contraindications to the procedure are uncommon and include pregnancy and lactation, and inflammatory and other T4 breast cancers [76]. The lymphazurin blue dye used for mapping has not been shown to be safe during pregnancy, and although the dose of radioactivity to the fetus is estimated to be below the recommended guidelines for radiation exposure during pregnancy [77, 78], the procedure has not been widely used in pregnant women. Limited experience in inflammatory breast cancer suggests an unacceptably high false-negative rate for SN biopsy in this circumstance [79, 80].
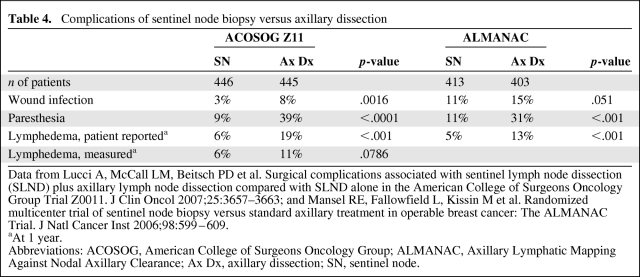
In an era when cancers are increasingly detected by screening mammography at a small size with a lower risk for axillary node involvement than was seen in the prescreening era, SN biopsy represents a real advance in the staging of breast cancer by avoiding the morbidity of axillary dissection in node-negative women. Morbidity outcomes in two randomized trials are shown in Table 4 [72, 81]. In the ACOSOG Z11 trial, the incidence of any side effect was 70% in the axillary dissection arm and only 25% in the SN arm (p < .001) [81]. In the ALMANAC (Axillary Lymphatic Mapping Against Nodal Axillary Clearance) trial, quality of life as measured by the Functional Assessment of Cancer Therapy B+4 questionnaire was significantly better at 1, 3, 6, and 12 months after surgery in the SN arm than in the axillary dissection arm (p = .001 at 12 months) [72].
Table 4.
Complications of sentinel node biopsy versus axillary dissection
Data from Lucci A, McCall LM, Beitsch PD et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol 2007;25:3657–3663; and Mansel RE, Fallowfield L, Kissin M et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J Natl Cancer Inst 2006;98:599–609.
aAt 1 year.
Abbreviations: ACOSOG, American College of Surgeons Oncology Group; ALMANAC, Axillary Lymphatic Mapping Against Nodal Axillary Clearance; Ax Dx, axillary dissection; SN, sentinel node.
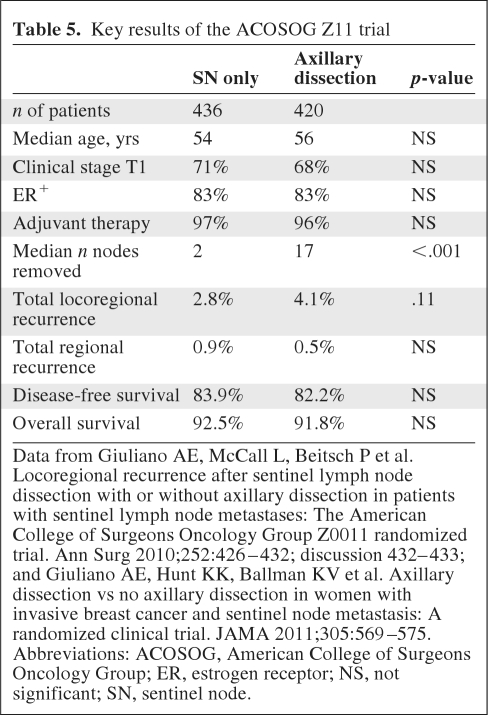
With the ability to stage patients as node positive or node negative with SN biopsy, the next clinical question to be addressed was the need for axillary dissection for local control, and perhaps survival, in the era of multimodality therapy. The ACOSOG Z11 trial addressed this question by randomizing clinically node-negative women with cancers ≤5 cm in size treated with lumpectomy and RT to completion axillary dissection versus no further surgery after the finding of a positive SN. Key results of the trial at a median 6.3 years of follow-up are summarized in Table 5 [82, 83]. No differences in local control or survival were observed between groups. The extremely low rate of LRR in both groups highlights the progress that has been made in breast cancer management in recent decades. It is important to recognize that these results apply to patients meeting the study entry criteria. Specifically, they should not be extrapolated to clinically node-positive women, those treated with neoadjuvant therapy, PBI, or mastectomy, or women with locally advanced breast cancer. In practice, the findings probably should be applied to women with involvement of one or two SNs only, because only 15 women with three or more involved SNs were treated within the SN biopsy-only study arm.
Table 5.
Key results of the ACOSOG Z11 trial
Data from Giuliano AE, McCall L, Beitsch P et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: The American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010;252:426–432; discussion 432–433; and Giuliano AE, Hunt KK, Ballman KV et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA 2011;305:569–575.
Abbreviations: ACOSOG, American College of Surgeons Oncology Group; ER, estrogen receptor; NS, not significant; SN, sentinel node.
Although these results are potentially practice changing, a number of concerns have been raised about the ACOSOG Z11 study. The study was originally designed to recruit 1,900 women, but closed after recruitment of 891 women because of slow accrual and a lower-than-anticipated event rate. In spite of this, the predefined statistical analysis plan was carried out and demonstrated noninferiority of SN biopsy alone with a p-value of .008 [82]. Equally important is the observation from the EBCTCG overview [3] that, for differences in local therapy to impact the 15-year breast cancer–specific survival rate, the difference in local control at 5 years must be >10%. The difference in regional node failure between the study arms in the ACOSOG Z11 trial is 0.4%, making it exceptionally unlikely that results would have changed had the full sample been recruited. Other studies examining outcomes after no axillary surgery at all, WBI, and systemic therapy reported nodal recurrence rates <4% [84–86], also below the threshold for a survival difference in the EBCTCG overview. Keeping in mind that the SNs were the only involved lymph nodes in 73% of patients in the axillary dissection arm, the very low rates of nodal failure in the ACOSOG Z11 trial are consistent with these findings.
Other concerns include the length of follow-up and whether or not the results can be applied to ER− women and young women. Women with ER+ breast cancer are known to have a longer time course to distant metastasis than those with ER− cancer [17], so it is reasonable to ask whether the same is true of regional recurrence. In the NSABP B04 trial, the median time to nodal recurrence was only 14.8 months [4], but that study is so old that the ER status of the participants is unknown. However, Greco et al. [87] and Martelli et al. [84] reported the time course of axillary recurrence in women treated with BCT and no axillary surgery in patient populations that were 75% and 92% ER+, respectively. The median times to axillary recurrence were 30.6 months and 33 months, respectively, strongly supporting the idea that the median follow-up of 6.3 years in the ACOSOG Z11 study was long enough to capture the majority of nodal recurrences.
Finally, there is the issue of the relatively small numbers of ER− women and young women in the ACOSOG Z11 trials. Because most breast cancer is ER+ and occurs in older women, the distribution of study participants is consistent with the epidemiology of the disease. No trends for a higher rate of nodal recurrence in ER− women or young women were observed. A multivariate analysis of predictors of regional nodal failure after axillary dissection in 1,500 women did not find age or ER status to be risk factors [88]. Further follow-up on these patient groups as the findings from the ACOSOG Z11 trial are implemented in clinical practice will help to clarify this issue.
In summary, just as the extent of surgery on the breast has become more individualized, so too has the surgical approach to the axilla. SN biopsy reliably identifies node-positive and node-negative women. In women with limited SN involvement (two or fewer involved nodes), removal of the SNs coupled with the tangent radiation that is part of BCT and systemic therapy is an effective method of maintaining local control. Elimination of axillary dissection in this subgroup does not decrease survival and reduces the morbidity of treatment. For women with greater numbers of involved lymph nodes and those undergoing mastectomy, the safety of this approach is uncertain, and axillary dissection remains the standard of care. However, based on the results of the NSABP B04 trial, it is clear that a substantial number of women with axillary node involvement do not experience axillary recurrence even in the absence of RT or systemic therapy. This suggests that the results of the ACOSOG Z11 study may apply to other subgroups of breast cancer patients, but that further research is needed to identify who can be safely managed this way.
Conclusions
The options available for the local therapy of breast cancer today represent a dramatic evolution from the era of the radical mastectomy. These therapeutic advances are a result of carefully conducted clinical trials and improved understanding of the biology of breast cancer. The explosion of knowledge regarding the molecular biology of breast cancer offers an opportunity not only to develop new drugs but also to continue to refine the biologic assumptions that underlie local treatment and to test these assumptions in clinical trials.
Author Contributions
Conception/Design: Monica Morrow
Provision of study material or patients: Monica Morrow
Collection and/or assembly of data: Monica Morrow
Data analysis and interpretation: Monica Morrow
Manuscript writing: Monica Morrow
Final approval of manuscript: Monica Morrow, Alice Ho
References
- 1.Adair F, Berg J, Joubert L, et al. Long-term followup of breast cancer patients: The 30-year report. Cancer. 1974;33:1145–1150. doi: 10.1002/1097-0142(197404)33:4<1145::aid-cncr2820330438>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B. Sounding board. Breast-cancer management: Alternatives to radical mastectomy. N Engl J Med. 1979;301:326–328. doi: 10.1056/NEJM197908093010611. [DOI] [PubMed] [Google Scholar]
- 3.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Redmond C, Fisher ER, et al. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985;312:674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- 6.Arriagada R, Lê MG, Rochard F, et al. Conservative treatment versus mastectomy in early breast cancer: Patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996;14:1558–1564. doi: 10.1200/JCO.1996.14.5.1558. [DOI] [PubMed] [Google Scholar]
- 7.Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: Six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr. 1992;(11):19–25. [PubMed] [Google Scholar]
- 8.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 9.Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: The National Cancer Institute randomized trial. Cancer. 2003;98:697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 10.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 11.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, Dignam J, Mamounas EP, et al. Sequential methotrexate and fluorouracil for the treatment of node-negative breast cancer patients with estrogen receptor-negative tumors: Eight-year results from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-13 and first report of findings from NSABP B-19 comparing methotrexate and fluorouracil with conventional cyclophosphamide, methotrexate, and fluorouracil. J Clin Oncol. 1996;14:1982–1992. doi: 10.1200/JCO.1996.14.7.1982. [DOI] [PubMed] [Google Scholar]
- 14.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 16.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: Results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28:1677–1683. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millar EK, Graham PH, O'Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 19.Kyndi M, Sørensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: The Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 20.Houssami N, Macaskill P, Marinovich ML, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010;46:3219–3232. doi: 10.1016/j.ejca.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 21.Holland R, Veling SH, Mravunac M, et al. Histologic multifocality of Tis, T1–2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. 1985;56:979–990. doi: 10.1002/1097-0142(19850901)56:5<979::aid-cncr2820560502>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: Systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248–3258. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 23.Hwang N, Schiller DE, Crystal P, et al. Magnetic resonance imaging in the planning of initial lumpectomy for invasive breast carcinoma: Its effect on ipsilateral breast tumor recurrence after breast-conservation therapy. Ann Surg Oncol. 2009;16:3000–3009. doi: 10.1245/s10434-009-0607-1. [DOI] [PubMed] [Google Scholar]
- 24.Solin LJ, Orel SG, Hwang WT, et al. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol. 2008;26:386–391. doi: 10.1200/JCO.2006.09.5448. [DOI] [PubMed] [Google Scholar]
- 25.Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg. 2009;209:180–187. doi: 10.1016/j.jamcollsurg.2009.04.010. quiz 294–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: Effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27:4082–4088. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302:1551–1556. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrow M, Harris JR. Practice guideline for the breast conservation therapy in the management of invasive breast cancer. J Am Coll Surg. 2007;205:362–376. doi: 10.1016/j.jamcollsurg.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 29.Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94:1189–1200. doi: 10.1002/bjs.5894. [DOI] [PubMed] [Google Scholar]
- 30.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 31.van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: Results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 32.Chen JH, Feig B, Agrawal G, et al. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer. 2008;112:17–26. doi: 10.1002/cncr.23130. [DOI] [PubMed] [Google Scholar]
- 33.Julius T, Kemp SE, Kneeshaw PJ, et al. MRI and conservative treatment of locally advanced breast cancer. Eur J Surg Oncol. 2005;31:1129–1134. doi: 10.1016/j.ejso.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881–10882 trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 35.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005;23:5526–5533. doi: 10.1200/JCO.2005.06.217. [DOI] [PubMed] [Google Scholar]
- 36.Fletcher GH. Hypofractionation: Lessons from complications. Radiother Oncol. 1991;20:10–15. doi: 10.1016/0167-8140(91)90106-q. [DOI] [PubMed] [Google Scholar]
- 37.START Trialists' Group. Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.START Trialists' Group. Bentzen SM, Agrawal RK, Aird EG, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 40.Haviland JS, Yarnold JR, Bentzen SM. Hypofractionated radiotherapy for breast cancer. N Engl J Med. 2010;362:1843. doi: 10.1056/NEJMc1002798. author reply 1843–1844. [DOI] [PubMed] [Google Scholar]
- 41.Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: An American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81:59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 42.Yarnold J, Bloomeld D, LeVay J. Prospective randomized trial testing 5.7 Gy and 6.0 Gy fractions of whole breast radiotherapy in women with early breast cancer (FAST) trial. Clin Oncol. 2004;16:S30. [Google Scholar]
- 43.Alonso-Basanta M, Ko J, Babcock M, et al. Coverage of axillary lymph nodes in supine vs. prone breast radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:745–751. doi: 10.1016/j.ijrobp.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 44.Kirby AM, Evans PM, Donovan EM, et al. Prone versus supine positioning for whole and partial-breast radiotherapy: A comparison of non-target tissue dosimetry. Radiother Oncol. 2010;96:178–184. doi: 10.1016/j.radonc.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Stegman LD, Beal KP, Hunt MA, et al. Long-term clinical outcomes of whole-breast irradiation delivered in the prone position. Int J Radiat Oncol Biol Phys. 2007;68:73–81. doi: 10.1016/j.ijrobp.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 46.Liljegren G, Holmberg L, Bergh J, et al. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: A randomized trial. J Clin Oncol. 1999;17:2326–2333. doi: 10.1200/JCO.1999.17.8.2326. [DOI] [PubMed] [Google Scholar]
- 47.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: Long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 48.MammoSite®. Hologic. [accessed June 3, 2011]. Available at http://www.mammosite.com/physicians/
- 49.Vicini F, Beitsch P, Quiet C, et al. Five-year analysis of treatment efficacy and cosmesis by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;79:808–817. doi: 10.1016/j.ijrobp.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 50.Arthur DW, Winter K, Kuske RR, et al. A phase II trial of brachytherapy alone after lumpectomy for select breast cancer: Tumor control and survival outcomes of RTOG 95–17. Int J Radiat Oncol Biol Phys. 2008;72:467–473. doi: 10.1016/j.ijrobp.2007.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chao KK, Vicini FA, Wallace M, et al. Analysis of treatment efficacy, cosmesis, and toxicity using the MammoSite breast brachytherapy catheter to deliver accelerated partial-breast irradiation: The William Beaumont Hospital experience. Int J Radiat Oncol Biol Phys. 2007;69:32–40. doi: 10.1016/j.ijrobp.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 52.Patel RR, Christensen ME, Hodge CW, et al. Clinical outcome analysis in “high-risk” versus “low-risk” patients eligible for National Surgical Adjuvant Breast and Bowel B-39/Radiation Therapy Oncology Group 0413 trial: Five-year results. Int J Radiat Oncol Biol Phys. 2008;70:970–973. doi: 10.1016/j.ijrobp.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Vicini FA, Antonucci JV, Wallace M, et al. Long-term efficacy and patterns of failure after accelerated partial breast irradiation: A molecular assay-based clonality evaluation. Int J Radiat Oncol Biol Phys. 2007;68:341–346. doi: 10.1016/j.ijrobp.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Dodwell DJ, Dyker K, Brown J, et al. A randomised study of whole-breast vs tumour-bed irradiation after local excision and axillary dissection for early breast cancer. Clin Oncol (R Coll Radiol) 2005;17:618–622. doi: 10.1016/j.clon.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 55.Ribeiro GG, Dunn G, Swindell R, et al. Conservation of the breast using two different radiotherapy techniques: Interim report of a clinical trial. Clin Oncol (R Coll Radiol) 1990;2:27–34. doi: 10.1016/s0936-6555(05)80215-8. [DOI] [PubMed] [Google Scholar]
- 56.Ribeiro GG, Magee B, Swindell R, et al. The Christie Hospital breast conservation trial: An update at 8 years from inception. Clin Oncol (R Coll Radiol) 1993;5:278–283. doi: 10.1016/s0936-6555(05)80900-8. [DOI] [PubMed] [Google Scholar]
- 57.Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): An international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
- 58.Yi M, Kronowitz SJ, Meric-Bernstam F, et al. Local, regional, and systemic recurrence rates in patients undergoing skin-sparing mastectomy compared with conventional mastectomy. Cancer. 2011;117:916–924. doi: 10.1002/cncr.25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanitis S, Tekkis PP, Sgourakis G, et al. Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer: A meta-analysis of observational studies. Ann Surg. 2010;251:632–639. doi: 10.1097/SLA.0b013e3181d35bf8. [DOI] [PubMed] [Google Scholar]
- 60.Lagios MD, Gates EA, Westdahl PR, et al. A guide to the frequency of nipple involvement in breast cancer. A study of 149 consecutive mastectomies using a serial subgross and correlated radiographic technique. Am J Surg. 1979;138:135–142. doi: 10.1016/0002-9610(79)90253-8. [DOI] [PubMed] [Google Scholar]
- 61.Laronga C, Kemp B, Johnston D, et al. The incidence of occult nipple-areola complex involvement in breast cancer patients receiving a skin-sparing mastectomy. Ann Surg Oncol. 1999;6:609–613. doi: 10.1007/s10434-999-0609-z. [DOI] [PubMed] [Google Scholar]
- 62.Brachtel EF, Rusby JE, Michaelson JS, et al. Occult nipple involvement in breast cancer: Clinicopathologic findings in 316 consecutive mastectomy specimens. J Clin Oncol. 2009;27:4948–4954. doi: 10.1200/JCO.2008.20.8785. [DOI] [PubMed] [Google Scholar]
- 63.Rusby JE, Smith BL, Gui GP. Nipple-sparing mastectomy. Br J Surg. 2010;97:305–316. doi: 10.1002/bjs.6970. [DOI] [PubMed] [Google Scholar]
- 64.Boneti C, Yuen J, Santiago C, et al. Oncologic safety of nipple skin-sparing or total skin-sparing mastectomies with immediate reconstruction. J Am Coll Surg. 2011;212:686–693. doi: 10.1016/j.jamcollsurg.2010.12.039. discussion 693–695. [DOI] [PubMed] [Google Scholar]
- 65.Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: Complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249:26–32. doi: 10.1097/SLA.0b013e31818e41a7. [DOI] [PubMed] [Google Scholar]
- 66.Gerber B, Krause A, Dieterich M, et al. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: An extended follow-up study. Ann Surg. 2009;249:461–468. doi: 10.1097/SLA.0b013e31819a044f. [DOI] [PubMed] [Google Scholar]
- 67.Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: One thousand and one cases of a five years experience at the European Institute of Oncology of Milan (EIO) Breast Cancer Res Treat. 2009;117:333–338. doi: 10.1007/s10549-008-0304-y. [DOI] [PubMed] [Google Scholar]
- 68.Benediktsson KP, Perbeck L, Geigant E, et al. Touch sensibility in the breast after subcutaneous mastectomy and immediate reconstruction with a prosthesis. Br J Plast Surg. 1997;50:443–449. doi: 10.1016/s0007-1226(97)90332-5. [DOI] [PubMed] [Google Scholar]
- 69.Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222:394–399. doi: 10.1097/00000658-199509000-00016. discussion 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 71.Posther KE, McCall LM, Blumencranz PW, et al. Sentinel node skills verification and surgeon performance: Data from a multicenter clinical trial for early-stage breast cancer. Ann Surg. 2005;242:593–599. doi: 10.1097/01.sla.0000184210.68646.77. discussion 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 73.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 74.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: Ten-year results of a randomized controlled study. Ann Surg. 2010;251:595–600. doi: 10.1097/SLA.0b013e3181c0e92a. [DOI] [PubMed] [Google Scholar]
- 76.Grube BJ, Giuliano AE. Sentinel lymph node dissection. In: Harris JR, Lippman ME, Morrow M, et al., editors. Diseases of the Breast. Fourth Edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. pp. 542–561. [Google Scholar]
- 77.Gentilini O, Cremonesi M, Trifirò G, et al. Safety of sentinel node biopsy in pregnant patients with breast cancer. Ann Oncol. 2004;15:1348–1351. doi: 10.1093/annonc/mdh355. [DOI] [PubMed] [Google Scholar]
- 78.Pandit-Taskar N, Dauer LT, Montgomery L, et al. Organ and fetal absorbed dose estimates from 99mTc-sulfur colloid lymphoscintigraphy and sentinel node localization in breast cancer patients. J Nucl Med. 2006;47:1202–1208. [PubMed] [Google Scholar]
- 79.Hidar S, Bibi M, Gharbi O, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in inflammatory breast cancer. Int J Surg. 2009;7:272–275. doi: 10.1016/j.ijsu.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 80.Stearns V, Ewing CA, Slack R, et al. Sentinel lymphadenectomy after neoadjuvant chemotherapy for breast cancer may reliably represent the axilla except for inflammatory breast cancer. Ann Surg Oncol. 2002;9:235–242. doi: 10.1007/BF02573060. [DOI] [PubMed] [Google Scholar]
- 81.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 82.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: The American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martelli G, Miceli R, Daidone MG, et al. Axillary dissection versus no axillary dissection in elderly patients with breast cancer and no palpable axillary nodes: Results after 15 years of follow-up. Ann Surg Oncol. 2011;18:125–133. doi: 10.1245/s10434-010-1217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.International Breast Cancer Study Group. Rudenstam CM, Zahrieh D, Forbes JF, et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: First results of International Breast Cancer Study Group Trial 10–93. J Clin Oncol. 2006;24:337–344. doi: 10.1200/JCO.2005.01.5784. [DOI] [PubMed] [Google Scholar]
- 86.Veronesi U, Orecchia R, Zurrida S, et al. Avoiding axillary dissection in breast cancer surgery: A randomized trial to assess the role of axillary radiotherapy. Ann Oncol. 2005;16:383–388. doi: 10.1093/annonc/mdi089. [DOI] [PubMed] [Google Scholar]
- 87.Greco M, Agresti R, Cascinelli N, et al. Breast cancer patients treated without axillary surgery: Clinical implications and biologic analysis. Ann Surg. 2000;232:1–7. doi: 10.1097/00000658-200007000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grills IS, Kestin LL, Goldstein N, et al. Risk factors for regional nodal failure after breast-conserving therapy: Regional nodal irradiation reduces rate of axillary failure in patients with four or more positive lymph nodes. Int J Radiat Oncol Biol Phys. 2003;56:658–670. doi: 10.1016/s0360-3016(03)00017-8. [DOI] [PubMed] [Google Scholar]