The prevalence of geriatric conditions in cancer patients aged ≥65 years acutely admitted to a general medicine ward were examined to determine functional decline and mortality within 12 months after admission and to assess which geriatric conditions and cancer-related variables are associated with 12-month mortality.
Keywords: Comprehensive geriatric assessment, Patient care, Prognostication, Elderly
Abstract
Introduction.
A comprehensive geriatric assessment systematically collects information on geriatric conditions and is propagated in oncology as a useful tool when assessing older cancer patients.
Objectives.
The objectives were: (a) to study the prevalence of geriatric conditions in cancer patients aged ≥65 years, acutely admitted to a general medicine ward; (b) to determine functional decline and mortality within 12 months after admission; and (c) to assess which geriatric conditions and cancer-related variables are associated with 12-month mortality.
Methods.
This was an observational cohort study of 292 cancer patients aged ≥65 years, acutely admitted to the general medicine and oncology wards of two university hospitals and one secondary teaching hospital. Baseline assessments included patient characteristics, reason for admission, comorbidity, and geriatric conditions. Follow-up at 3 and 12 months was aimed at functional decline (loss of one or more activities of daily living [ADL]) and mortality.
Results.
The median patient age was 74.9 years, and 95% lived independently; 126 patients (43%) had metastatic disease. A high prevalence of geriatric conditions was found for instrumental ADL impairment (78%), depressive symptoms (65%), pain (65%), impaired mobility (48%), malnutrition (46%), and ADL impairment (38%).
Functional decline was observed in 8% and 33% of patients at 3 and 12 months, respectively. Mortality rates were 38% at 3 months and 64% at 12 months. Mortality was associated with cancer-related factors only.
Conclusion.
In these acutely hospitalized older cancer patients, mortality was only associated with cancer-related factors. The prevalence of geriatric conditions in this population was high. Future research is needed to elucidate if addressing these conditions can improve quality of life.
Introduction
Although malignant tumors occur at all ages, cancer disproportionately strikes individuals aged ≥65 years [1]. Data from the National Cancer Institute's Surveillance, Epidemiology, and End Results program reveal that over half of all newly diagnosed cancer patients and more than two thirds of cancer deaths are in this age group [2]. In western societies, the number of older cancer patients will increase substantially in the coming decades as a result of increasing life expectancy and aging of the population. Oncologists are faced with the challenge of how to determine what treatment is suitable for their older cancer patients, with their heterogeneity in comorbidity, physical reserve, disability, and geriatric conditions. Age and performance status are too limited to do justice to this diversity [3, 4], and because guidelines for cancer treatment are often based on trials from which older patients and patients with comorbidity have been excluded [5], these guidelines cannot automatically be extrapolated to all ages.
Therefore, the use of a comprehensive geriatric assessment (CGA)—a systematic procedure to appraise the objective health status of older people, focusing on somatic, functional, and psychosocial domains—is frequently propagated in oncology as the tool to fill in these gaps [4, 6–11]. It is thought that identifying those factors associated with poor outcome will aid in prognostication and decision making regarding treatment for the individual patient [10, 12]. Furthermore, modifying the conditions identified with a CGA could improve outcome and health-related quality of life [7, 13, 14], particularly because geriatric conditions are often missed if they are not specifically looked for [7–9, 15].
Although many editorials and review articles endorse the use of a CGA in geriatric oncology, publication of evidence supporting this assumption of an added value of systematic CGA above usual care is far less frequent [4, 5, 7–10, 12, 14, 16–19]. In addition, as a result of heterogeneity in study populations (inpatients versus outpatients) and study settings (oncology versus geriatric medicine versus general medicine), as well as the large variation in the extensiveness of the CGA administered, the data remain fragmentary and inconclusive on the association between CGA and outcome.
One setting in which CGA may be of added value is for older cancer patients requiring acute hospitalization, for example, when an acute illness reveals the presence of malignancy or because of cancer- or treatment-related complications in the course of the disease. Independent of the reason for admission, choices need to be made about the future course of treatment during hospitalization. Furthermore, acutely ill patients could have a higher risk for geriatric conditions and functional decline.
Therefore, we studied the value of CGA for older cancer patients acutely admitted to the general medicine and oncology departments of three hospitals. The aims of the present study were threefold: (a) to study the prevalence of geriatric conditions in cancer patients aged ≥65 years acutely admitted to a general medicine ward, (b) to determine functional decline and mortality within 12 months after admission, and (c) to assess which geriatric conditions and cancer-related variables are associated with 12-month mortality.
Methods
Patients
This is an observational substudy of cancer patients who were included in the Development of strategies Enabling Frail Elderly New Complications to Evade (DEFENCE) I [20] and DEFENCE II studies [unpublished manuscript]. The DEFENCE I study (n = 647) was conducted at the Academic Medical Center (AMC) Amsterdam, The Netherlands; inclusion ran from November 2002 until March 2006 [20]. The DEFENCE II study (n = 639) ran from April 2006 until March 2008 at the AMC, University Medical Center Utrecht, and Spaarne Hospital Hoofddorp [unpublished manuscript].
In these prospective cohort studies, all patients aged ≥65 years acutely admitted to the general medicine or oncology ward were included. Patients were excluded if: (a) they or their relatives did not give informed consent; (b) they were too ill to participate according to their attending physician; (c) they came from another ward inside or outside the hospital; (d) they were transferred to the intensive care unit, coronary care unit, or another ward inside or outside the hospital within 48 hours after admission; or (e) they were unable to speak or understand Dutch. Inclusion had to take place within 48 hours after admission. The Medical Ethics Committee of the AMC approved both studies.
In this substudy, only patients with a known malignancy at the time of admission or a malignancy first diagnosed during admission were included.
Data Collection
The methods of the two studies were similar: within 48 hours of admission, a multidisciplinary evaluation was performed by a geriatric consultation team. This team consisted of two medical specialists, a geriatric resident, a clinical nurse specialist, and two research nurses trained in geriatric medicine.
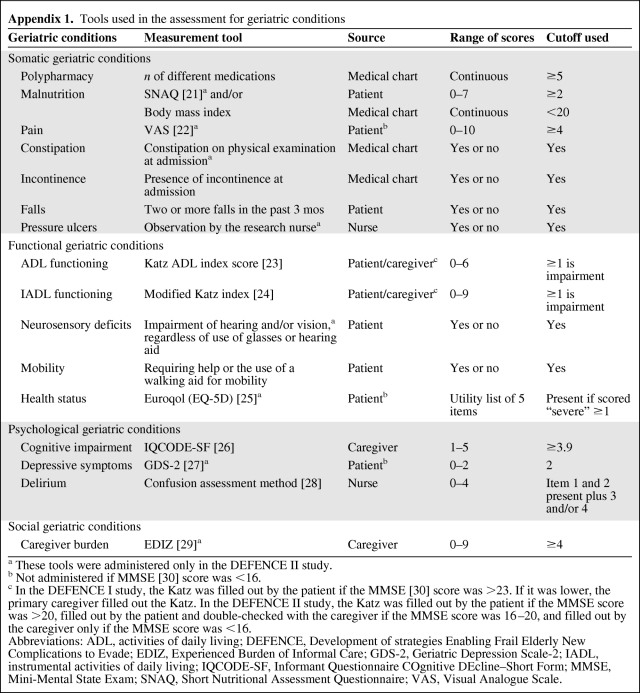
Data on social and demographic status were collected. Patients were assessed for the following geriatric conditions: polypharmacy, malnutrition, incontinence, falls, the ability to perform activities of daily living (ADL) and instrumental ADL (IADL), cognitive impairment 2 weeks prior to admission, neurosensory deficits, mobility disorders, and delirium. Furthermore, patients in the DEFENCE II study were assessed for the presence of pain, constipation, pressure ulcers, health status, depressive symptoms, and caregiver burden. Appendix 1 lists the tools used in the assessment [21–30]. All variables were dichotomized, using the cutoffs described in Appendix 1.
Medical history and oncologic treatment prior to, during, and after the hospital stay were collected from patients' medical records by a geriatrician. Based on the treatment during and after hospitalization, patients were subdivided into two groups: those still receiving active anticancer treatment (both curative and palliative, i.e., chemotherapy, radiotherapy, or surgical therapy) and those receiving supportive or symptomatic care only. The reason for admission was collected from the discharge report and classified as directly tumor related, treatment related, or because of another cause.
These reports were also used to derive the Charlson comorbidity index [31], excluding the current malignancy. The Charlson score is a continuous variable with scores in the range of 0–31, with higher scores indicating more or more severe comorbidities.
Follow-Up and Definition of Outcomes
Follow-up consisted of a telephone interview by a research nurse at 3 and 12 months after discharge, in which the modified Katz ADL index was readministered. Follow-up was completed by the same person (patient or primary caregiver) interviewed at baseline.
Functional decline was defined as a loss of one or more ADL abilities at 3 or 12 months, compared with premorbid function 2 weeks prior to hospital admission. Data on mortality were collected from the municipal data registry.
Statistical Analysis
Patients receiving active treatment and those receiving supportive care only after admission were compared with one another for differences in age, comorbidity, the presence of geriatric syndromes, as well as for mortality and functional decline. The χ2 test and risk analysis were used for nominal and ordinal variables, as well as for continuous variables with a non-normal distribution; for continuous variables with a normal distribution, the Student t-test was used.
To determine which baseline factors and geriatric conditions were associated with mortality in the 12 months following admission, a Cox regression analysis was performed. For each variable, the Cox proportional hazards assumption was tested using the log minus log plot. Next, a univariate Cox regression analysis was performed to determine which variables were associated with mortality in the 12 months following admission. Factors with a p-value <.20 in the univariate analysis and with <20% missing data were included in the multivariate analysis. A backward selection procedure was applied, accepting a p-value of <.05. Kaplan–Meier survival plots with a log-rank analysis were used to determine survival in the 12 months after admission. SPSS version 16.0 (SPSS Inc., Chicago, IL) was used for the analyses.
Results
Characteristics of Acutely Hospitalized Older Cancer Patients
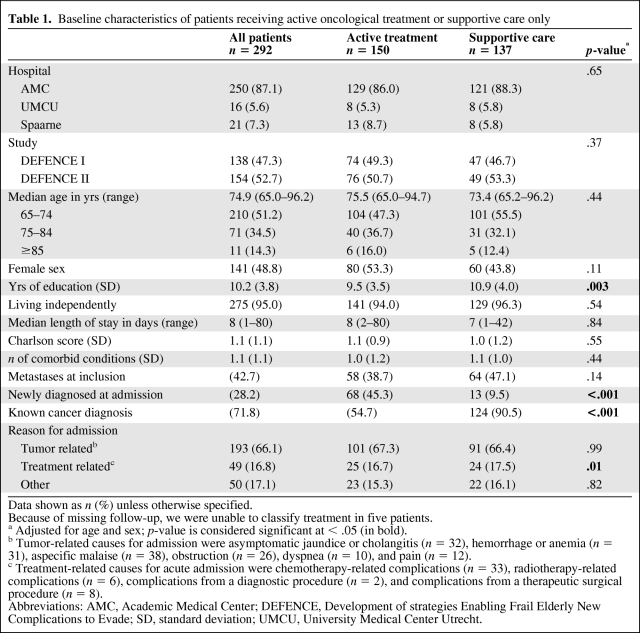
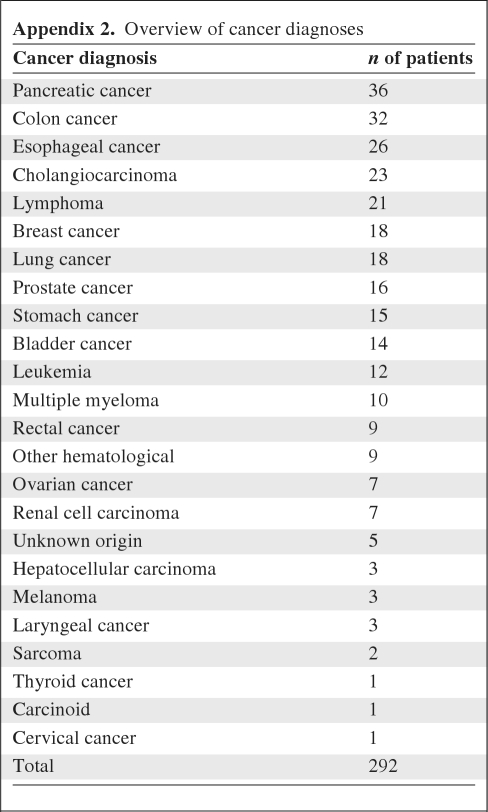
In total, 1,286 patients were included in the two studies, of whom 208 had a known, active malignancy (16%) and 84 were diagnosed with cancer during admission (7%). Their baseline characteristics are listed in Table 1. The median age was 74.9 years (range, 65.0–96.2 years) and 95% of the patients lived independently. In total, 27 different types of malignancies were present (Appendix 2). In total, 126 patients (43%) had metastatic disease.
Table 1.
Baseline characteristics of patients receiving active oncological treatment or supportive care only
Data shown as n (%) unless otherwise specified.
Because of missing follow-up, we were unable to classify treatment in five patients.
a Adjusted for age and sex; p-value is considered significant at < .05 (in bold).
b Tumor-related causes for admission were asymptomatic jaundice or cholangitis (n = 32), hemorrhage or anemia (n = 31), aspecific malaise (n = 38), obstruction (n = 26), dyspnea (n = 10), and pain (n = 12).
c Treatment-related causes for acute admission were chemotherapy-related complications (n = 33), radiotherapy-related complications (n = 6), complications from a diagnostic procedure (n = 2), and complications from a therapeutic surgical procedure (n = 8).
Abbreviations: AMC, Academic Medical Center; DEFENCE, Development of strategies Enabling Frail Elderly New Complications to Evade; SD, standard deviation; UMCU, University Medical Center Utrecht.
Prevalence of Geriatric Conditions
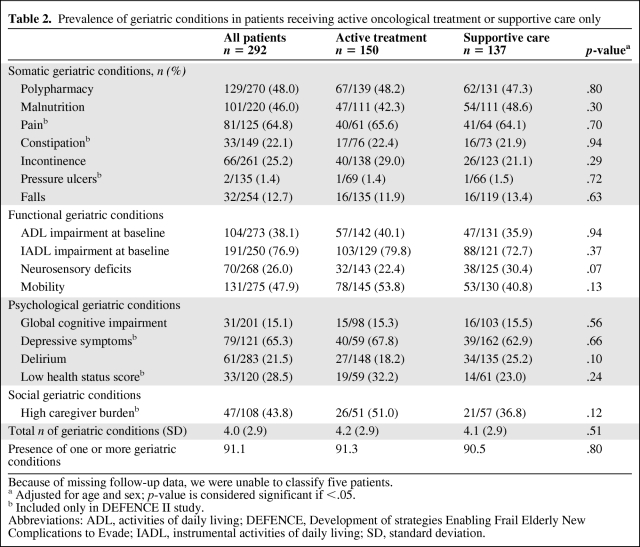
A high prevalence of geriatric conditions was seen, particularly in IADL impairment (77%), pain (65%), depressive symptoms (65%), polypharmacy (48%), mobility problems (48%), malnutrition (46%), high caregiver burden (44%), and ADL impairment (38%) (Table 2). On average, patients had four geriatric conditions, whereas only 9% had no geriatric condition. The prevalence increased with age— on average, patients aged 65–69 years had 2.9 conditions, patients aged 70–79 years had 4.1 conditions, and those aged ≥80 years had 5.5 conditions (p < .001). The mean Charlson comorbidity index, excluding the current malignancy, was 1.1 (standard deviation, 1.1).
Table 2.
Prevalence of geriatric conditions in patients receiving active oncological treatment or supportive care only
Because of missing follow-up data, we were unable to classify five patients.
a Adjusted for age and sex; p-value is considered significant if <.05.
b Included only in DEFENCE II study.
Abbreviations: ADL, activities of daily living; DEFENCE, Development of strategies Enabling Frail Elderly New Complications to Evade; IADL, instrumental activities of daily living; SD, standard deviation.
Differences Between Patients Receiving Active and Supportive Care
We were unable to classify five patients as either receiving active or supportive care as a result of missing follow-up data. Of the remaining patients, 137 (48%) received only symptomatic or supportive care; for newly diagnosed patients, this percentage was much lower than for patients with a known cancer diagnosis (16% versus 60%; p < .001). Patients receiving supportive care only had a higher level of education and a higher Charlson comorbidity index. Interestingly, they did not differ in age (Table 1) or the presence of geriatric conditions (Table 2) from those receiving active care.
Outcomes
Mortality rates were 11% in hospital, 38% at 3 months, and 64% at 12 months. Of patients still alive at follow-up, 8% experienced functional decline at 3 months, as determined by a decline in one or more ADL abilities in comparison with premorbid function 2 weeks prior to hospitalization. At 12 months, 33% of patients showed functional decline.
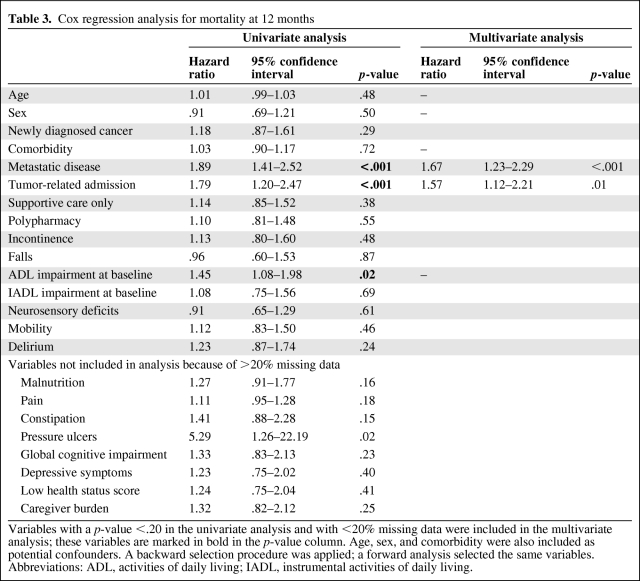
Table 3 shows results of the Cox regression analysis for factors associated with the 12-month mortality rate. Only metastatic disease (hazard ratio [HR], 1.67; 95% confidence interval [CI], 1.23–2.29) and a tumor-related reason for admission (HR, 1.57; 95% CI, 1.12–2.21) were independent predictors of outcome, whereas age, sex, and comorbidity were not.
Table 3.
Cox regression analysis for mortality at 12 months
Variables with a p-value <.20 in the univariate analysis and with <20% missing data were included in the multivariate analysis; these variables are marked in bold in the p-value column. Age, sex, and comorbidity were also included as potential confounders. A backward selection procedure was applied; a forward analysis selected the same variables.
Abbreviations: ADL, activities of daily living; IADL, instrumental activities of daily living.
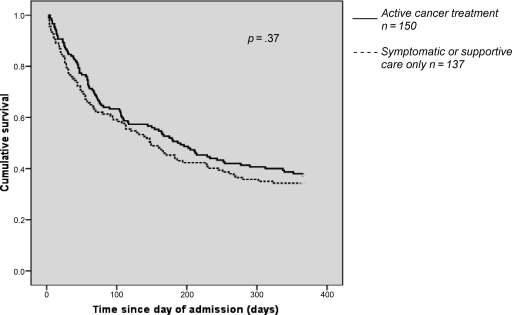
Active oncological treatment did not give any significant survival benefit over supportive care only (Fig. 1); at 3 months, the mortality rates were 36.7% for patients receiving active treatment and 38.7% for those receiving supportive care only. At 12 months, the mortality rates were 62.7% and 65.7%, respectively.
Figure 1.
Kaplan–Meier survival plotted in relation to active treatment versus supportive care, after correcting for stage of disease and reason for admission. Solid line, active cancer treatment (n = 150); dashed line, symptomatic or supportive care only (n = 137).
Because several geriatric conditions were not included in the DEFENCE I study, we also performed a separate multivariate analysis using only the data from the DEFENCE II study. That analysis yielded similar results for factors associated with mortality within 12 months.
Discussion
To our knowledge, this is the first prospective, inpatient study addressing the prevalence of geriatric conditions and their association with outcome in acutely hospitalized older cancer patients. Recent observations demonstrated that several geriatric conditions were predictive of outcome in older cancer patients in outpatient settings [6, 11, 12]. In the present study, we demonstrated that, in our population of 292 acutely hospitalized older cancer patients, most of whom were living independently prior to admission, geriatric conditions were highly prevalent, but none of the elements of the CGA were associated with mortality.
The high prevalence of geriatric conditions that we found was also seen in a hospital-based, retrospective study, which reported comparable rates of functional limitations, impaired mobility, malnutrition, and depression [17]. Interestingly, we found no differences in the presence of geriatric conditions between patients receiving active care and those receiving supportive care only. Also, receiving active oncologic treatment did not influence mortality rates, compared with receiving supportive care only. Of course, oncologic treatment is aimed not only at improving survival but also at improving quality of life, a factor not evaluated in this study.
In our study, 8% of cancer patients experienced greater ADL dependency 3 months after discharge, and at 12 months this rate was >33%. We found no prior studies that addressed functional decline after acute hospitalization in older cancer patients. A study by Covinsky et al. [32] looked at loss of ADL abilities after hospitalization for any acute medical illness in patients aged ≥70 years without specifically investigating cancer patients. In their cohort, as many as 35%–50% of older patients experienced greater disability (defined by a loss of one or more ADL abilities) 3 months after acute hospitalization. The difference with our findings could be explained by differences in the study population, because the cancer patients included in our analysis were younger and had less comorbidity, less functional impairment, and less cognitive impairment at baseline, all of which are factors potentially associated with functional decline [33].
For acutely hospitalized patients, the value of a CGA in predicting mortality seems to be low. We found that none of the geriatric conditions were associated with mortality within 12 months of admission. This outcome differs from several other studies in the outpatient setting addressing the association between geriatric conditions and outcome—age [12], comorbidity [12], ADL and IADL dependency [11], and depression [6] were all found to predict mortality in older cancer patients in that setting. However, in those patients, geriatric assessment was often administered prior to the onset of oncologic treatment with curative intent. Therefore, patients were in a different phase of disease than the patients in our study. Most likely, for these acutely admitted older cancer patients, the presence or absence of geriatric conditions had little further impact on outcome, probably because of the severity of the cancer and cancer-related symptoms: 66% of the patients were admitted for a directly tumor-related reason, >43% had metastases, 48% received only supportive care, and 64% died within 12 months. This could also explain why mortality was not associated with age or comorbidity.
In patients with a poor prognosis, the goal of care generally shifts from curation to palliation. Potentially, identifying geriatric conditions at the time of hospital admission can provide the treating physician with leads for improving quality of life. For example, in a study comparing geriatric care for older patients with cancer care as usual, geriatric care improved quality of life, although there was no difference in survival [14]. Because of the high prevalence of geriatric conditions in our study, and the fact that geriatric conditions are easily missed if not specifically looked for [7–9, 15], assessing patients for modifiable geriatric conditions seems appropriate when aiming to optimize the quality of hospital and palliative care for older cancer patients.
There are several limitations to this study. First of all, the study population forms a heterogeneous group, with different types of malignancies in different stages of disease. Furthermore, because two of the three hospitals are tertiary referral centers, there is a potential for referral bias, resulting in a less frail population as well as an overrepresentation of upper gastrointestinal (GI) tract tumors—for which the AMC is a national center—compared with regional hospitals. This potentially influences the generalizability of our findings. For example, the low cancer-specific survival rates of patients with upper GI tract malignancies could have decreased the value of the CGA for predicting mortality. A second limitation is that this is a post hoc analysis of two studies, whose designs were highly but not entirely similar. Some geriatric conditions addressed in the DEFENCE II study were not included in the DEFENCE I study, therefore resulting in missing data for these items. The effect on the outcomes of this study seems low, however, because analyses with only the data from the DEFENCE II study did not lead to different results. Because of the high mortality rates at 3 and 12 months, we were unable to test which factors at baseline were associated with functional decline.
In conclusion, our study demonstrates that geriatric conditions are highly prevalent in older cancer patients admitted for an acute illness. Based on prior literature, we assume that using a CGA for older cancer patients acutely admitted to hospital may have added value for improving quality of life. None of the elements of the CGA were of value in predicting mortality, because outcome in this population was only associated with cancer-related factors. To elucidate the exact role of CGA, future research comparing quality of life and outcome in patients receiving either care as usual or specific interventions aimed at modifying the geriatric conditions identified by a CGA is needed.
Appendix 1.
Tools used in the assessment for geriatric conditions
a These tools were administered only in the DEFENCE II study.
b Not administered if MMSE [30] score was <16.
c In the DEFENCE I study, the Katz was filled out by the patient if the MMSE [30] score was >23. If it was lower, the primary caregiver filled out the Katz. In the DEFENCE II study, the Katz was filled out by the patient if the MMSE score was >20, filled out by the patient and double-checked with the caregiver if the MMSE score was 16–20, and filled out by the caregiver only if the MMSE score was <16.
Abbreviations: ADL, activities of daily living; DEFENCE, Development of strategies Enabling Frail Elderly New Complications to Evade; EDIZ, Experienced Burden of Informal Care; GDS-2, Geriatric Depression Scale-2; IADL, instrumental activities of daily living; IQCODE-SF, Informant Questionnaire COgnitive DEcline–Short Form; MMSE, Mini-Mental State Exam; SNAQ, Short Nutritional Assessment Questionnaire; VAS, Visual Analogue Scale.
Appendix 2.
Overview of cancer diagnoses
Author Contributions
Conception/Design: Marije E. Hamaker, Barbara C. van Munster, Bianca M. Buurman, Carolien H. Smorenburg, Sophia E. de Rooij
Provision of study material or patients: Barbara C. van Munster, Sophia E. de Rooij
Collection and/or assembly of data: Marije E. Hamaker, Barbara C. van Munster, Bianca M. Buurman, Sophia E. de Rooij
Data analysis and interpretation: Marije E. Hamaker, Barbara C. van Munster, Bianca M. Buurman, Ingeborg M.J.A. Kuper, Carolien H. Smorenburg, Sophia E. de Rooij
Manuscript writing: Marije E. Hamaker, Barbara C. van Munster, Bianca M. Buurman, Ingeborg M.J.A. Kuper, Carolien H. Smorenburg, Sophia E. de Rooij
Final approval of manuscript: Marije E. Hamaker, Barbara C. van Munster, Bianca M. Buurman, Ingeborg M.J.A. Kuper, Carolien H. Smorenburg, Sophia E. de Rooij
References
- 1.Muss HB. Cancer in the elderly: A societal perspective from the United States. Clin Oncol (R Coll Radiol) 2009;21:92–98. doi: 10.1016/j.clon.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Yancik R, Ries LA. Cancer in older persons: An international issue in an aging world. Semin Oncol. 2004;31:128–136. doi: 10.1053/j.seminoncol.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Smorenburg CH, Sijp JR. [Breast cancer in the elderly] Ned Tijdschr Oncol. 2006;3:247–252. In Dutch. [Google Scholar]
- 4.Extermann M, Overcash J, Lyman GH, et al. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 5.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 6.Maas HAAM, Janssen-Heijnen MLG, Olde Rikkert MGM, et al. Comprehensive geriatric assessment and its impact in oncology. Eur J Cancer. 2007;43:2161–2169. doi: 10.1016/j.ejca.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Extermann M, Meyer J, McGinnis M, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Crit Rev Oncol Hematol. 2004;49:69–75. doi: 10.1016/s1040-8428(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 8.Flood KL, Carroll MB, Le CV, et al. Geriatric syndromes in elderly patients admitted to an oncology-acute care for elders unit. J Clin Oncol. 2006;24:2298–2303. doi: 10.1200/JCO.2005.02.8514. [DOI] [PubMed] [Google Scholar]
- 9.Terrett C, Albrand G, Droz JP Geriatric Oncology Program. Multidimensional geriatric assessment reveals unknown medical problems in elderly cancer patients. J Clin Oncol. 2004;22(14 suppl) Abstract 8167. [Google Scholar]
- 10.Zagonel V, Fratino L, Piselli P, et al. The comprehensive geriatric assessment predicts mortality among elderly cancer patients. Proc Am Soc Clin Oncol. 2002;21 Abstract 1458. [Google Scholar]
- 11.Terret C, Zulian GB, Naiem A, et al. Multidisciplinary approach to the geriatric oncology patient. J Clin Oncol. 2007;25:1876–1881. doi: 10.1200/JCO.2006.10.3291. [DOI] [PubMed] [Google Scholar]
- 12.Wedding U, Röhrig B, Klippstein A, et al. Age, severe comorbidity and functional impairment independently contribute to poor survival in cancer patients. J Cancer Res Clin Oncol. 2007;133:945–950. doi: 10.1007/s00432-007-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCorkle R, Strumph NE, Nuamah IF, et al. A specialized home care intervention improves survival among older post-surgical cancer patients. J Am Geriatr Soc. 2000;48:1707–1713. doi: 10.1111/j.1532-5415.2000.tb03886.x. [DOI] [PubMed] [Google Scholar]
- 14.Rao AV, Hsieh F, Feussner JR, et al. Geriatric evaluation and management units in the care of the frail elderly cancer patient. J Gerontol A Biol Sci Med Sci. 2005;60:798–803. doi: 10.1093/gerona/60.6.798. [DOI] [PubMed] [Google Scholar]
- 15.Inouye SK, Foreman MD, Mion LC, et al. Nurses' recognition of delirium and its symptoms: Comparison of nurse and researcher ratings. Arch Intern Med. 2001;161:2467–2473. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 16.Serraino D, Fratino L, Zagonel V GIOGer Study Group (Italy) Prevalence of functional disability among elderly patients with cancer. Crit Rev Oncol Hematol. 2001;39:269–273. doi: 10.1016/s1040-8428(00)00130-x. [DOI] [PubMed] [Google Scholar]
- 17.Retornaz F, Seux V, Pauly V, et al. Geriatric assessment and care for older cancer inpatients admitted in acute care for elders unit. Crit Rev Oncol Hematol. 2008;68:165–171. doi: 10.1016/j.critrevonc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Retornaz F, Seux V, Sourial N, et al. Comparison of the health and functional status between older inpatients with and without cancer admitted to a geriatric/internal medicine unit. J Gerontol A Biol Sci Med Sci. 2007;62:917–922. doi: 10.1093/gerona/62.8.917. [DOI] [PubMed] [Google Scholar]
- 19.Hurria A, Lichtman SM, Gardes J, et al. Identifying vulnerable older adults with cancer: Integrating geriatric assessment into oncology practice. J Am Geriatr Soc. 2007;55:1604–1608. doi: 10.1111/j.1532-5415.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 20.Buurman BM, van Munster BC, Korevaar JC, et al. Prognostication in acutely admitted older patients by nurses and physicians. J Gen Intern Med. 2008;23:1883–1889. doi: 10.1007/s11606-008-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruizenga HM, Seidell JC, de Vet HC, et al. Development and validation of a hospital screening tool for malnutrition: The short nutritional assessment questionnaire (SNAQ) Clin Nutr. 2005;24:75–82. doi: 10.1016/j.clnu.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: What is moderate pain in millimetres? Pain. 1997;72:95–97. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 23.Katz S, Ford AB, Moskowits RW, et al. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychological function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 24.Weinberger M, Samsa GP, Schmader K, et al. Comparing proxy and patients' perceptions of patients' functional status: Results from an outpatient geriatric clinic. J Am Geriatr Soc. 1992;40:585–588. doi: 10.1111/j.1532-5415.1992.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 25.The EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 26.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 27.Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: Cross sectional study. BMJ. 2003;327:1144–1146. doi: 10.1136/bmj.327.7424.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 29.Pot AM, van Dyck R, Deeg DJ. [Perceived stress caused by informal caregiving. Construction of a scale] Tijdschr Gerontol Geriatr. 1995;26:214–219. In Dutch. [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: Increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 33.Boyd CM, Ricks M, Fried LP, et al. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: The Women's Health and Aging Study I. J Am Geriatr Soc. 2009;57:1757–1766. doi: 10.1111/j.1532-5415.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]