Racial disparities between blacks and whites in lung cancer incidence, morbidity, and mortality and in smoking outcomes are examined.
Keywords: Racial disparities, Blacks, Lung cancer, Tobacco treatment, Treatment outcomes
Abstract
Racial disparities exist in lung cancer incidence, morbidity, and mortality. Smoking is responsible for the majority of lung cancers, and racial disparities also exist in smoking outcomes. Black smokers are less likely than white smokers to engage in evidence-based tobacco treatment, and black smokers are less likely than white smokers to stop smoking. Continued smoking following a lung cancer diagnosis is a potential indicator of poor lung cancer treatment outcomes, yet lung cancer patients who smoke are unlikely to receive evidence-based tobacco treatment. The risks from continued smoking after diagnosis deserve attention as a modifiable factor toward lessening racial disparities in lung cancer outcomes.
Blacks Are at Risk for Lung Cancer and Poor Treatment Outcomes
Lung cancer is the leading cause of cancer death for both men and women. With 222,000 new cases per year [1], lung cancer accounts for more deaths in the U.S. than breast cancer, prostate cancer, and colon cancer combined. Although the lung cancer incidence is higher among blacks than whites within all disease stages [2], these findings are driven by disparities between black men and white men. Black males have a higher annual lung cancer incidence (104.8 cases per 100,000 black males versus 85.9 cases per 100,000 white males) and mortality rate (90.1 cases per 100,000 black males versus 69.9 cases per 100,000 white males) [1, 3, 4], whereas lung cancer mortality rates among black and white women are now comparable (41.9 cases per 100,000 white females versus 40.0 cases per 100,000 black females) [5–7]. Black lung cancer patients are diagnosed with lung cancer at a younger age [8] and with more advanced disease (53.4% of blacks versus 47.8% of whites present with distant disease; p < .001) [9], and they face higher mortality rates than white lung cancer patients with comparable disease severity [9, 10].
Racial differences in lung cancer outcomes have been attributed to numerous risk factors [6, 11–13], such as higher rates of poverty [8, 14, 15] and medical comorbidity (e.g., hypertension, diabetes) [8] among black patients, treatment access [9], and patient–provider interactions [16, 17]. Using the Surveillance Epidemiology and End Results data, Lathan and colleagues (2006) reported that black lung cancer patients were less likely than early-stage white lung cancer patients to undergo staging (odds ratio, 0.75; 95% confidence interval [CI], 0.67–0.83) and receive a recommendation for surgery (67.0% versus 71.4%; p < .05) [16]. McCann and colleagues reported that black lung cancer patients were more likely than white lung cancer patients to decline surgery (18% versus 5%; p = .002) [17]. There is evidence of less effective communication patterns between patients and providers who are race discordant versus race concordant [18]. Lastly, black cancer patients have lower rates of enrollment in cancer clinical trials [19].
Taken together, studies of racial disparities in lung cancer outcomes have increased our understanding of the underlying risk factors but have not completely accounted for the elevated risk among black patients. Despite the anticipated role of smoking in lung cancer and adverse treatment outcomes, no research has focused on the contribution of continued smoking behavior and related factors in disparities in lung cancer treatment outcomes.
Blacks Are at Risk for Continued Smoking and Poor Cessation Outcomes
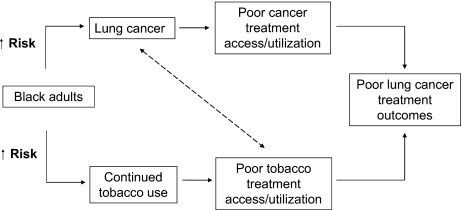
Cigarette smoking is responsible for 87% and 70% of lung cancer deaths in men and women, respectively [20]. Figure 1 illustrates how a higher risk for continued tobacco use coinciding with a lung cancer diagnosis can lead to poor lung cancer treatment outcomes. As in lung cancer treatment, disparities also loom in tobacco treatment and are likely a result of multiple predisposing risk factors. blacks initiate smoking later (average age at onset, 17.4 years for blacks versus 14.7 years for whites; p < .05) [21] and smoke fewer cigarettes per day than whites (14.1 versus 18.4 cigarettes per day) [21, 22]. However, despite later initiation, black adults smoke at rates similar to whites (black men, 23.9%; black women, 19.2%; white men, 24.5%; white women, 19.8%) [23]. This is of particular concern because evidence suggests that racial differences in nicotine exposure (nicotine intake per cigarette is 30.0% greater) and metabolism of tobacco (slower clearance of nicotine) place black smokers, compared with white smokers, at a higher risk for tobacco-related diseases [24, 25]. Blacks also have a lower rate of successful quitting [4, 26]. According to a Morbidity and Mortality Weekly Report of the National Health Interview Survey findings, successful quit rates of ever smokers were 51.0% (95% CI, ±1.1%) among whites and 37.3% (95% CI, ±2.7%) among blacks [27].
Figure 1.
A higher risk for continued tobacco use coinciding with a lung cancer diagnosis leads to a poor lung cancer treatment outcome.
There could be several reasons for low quit rates among blacks. Racial differences in continued smoking may be attributable to socioeconomic vulnerabilities, such as poverty, stress, and secondhand smoke exposure [28]. Although the majority of black smokers express a desire to quit [29], they are less likely to receive and use evidence-based treatments [30, 31] (e.g., screening for tobacco use and advice to quit [32–34], smoking cessation pharmacotherapy [35–38], and counseling [31]). In addition, black smokers are less likely to enroll in smoking cessation trials [39]. Blacks are more likely to smoke mentholated cigarettes, and mentholated cigarettes might be harder to quit than nonmentholated cigarettes [21], which leads to poorer cessation outcomes [40]. Blacks report less accurate knowledge about the risks and prevalence of smoking [41–43] and about the benefits and risks of effective smoking cessation treatments. However, culturally targeted treatments can increase blacks' smoking risk perceptions [44], and evidenced-based smoking cessation treatment can improve blacks' quit rates [45]. Thus, although the underlying causes of racial differences in smoking patterns and related outcomes are not fully understood, improvements in tobacco treatment use and targeted treatments are needed.
Lung Cancer Patients Who Continue to Smoke May Be Vulnerable to Poorer Cancer Treatment Outcomes
Recent estimates of smoking at the time of a lung cancer diagnosis are 20%–30% [46–48]. However, whereas estimates of quitting around the time of diagnosis are in the range of 35%–79% [49, 50], about one third of nonadvanced-stage lung cancer patients resume smoking within the first year after surgery [50–52]. Dr. Park and colleagues examined data from the Cancer Care Outcomes Research and Surveillance cohort survey and reported that 90% of lung cancer patients had a history of ever smoking and approximately one third of lung cancer patients reported smoking around the time of diagnosis [53]. It is important to note that many participants reported quitting tobacco use prior to, during, and immediately following a cancer diagnosis, resulting in a significant group of lung cancer patients who were relatively new former smokers and thus vulnerable to smoking relapse [50].
Research on the risks of continued smoking during cancer treatment suggests that quitting tobacco use upon a lung cancer diagnosis can improve the chance for treatment efficacy, reduce the chance for secondary tumors, and may double the chance for survival [54–58]. In a recent systematic review of observational studies using meta-analysis, continued smoking was associated with a significantly greater risk for all-cause mortality in early-stage non-small cell lung cancer patients (hazard ratio, 2.94; 95% CI, 1.15–7.54) and in limited-stage small cell lung cancer patients (hazard ratio, 1.86; CI, 1.33–2.59) [59]. Examinations of the effect of smoking on adverse events suggest that continued smoking is associated with treatment delays and more complications from surgery, including more complications from general anesthesia, a higher risk for severe pulmonary complications, and detrimental effects on wound healing [49, 60]. Continued smoking may also affect processes that increase the risk for complications of radiation and chemotherapy [55, 58]. Complications from smoking while undergoing radiation therapy include worse treatment efficacy and greater toxicity and side effects [61, 62]. Smoking while receiving chemotherapy exacerbates drug toxicity side effects and increases the incidence of infection [46, 63–65]. Future work examining the effects of continued smoking on treatment-related adverse events in lung cancer patients, particularly black adults, is needed.
Lung Cancer Patients, Particularly Those Who Continue to Smoke, Experience Psychosocial Problems
Depression affects over one third [66–68] of the cancer patient population and is more prevalent [68, 69] and persistent [70] among lung cancer patients than among patients with other common tumor types. Depression is prevalent among smokers as well; the role of depression in smoking may be bidirectional, with smokers being more likely to be depressed than nonsmokers, and, in turn, depression posing a barrier to tobacco abstinence [71–74]. In fact, depressed smokers may perceive quitting as difficult and stressful, and may use cigarettes to self-medicate depressive feelings [75, 76]. Depression is associated with continued smoking following a lung cancer diagnosis [48, 77], which may worsen the prognosis (e.g., through behavioral and biological pathways such as chronic hypothalamic–pituitary–adrenal axis activation) [78]. Thus, the psychological effects of having lung cancer and depression concurrently may lead to a heightened sense of vulnerability, delaying quit attempts [75]. This is of particular concern for blacks, because studies from the general population indicate that depression is more severe, chronic, debilitating, and undertreated [79] among blacks than among whites, with substantial racial disparities in the level and type of mental health care received.
Lung Cancer and Stigma
Higher rates of depression in lung cancer patients and lung cancer patients who smoke may be a result, in part, of perceived and experienced stigma. Stigma is associated with cancer patients whose behavior may have contributed to their disease [80], which is especially true for lung cancer patients [81, 82]. Lung cancer patients are more likely than breast and prostate cancer patients to report internal causal attributions for their cancer [83, 84]. Within the past 20 years, cancer patient populations, such as breast cancer patients, have received greater sympathy and social awareness [85], but lung cancer patients are less likely to receive sympathy and support.
Lung cancer patients report feeling stigmatized regardless of their smoking status [81, 86]. However, patients who continue to smoke after diagnosis are at further risk for perceived stigma as well as barriers to seeking support for quitting tobacco use. In a survey of current smokers living in New York City, participants who perceived high compared with low levels of smoker-related stigma reported being less likely to disclose their smoking status [87]. Social norms, including disapproval of smoking and the poor prognosis for lung cancer patients, are factors in smoking stigma and may explain the heightened stigma experienced by lung cancer patients who are smokers [88]. Because of the association of lung cancer with smoking [80–82], lung cancer patients who continue to smoke experience shame [81, 83] for causing their disease and thus may be reluctant to discuss smoking with oncology providers.
Lung Cancer Patients, Particularly Blacks, Are a Vulnerable Group for Whom Smoking Cessation Treatment Is Extremely Important
Most lung cancer patients who smoke want to quit smoking [49, 50] but need help in order to do so. In the 2 years following a lung cancer diagnosis, 86.4% of smokers try to quit [50]. Recently, the American Society for Clinical Oncology's Quality Oncology Practice Initiative included among its core measures that: (a) cigarette smoking status be documented by the second medical visit and (b) tobacco treatment counseling be recommended to patients receiving cancer treatment [89]. Unfortunately, despite these new guidelines, half of comprehensive cancer centers do not have tobacco treatment programs [90]. Lung cancer patients generally do not receive tobacco treatment from their oncology providers, nor is smoking routinely addressed in the primary care setting following diagnosis [52, 91–93]. Consequently, most lung cancer patients who smoke have not used established behavioral and pharmacological tobacco treatment [48], and a lack of patient–provider communication in the context of smoking and a lung cancer diagnosis might exacerbate this gap in treatment.
General Discussion
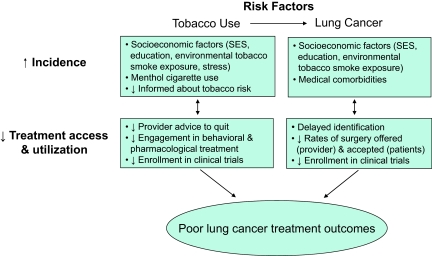
Black lung cancer patients who smoke experience a confluence of risk factors for poor lung cancer treatment outcomes. Figure 2 summarizes how risk factors for a higher incidence of tobacco use and lung cancer and poorer treatment access and utilization for tobacco use and lung cancer may lead to poor lung cancer treatment outcomes among black adults. Black smokers are less likely to receive effective tobacco treatment and are more likely to have poor tobacco treatment outcomes. Because blacks and smokers are vulnerable to inadequate treatments for smoking and risk factors for smoking relapse (e.g., depression), they may be more likely to have adverse events from lung cancer treatment, poorer lung cancer treatment outcomes and, ultimately, a higher mortality rate from lung cancer. Thus, some of the disparities in lung cancer outcomes in blacks could be a result of smoking and related disparities in tobacco treatment.
Figure 2.
Risk factors for tobacco use and lung cancer are associated with lower treatment access/use among black adults and may lead to a poor lung cancer treatment outcome.
Abbreviation: SES, socioeconomic status.
Smoking could be a modifiable risk factor for poor lung cancer outcomes in blacks, and addressing smoking could have immediate effects on treatment efficacy, adverse events, and quality of life. Models have been posited for addressing smoking during oncology care [48] and for targeting treatment to black smokers [94]. Important cancer-related factors to consider when targeting treatment to lung cancer patients include: the benefits of quitting following a cancer diagnosis (for treatment and for preventing secondary tumors and other smoking-related disease), the shame and stigma about a cancer diagnosis, preparing for cancer treatment, pain and symptom management, fatalism, and disease-targeted materials [48]. Important factors to target smoking cessation treatment for black smokers include health disparities (both in the rate of lung cancer and in treatment outcome), emotional and psychological concerns (targeted to unique stressors for this population), family and religion, and culturally appropriate materials [44, 94]. These factors should be considered in future work to decrease the prevalence of continued smoking after diagnosis, as a step toward lessening racial disparities in lung cancer outcomes.
Author Contributions
Conception/Design: Elyse R. Park, Sandra J. Japuntich, Lara Traeger, Sheila Cannon
Manuscript writing: Elyse R. Park, Sandra J. Japuntich, Lara Traeger, Sheila Cannon, Hannah Pajolek
Final approval of manuscript: Elyse R. Park, Sandra J. Japuntich, Lara Traeger, Sheila Cannon, Hannah Pajolek
References
- 1.American Cancer Society. Atlanta: American Cancer Society; 2010. Cancer Facts & Figures 2010; pp. 1–62. [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005, National Cancer Institute, based on November 2007 SEER data submission, posted to the SEER web site, 2008. [accessed August 8, 2011]. Available at http://seer.cancer.gov/csr/1975_2005/
- 3.Flenaugh EL, Henriques-Forsythe MN. Lung cancer disparities in African Americans: Health versus health care. Clin Chest Med. 2006;27:431–439. vi. doi: 10.1016/j.ccm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 5.Abidoye O, Ferguson MK, Salgia R. Lung carcinoma in African Americans. Nat Clin Pract Oncol. 2007;4:118–129. doi: 10.1038/ncponc0718. [DOI] [PubMed] [Google Scholar]
- 6.Bryant AS, Cerfolio RJ. Impact of race on outcomes of patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:711–715. doi: 10.1097/JTO.0b013e31817c60c7. [DOI] [PubMed] [Google Scholar]
- 7.Shugarman LR, Mack K, Sorbero ME, et al. Race and sex differences in the receipt of timely and appropriate lung cancer treatment. Med Care. 2009;47:774–781. doi: 10.1097/MLR.0b013e3181a393fe. [DOI] [PubMed] [Google Scholar]
- 8.Yang R, Cheung MC, Byrne MM, et al. Do racial or socioeconomic disparities exist in lung cancer treatment? Cancer. 2010;116:2437–2447. doi: 10.1002/cncr.24986. [DOI] [PubMed] [Google Scholar]
- 9.Lally BE, Geiger AM, Urbanic JJ, et al. Trends in the outcomes for patients with limited stage small cell lung cancer: An analysis of the Surveillance, Epidemiology, and End Results database. Lung Cancer. 2009;64:226–231. doi: 10.1016/j.lungcan.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 10.DeLancey JO, Thun MJ, Jemal A, et al. Recent trends in Black-White disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 2008;17:2908–2912. doi: 10.1158/1055-9965.EPI-08-0131. [DOI] [PubMed] [Google Scholar]
- 11.Lathan CS, Neville BA, Earle CC. Racial composition of hospitals: Effects on surgery for early-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:4347–4352. doi: 10.1200/JCO.2007.15.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Said R, Terjanian T, Taioli E. Clinical characteristics and presentation of lung cancer according to race and place of birth. Future Oncol. 2010;6:1353–1361. doi: 10.2217/fon.10.89. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui F, Bae K, Langer CJ, et al. The influence of gender, race, and marital status on survival in lung cancer patients: Analysis of Radiation Therapy Oncology Group trials. J Thorac Oncol. 2010;5:631–639. doi: 10.1097/jto.0b013e3181d5e46a. [DOI] [PubMed] [Google Scholar]
- 14.Esnaola NF, Gebregziabher M, Knott K, et al. Underuse of surgical resection for localized, non-small cell lung cancer among whites and African Americans in South Carolina. Ann Thorac Surg. 2008;86:220–226. doi: 10.1016/j.athoracsur.2008.02.072. discussion 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farjah F, Wood DE, Yanez ND, 3rd, et al. Racial disparities among patients with lung cancer who were recommended operative therapy. Arch Surg. 2009;144:14–18. doi: 10.1001/archsurg.2008.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 17.McCann J, Artinian V, Duhaime L, et al. Evaluation of the causes for racial disparity in surgical treatment of early stage lung cancer. Chest. 2005;128:3440–3446. doi: 10.1378/chest.128.5.3440. [DOI] [PubMed] [Google Scholar]
- 18.Gordon HS, Street RL, Jr, Sharf BF, et al. Racial differences in doctors' information-giving and patients' participation. Cancer. 2006;107:1313–1320. doi: 10.1002/cncr.22122. [DOI] [PubMed] [Google Scholar]
- 19.Park ER, Weiss ES, Moy B. Recruiting and enrolling minority patients into cancer clinical trials. Community Oncol. 2007;4:254–257. [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 21.Finkenauer R, Pomerleau CS, Snedecor SM, et al. Race differences in factors relating to smoking initiation. Addict Behav. 2009;34:1056–1059. doi: 10.1016/j.addbeh.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trinidad DR, Gilpin EA, Lee L, et al. Has there been a delay in the age of regular smoking onset among African Americans? Ann Behav Med. 2004;28:152–157. doi: 10.1207/s15324796abm2803_2. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Vital signs: Current cigarette smoking among adults aged ≥18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1135–1140. [PubMed] [Google Scholar]
- 24.Signorello LB, Cai Q, Tarone RE, et al. Racial differences in serum cotinine levels of smokers. Dis Markers. 2009;27:187–192. doi: 10.3233/DMA-2009-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez-Stable EJ, Herrera B, Jacob P, 3rd, et al. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 26.Fagan P, Moolchan ET, Lawrence D, et al. Identifying health disparities across the tobacco continuum. Addiction. 2007;102(suppl 2):5–29. doi: 10.1111/j.1360-0443.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults—United States 2000. MMWR Morb Mortal Wkly Rep. 2002;51:642–645. [PubMed] [Google Scholar]
- 28.Winickoff JP, Gottlieb M, Mello MM. Regulation of smoking in public housing. N Engl J Med. 2010;362:2319–2325. doi: 10.1056/NEJMhle1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yerger VB, Wertz M, McGruder C, et al. Nicotine replacement therapy: Perceptions of African-American smokers seeking to quit. J Natl Med Assoc. 2008;100:230–236. doi: 10.1016/s0027-9684(15)31211-6. [DOI] [PubMed] [Google Scholar]
- 30.Cokkinides VE, Halpern MT, Barbeau EM, et al. Racial and ethnic disparities in smoking-cessation interventions: Analysis of the 2005 National Health Interview Survey. Am J Prev Med. 2008;34:404–412. doi: 10.1016/j.amepre.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Fu SS, Burgess D, van Ryn M, et al. Views on smoking cessation methods in ethnic minority communities: A qualitative investigation. Prev Med. 2007;44:235–240. doi: 10.1016/j.ypmed.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Sonnenfeld N, Schappert SM, Lin SX. Racial and ethnic differences in delivery of tobacco-cessation services. Am J Prev Med. 2009;36:21–28. doi: 10.1016/j.amepre.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 33.Franks P, Fiscella K, Meldrum S. Racial disparities in the content of primary care office visits. J Gen Intern Med. 2005;20:599–603. doi: 10.1111/j.1525-1497.2005.0109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houston TK, Scarinci IC, Person SD, et al. Patient smoking cessation advice by health care providers: The role of ethnicity, socioeconomic status, and health. Am J Public Health. 2005;95:1056–1061. doi: 10.2105/AJPH.2004.039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu SS, Kodl MM, Joseph AM, et al. Racial/ethnic disparities in the use of nicotine replacement therapy and quit ratios in lifetime smokers ages 25 to 44 years. Cancer Epidemiol Biomarkers Prev. 2008;17:1640–1647. doi: 10.1158/1055-9965.EPI-07-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu SS, Sherman SE, Yano EM, et al. Ethnic disparities in the use of nicotine replacement therapy for smoking cessation in an equal access health care system. Am J Health Promot. 2005;20:108–116. doi: 10.4278/0890-1171-20.2.108. [DOI] [PubMed] [Google Scholar]
- 37.Piper ME, Fox BJ, Welsch SK, et al. Gender and racial/ethnic differences in tobacco-dependence treatment: A commentary and research recommendations. Nicotine Tob Res. 2001;3:291–297. doi: 10.1080/14622200110050448. [DOI] [PubMed] [Google Scholar]
- 38.Zhu S, Melcer T, Sun J, et al. Smoking cessation with and without assistance: A population-based analysis. Am J Prev Med. 2000;18:305–311. doi: 10.1016/s0749-3797(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 39.King AC, Cao D, Southard CC, et al. Racial differences in eligibility and enrollment in a smoking cessation clinical trial. Health Psychol. 2011;30:40–48. doi: 10.1037/a0021649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gundersen DA, Delnevo CD, Wackowski O. Exploring the relationship between race/ethnicity, menthol smoking, and cessation, in a nationally representative sample of adults. Prev Med. 2009;49:553–557. doi: 10.1016/j.ypmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Reimer RA, Gerrard M, Gibbons FX. Racial disparities in smoking knowledge among current smokers: Data from the health information national trends surveys. Psychol Health. 2010;25:943–959. doi: 10.1080/08870440902935913. [DOI] [PubMed] [Google Scholar]
- 42.Davis KC, Nonnemaker JM, Asfaw HA, et al. Racial/ethnic differences in perceived smoking prevalence: Evidence from a national survey of teens. Int J Environ Res Public Health. 2010;7:4152–4168. doi: 10.3390/ijerph7124152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwards CL, Bennett GG, Wolin KY, et al. Misestimation of peer tobacco use: Understanding disparities in tobacco use. J Natl Med Assoc. 2008;100:299–302. doi: 10.1016/s0027-9684(15)31242-6. [DOI] [PubMed] [Google Scholar]
- 44.Webb MS, Baker EA, Rodríguez de Ybarra D. Effects of culturally specific cessation messages on theoretical antecedents of behavior among low-income African American smokers. Psychol Addict Behav. 2010;24:333–341. doi: 10.1037/a0018700. [DOI] [PubMed] [Google Scholar]
- 45.Webb MS. Treating tobacco dependence among African Americans: A meta-analytic review. Health Psychol. 2008;27(3 suppl):S271–S282. doi: 10.1037/0278-6133.27.3(suppl.).s271. [DOI] [PubMed] [Google Scholar]
- 46.Gritz ER. Rationale for treating tobacco dependence in the cancer setting. Presented at Treating Tobacco Dependence at the National Cancer Institute's Cancer Centers; December 07, 2009; Bethesda, MD. [Google Scholar]
- 47.Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126:1733–1741. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- 48.Park ER, Japuntich S, Temel J, et al. A smoking cessation intervention for thoracic surgery and oncology clinics: A pilot trial. J Thorac Oncol. 2011;6:1059–1065. doi: 10.1097/JTO.0b013e318215a4dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dresler CM, Bailey M, Roper CR, et al. Smoking cessation and lung cancer resection. Chest. 1996;110:1199–1202. doi: 10.1378/chest.110.5.1199. [DOI] [PubMed] [Google Scholar]
- 50.Gritz ER, Nisenbaum R, Elashoff RE, et al. Smoking behavior following diagnosis in patients with stage I non-small cell lung cancer. Cancer Causes Control. 1991;2:105–112. doi: 10.1007/BF00053129. [DOI] [PubMed] [Google Scholar]
- 51.Walker MS, Vidrine DJ, Gritz ER, et al. Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2370–2377. doi: 10.1158/1055-9965.EPI-06-0509. [DOI] [PubMed] [Google Scholar]
- 52.Cooley ME, Sarna L, Kotlerman J, et al. Smoking cessation is challenging even for patients recovering from lung cancer surgery with curative intent. Lung Cancer. 2009;66:218–225. doi: 10.1016/j.lungcan.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park ER, Traeger L, Japuntich SJ, et al. Continued smoking among lung or colorectal cancer patients: A population-based study. Presented at the 2010 Annual Meeting of the Society for Research on Nicotine and Tobacco; February 24–27, 2010; Baltimore, MD. [Google Scholar]
- 54.Gritz ER, Fingeret MC, Vidrine DJ, et al. Successes and failures of the teachable moment: Smoking cessation in cancer patients. Cancer. 2006;106:17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- 55.Dresler CM. Is it more important to quit smoking than which chemotherapy is used? Lung Cancer. 2003;39:119–124. doi: 10.1016/s0169-5002(02)00455-5. [DOI] [PubMed] [Google Scholar]
- 56.Tucker MA, Murray N, Shaw EG, et al. Second primary cancers related to smoking and treatment of small-cell lung cancer. Lung Cancer Working Cadre. J Natl Cancer Inst. 1997;89:1782–1788. doi: 10.1093/jnci/89.23.1782. [DOI] [PubMed] [Google Scholar]
- 57.Garces YI, Schroeder DR, Nirelli LM, et al. Second primary tumors following tobacco dependence treatments among head and neck cancer patients. Am J Clin Oncol. 2007;30:531–539. doi: 10.1097/COC.0b013e318059adfc. [DOI] [PubMed] [Google Scholar]
- 58.Cataldo JK, Dubey S, Prochaska JJ. Smoking cessation: An integral part of lung cancer treatment. Oncology. 2010;78:289–301. doi: 10.1159/000319937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: Systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaporciyan AA, Merriman KW, Ece F, et al. Incidence of major pulmonary morbidity after pneumonectomy: Association with timing of smoking cessation. Ann Thorac Surg. 2002;73:420–425. doi: 10.1016/s0003-4975(01)03443-9. discussion 425–426. [DOI] [PubMed] [Google Scholar]
- 61.Monson JM, Stark P, Reilly JJ, et al. Clinical radiation pneumonitis and radiographic changes after thoracic radiation therapy for lung carcinoma. Cancer. 1998;82:842–850. [PubMed] [Google Scholar]
- 62.Zevallos JP, Mallen MJ, Lam CY, et al. Complications of radiotherapy in laryngopharyngeal cancer: Effects of a prospective smoking cessation program. Cancer. 2009;115:4636–4644. doi: 10.1002/cncr.24499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: Ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14:2287–2293. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J, Kamdar O, Le W, et al. Nicotine induces resistance to chemotherapy by modulating mitochondrial signaling in lung cancer. Am J Respir Cell Mol Biol. 2009;40:135–146. doi: 10.1165/rcmb.2007-0277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu J, Huang H, Pan C, et al. Nicotine inhibits apoptosis induced by cisplatin in human oral cancer cells. Int J Oral Maxillofac Surg. 2007;36:739–744. doi: 10.1016/j.ijom.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 66.Ell K, Xie B, Quon B, et al. Randomized controlled trial of collaborative care management of depression among low-income patients with cancer. J Clin Oncol. 2008;26:4488–4496. doi: 10.1200/JCO.2008.16.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pirl WF. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. J Natl Cancer Inst Monogr. 2004;(32):32–39. doi: 10.1093/jncimonographs/lgh026. [DOI] [PubMed] [Google Scholar]
- 68.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 69.Akechi T, Okamura H, Okuyama T, et al. Psychosocial factors and survival after diagnosis of inoperable non-small cell lung cancer. Psychooncology. 2009;18:23–29. doi: 10.1002/pon.1364. [DOI] [PubMed] [Google Scholar]
- 70.Hopwood P, Stephens RJ. Depression in patients with lung cancer: Prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18:893–903. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- 71.Flensborg-Madsen T, von Scholten MB, Flachs EM, et al. Tobacco smoking as a risk factor for depression. A 26-year population-based follow-up study. J Psychiatr Res. 2011;45:143–149. doi: 10.1016/j.jpsychires.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Berlin I, Covey LS, Glassman AH. Smoking and depression: A co-morbidity. J Dual Diagn. 2009;5:149–158. [Google Scholar]
- 73.Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 74.Grant BF, Hasin DS, Chou SP, et al. Nicotine dependence and psychiatric disorders in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- 75.Floyd AH, Westmaas JL, Targhetta V, et al. Depressive symptoms and smokers' perceptions of lung cancer risk: Moderating effects of tobacco dependence. Addict Behav. 2009;34:154–163. doi: 10.1016/j.addbeh.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 76.Catley D, Harris KJ, Okuyemi KS, et al. The influence of depressive symptoms on smoking cessation among African Americans in a randomized trial of bupropion. Nicotine Tob Res. 2005;7:859–870. doi: 10.1080/14622200500330118. [DOI] [PubMed] [Google Scholar]
- 77.Cooley ME, Sarna L, Brown JK, et al. Tobacco use in women with lung cancer. Ann Behav Med. 2007;33:242–250. doi: 10.1007/BF02879906. [DOI] [PubMed] [Google Scholar]
- 78.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 79.Williams DR, Gonzàlez HM, Neighbors H, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: Results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305–315. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]
- 80.Lebel S, Devins GM. Stigma in cancer patients whose behavior may have contributed to their disease. Future Oncol. 2008;4:717–733. doi: 10.2217/14796694.4.5.717. [DOI] [PubMed] [Google Scholar]
- 81.Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: Qualitative study. BMJ. 2004;328:1470. doi: 10.1136/bmj.38111.639734.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tod AM, Craven J, Allmark P. Diagnostic delay in lung cancer: A qualitative study. J Adv Nurs. 2008;61:336–343. doi: 10.1111/j.1365-2648.2007.04542.x. [DOI] [PubMed] [Google Scholar]
- 83.Else-Quest NM, LoConte NK, Schiller JH, et al. Perceived stigma, self-blame, and adjustment among lung, breast and prostate cancer patients. Psychol Health. 2009;24:949–964. doi: 10.1080/08870440802074664. [DOI] [PubMed] [Google Scholar]
- 84.LoConte NK, Else-Quest NM, Eickhoff J, et al. Assessment of guilt and shame in patients with non-small-cell lung cancer compared with patients with breast and prostate cancer. Clin Lung Cancer. 2008;9:171–178. doi: 10.3816/CLC.2008.n.026. [DOI] [PubMed] [Google Scholar]
- 85.Gulyn LM, Youssef F. Attribution of blame for breast and lung cancers in women. J Psychosoc Oncol. 2010;28:291–301. doi: 10.1080/07347331003689052. [DOI] [PubMed] [Google Scholar]
- 86.Raleigh ZT. A biopsychosocial perspective on the experience of lung cancer. J Psychosoc Oncol. 2010;28:116–125. doi: 10.1080/07347330903438990. [DOI] [PubMed] [Google Scholar]
- 87.Stuber J, Galea S. Who conceals their smoking status from their health care provider? Nicotine Tob Res. 2009;11:303–307. doi: 10.1093/ntr/ntn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Conlon A, Gilbert D, Jones B, et al. Stacked stigma: Oncology social workers' perceptions of the lung cancer experience. J Psychosoc Oncol. 2010;28:98–115. doi: 10.1080/07347330903438982. [DOI] [PubMed] [Google Scholar]
- 89.American Society of Clinical Oncology. The Quality Oncology Practice Initiative: Program Overview. [accessed January 26, 2010]. Available at http://qopi.asco.org/Documents/QOPIProgramOverview9–21-09_000.pdf.
- 90.Morgan G, Schnoll R, Alfano C, et al. National Cancer Institute Conference on Treating Tobacco Dependence at Cancer Centers. J Oncol Pract. 2011;7:178–182. doi: 10.1200/JOP.2010.000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pentz RD, Berg CJ. Smoking and ethics: What are the duties of oncologists? The Oncologist. 2010;15:987–993. doi: 10.1634/theoncologist.2010-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simmons VN, Litvin EB, Patel RD, et al. Patient-provider communication and perspectives on smoking cessation and relapse in the oncology setting. Patient Educ Couns. 2009;77:398–403. doi: 10.1016/j.pec.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coups EJ, Dhingra LK, Heckman CJ, et al. Receipt of provider advice for smoking cessation and use of smoking cessation treatments among cancer survivors. J Gen Intern Med. 2009;24(suppl 2):S480–S486. doi: 10.1007/s11606-009-0978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Webb MS, Francis J, Hines BC, et al. Health disparities and culturally specific treatment: Perspectives and expectancies of African American smokers. J Clin Psychol. 2007;63:1247–1263. doi: 10.1002/jclp.20437. [DOI] [PubMed] [Google Scholar]