A hand–foot syndrome–specific quality of life questionnaire (HFS-14) for patients treated with chemotherapy and targeted therapy was developed and validated.
Keywords: Chemotherapy, Hand–foot syndrome, HFS-14 scale, Quality of life, Tyrosine kinase inhibitors, Skindex-16
Abstract
Background.
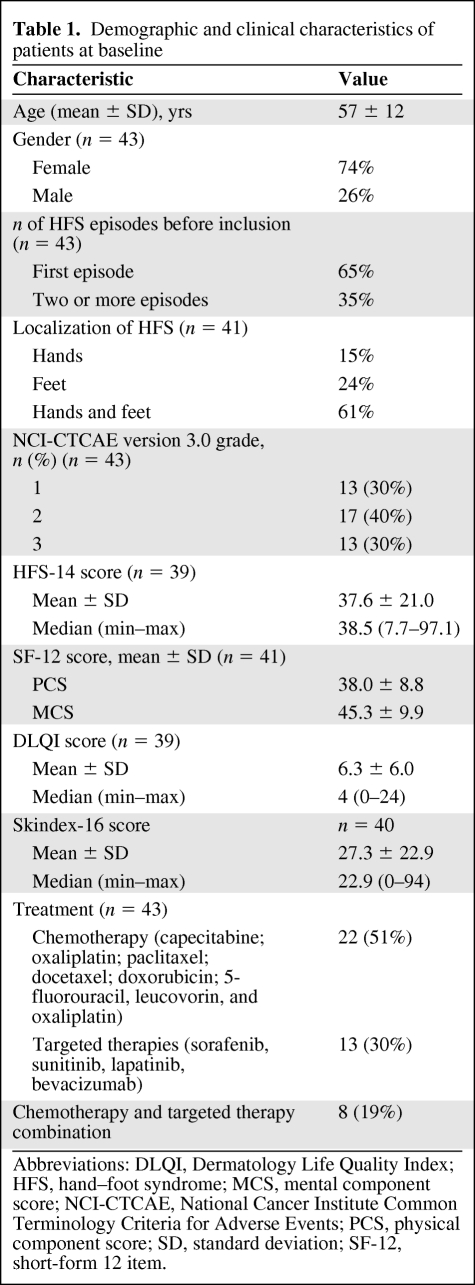
Hand–foot syndrome (HFS) is a common reaction to certain chemotherapies and new targeted therapies, impairing patient quality of life (QoL). However, there is currently no specific tool to measure QoL in patients with HFS.
Objective.
The objective was to develop and validate a HFS-specific QoL questionnaire (HFS-14).
Patients and Methods.
From a list of 31 items identified from a literature review and patient interview notes, item reduction and pilot testing by cognitive debriefing resulted in a final 14-item questionnaire with excellent internal reliability. Clinical validity was assessed in 43 patients with HFS by comparing the HFS-14 score according to HFS clinical grade based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 3.0, and by measuring its correlation with the Dermatology Life Quality Index (DLQI), Skindex-16, and short-form 12 health-related questionnaires and pain measurement.
Results.
The mean HFS-14 score was significantly higher in patients with clinical grade 2 and grade 3 HFS than in those with grade 1 HFS. The higher the HFS-14 score, the greater the QoL impairment. The HFS-14 score was highly correlated with the DLQI and Skindex-16 scores. In the population of patients with severe grade 3 NCI-CTCAE HFS, the HFS-14 score was significantly higher in patients having both hands and feet severely involved than in those with severe involvement of one limb (hands or feet) with the other one less severely affected.
Conclusions.
This scale specifically developed for patients with HFS is a valid and valuable tool for measuring HFS-related QoL impairment.
Introduction
Palmar–plantar erythrodysesthesia, also referred to as hand–foot syndrome (HFS), is a very common adverse event of chemotherapy. The drugs most frequently associated with HFS are 5-fluorouracil, pegylated liposomal doxorubicin, and capecitabine [1]. The HFS incidence in patients receiving these chemotherapy agents is in the range of 7.3%–63% [1]. HFS is characterized at first by dysesthesia and tingling in the palms, fingers, and soles [2–4]. It may then progress, in a few days, to burning pain with diffuse erythema and swelling. In severe cases, it may be accompanied by scaling, blistering, erosions, or, more rarely, ulcerations [1–5].
Over the past several years, similar cutaneous toxicity effects have also frequently been reported in association with certain new targeted cancer therapies, namely, the two multikinase inhibitors (MKIs) sorafenib and sunitinib. This MKI-associated HFS (also referred to as hand–foot skin reaction) differs from HFS induced by chemotherapy because it presents as more localized hyperkeratosic inflammatory lesions surrounded by an erythematous halo, predominating on the feet and triggered by factors such as pressure, friction, and trauma [6–8]. The lesions can be very painful and can interfere considerably with even the simplest everyday activities, such as walking or gripping objects [9].
Although MKI- and chemotherapy-associated HFS presentations are not life threatening, both seriously impair patient quality of life (QoL) [1, 10]. Most reports have indicated that severe HFS could lead to significant morbidity and poor patient compliance with cancer treatment [11]. Currently available QoL measurement tools such as the Skindex-16 [12] and the Dermatology Life Quality Index (DLQI) questionnaire [13] can assess skin-related QoL in patients receiving chemotherapy or MKIs [10], but they are not specifically designed for this population [14].
The aim of our study was, therefore, to develop a simple and easy-to-use HFS-specific QoL scale using a well-established methodology and to validate this tool by studying its correlation with clinical grade and several generic and dermatologic QoL scales in patients suffering from HFS of varying severity induced by chemotherapies or targeted therapies.
Patients and Methods
Development of the HFS-14 Questionnaire
The HFS-14 questionnaire was developed using standardized methodology for QoL questionnaire development [15], which consisted of the following steps: gathering patient input, generating an exhaustive list of items, reducing the list of items, allocating items to domains, pilot testing, calculation of scores, and validation. A working group of experts including oncologists, a dermatologist, a public health physician, and health professionals having a good knowledge of HFS and day-to-day experience in the management of this condition was constructed for the development of this questionnaire.
Gathering Patient Input
Before the beginning of the study, oncologists and nurses in the oncology department who were part of the working group were instructed to be receptive to the complaints of patients suffering from HFS and to refer patients who presented with HFS symptoms to the dermatologist. Among the patients referred for dermatology consultation, 20 of them suffering from HFS were interviewed by the dermatologist. They were asked about their major complaints, their activities before they had HFS, and what had changed in their lives (e.g., which activities they found more difficult to perform or could not perform anymore because of their HFS).
Generation of a List of Items
The working group then identified a first pool of items. The selection was based on a critical review of the literature (PubMed search), the opinion of the working group, and interview notes collected by the dermatologist during his consultations dedicated to HFS patients. The public health expert converted each statement or sentence from this selection into a question to generate a preliminary questionnaire. The selected items mainly related to the personal experience of HFS, the impact of hand and/or foot involvement, social concerns, and disturbances in daily life resulting from HFS. Items were then categorized into three domains: hands, feet (disability caused by HFS-related impairment of hands and feet, respectively), and social (HFS social impact).
Item Reduction and Questionnaire Consolidation
From this preliminary list, the public health expert grouped redundant items with similar meanings (e.g., items related to difficulty in applying makeup, washing, or shaving) together and suppressed rarely quoted items and items with a low impact in terms of disability in daily life. To avoid any confusion with QoL impairment related to other symptoms of comorbidities, each item was concluded by the expression “because of my HFS.”
Pilot Testing (Cognitive Debriefing)
This final questionnaire was tested in a sample of subjects with a French mother tongue during an individual, cognitive debriefing interview to determine the issues related to question and answer wording (ambiguity, misunderstanding, etc.). Pilot testing was performed by Lionbridge company.
Allocation of Items to Domains and Internal Consistency
The selected items were allocated to the three predefined domains, and the final questionnaire included similar numbers of items allocated to the hands and feet domains. Internal consistency was measured by calculating Cronbach's α coefficients for the hands and feet domains and for the final questionnaire.
English Translation and Linguistic Validation
An English version of the questionnaire was developed by Lionbridge company using a standardized method based on the International Society for Pharmacoeconomics and Outcomes Research Good Practices for Translation [16], which included forward and back translation, back translation review steps, and finally pilot testing by individual cognitive debriefing in a British population sample.
Calculation of Scores
Each item was scored on a three-point Likert scale: 0, “no, never”; 1, “yes, from time to time”; 2, “yes, always.” Two separate questions were also added, one to measure limb involvement (type of limb affected by HFS, one or both) and one to measure pain. The limb involvement item was scored either 1 if only the hands or feet were affected or 3 if both the hands and feet were affected. The pain item was scored on a three-point scale: 1, not painful; 2, moderately painful; 3, very painful. The total HFS-14 score was calculated by summing the scores of all items and was adjusted to 100 by applying a rule of three. HFS-14 scores were in the range of 2–100, with the higher the score, the greater the QoL impairment.
Study Validation
Patients and Study Design
This was a single-center cross-sectional clinical study carried out from March to July 2009 at the Institut Claudius Regaud (Toulouse, France). Participants were recruited from the dermatology and oncology departments of the Institut and were included consecutively. Eligible patients were suffering from HFS induced by chemotherapy or targeted therapies and were exhibiting all forms of HFS severity defined according to clinical grade based on the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 3.0 [17]. Furthermore, they had to be of French mother tongue and to be able to read and understand the questionnaire in French. All patients included in the study were previously informed about the HFS condition, before the initiation of chemotherapeutic treatment or targeted therapy, and were aware of the potential occurrence of HFS symptoms related to cancer treatment.
Study Procedures
At inclusion, the investigators recorded the type of treatment received and the number of HFS episodes before inclusion, and graded patients' HFS according to the NCI-CTCAE. Moreover, they assessed clinical limb involvement by grading, according to the NCI-CTCAE, each limb extremity (hands or feet) separately to obtain a specific grade for hands and for feet.
The patients anonymously completed the HFS-14 questionnaire and three other validated health-related QoL questionnaires: (a) The DLQI [13], a 10-item skin disease–specific QoL instrument for adults aged ≥16 years that ranges from 0 to 30 (the higher the score, the poorer the QoL). (b) The Skindex-16 [12], a skin-specific questionnaire already used for QoL evaluation in patients with HFS [10] and relating to the most embarrassing skin problems experienced by the patient over the last week. This 16-item questionnaire covers three domains—symptoms, emotions, and functioning—and ranges from 0 to 100 (the higher the score, the poorer the QoL). (c) The short-form (SF)-12 questionnaire [18], which is an abridged version of the medical outcomes study SF-36 general health survey [19], consisting of two components—the physical component summary (PCS) and mental health component summary (MCS) scores—and ranging from 0 to 100 (the lower the score, the poorer the QoL). Finally, patients self-assessed their pain using a 100-mm visual analog scale (VAS), from no pain to the maximum pain imaginable.
Evaluation Criteria
Main Criterion.
The main criterion to assess HFS-14 scale clinical validity was a comparison of HFS-14 scores according to HFS clinical grade (using the NCI-CTCAE).
Secondary Criteria.
Secondary criteria included an analysis of the SF-12, DLQI, and Skindex-16 scores according to HFS clinical grade (NCI-CTCAE), an assessment of the correlation between the HFS-14 score and SF-12, DLQI, and Skindex-16 scores, an assessment of the correlation between the HFS-14 score and pain intensity measured by the VAS, and an analysis of the HFS-14, DLQI, and Skindex-16 scores according to limb involvement (clinical grade for each limb).
Statistical Analysis
Data analysis was performed using SAS software, version 8.2 (SAS Institute, Cary, NC). Quantitative data are described using the number and percentage of patients, mean ± standard deviation (SD). Qualitative data are described as total numbers and percentages of patients. Comparisons between the clinical grade and HFS-14 score and the other QoL scores were analyzed with Tukey's test. The Cronbach's α coefficient had to be >0.70 for a conclusion of good internal reliability. Correlations between the HFS-14 score and the SF-12, DLQI, and Skindex-16 scores were analyzed using the Spearman correlation coefficient (r) after verifying the linearity of the relationship between the two scores. For a conclusion of consistency between two questionnaires, the correlation coefficient had to be ≥0.7, with a lower limit of the 95% confidence interval of ≥0.65. All tests were two-sided and the α risk was set at 5% for the whole study.
Results
Development of the HFS-14 Questionnaire
The initial list was comprised of 31 items. Of this list, nonrelevant items were suppressed and redundant items were grouped to obtain a short questionnaire of 14 items (Fig. 1). Eight items were allocated to the hands domain (items 1–8), eight items were allocated to the feet domain (items 3 and 5–11), with five of these being allocated both to the hands and feet domains (items 3 and 5–8). The social domain was made up of three items (items 12–14). Two additional questions defined in Patients and Methods were also included in the final questionnaire (Fig. 1). Cronbach's α values were 0.91 and 0.92 for the hands and feet domains, respectively, and 0.93 for the final questionnaire.
Figure 1.
Final hand–foot syndrome (HFS)-14 questionnaire.
Validation Study
Study Population Characteristics
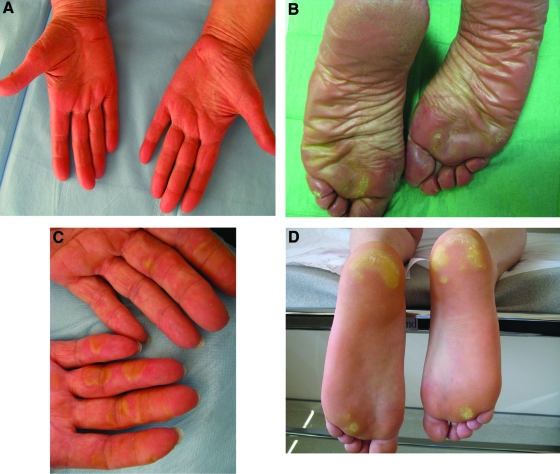
The validation study included 43 patients (mean age, 57 ± 12 years), of whom 74% were female. The demographic and clinical characteristics of the patients are shown in Table 1. In this population, 51% of patients were treated using chemotherapy that mainly included capecitabine (Fig. 2A, 2B), 30% were treated using targeted therapies such as sorafenib (Fig. 2C, 2D) or sunitinib, and 19% received both types of treatment. The majority of patients (65%) declared exhibiting their first HFS episode, and for 61% of patients, HFS involved both the hands and feet. The patient population showed all grades of HFS severity, with the most common being grade 2 (Table 1). The mean (±SD) SF-12 component scores were 38.0 (±8.8) and 45.3 (±9.9) for the PCS and MCS scores, respectively, and the mean (±SD) DLQI and Skindex-16 scores were 6.3 (±6.0) and 27.3 (±22.9), respectively.
Table 1.
Demographic and clinical characteristics of patients at baseline
Abbreviations: DLQI, Dermatology Life Quality Index; HFS, hand–foot syndrome; MCS, mental component score; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; PCS, physical component score; SD, standard deviation; SF-12, short-form 12 item.
Figure 2.
Diffuse hand–foot syndrome (HFS) induced by capecitabine (A, B) and HFS induced by sorafenib (C, D), with inflammatory lesions on pressure areas and interphalangeal joints.
Analysis of HFS-14 Scores According to HFS Clinical Grade (Main Criterion)
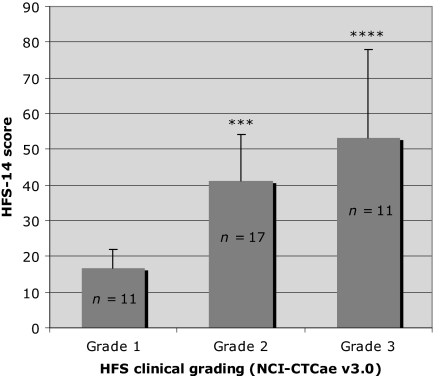
The mean (±SD) HFS-14 score in patients who completed the questionnaire (n = 39) was 37.6 (±21.0). Significant differences were observed in mean HFS-14 scores calculated according to clinical severity grade (p < .0001) (Fig. 3). Mean HFS-14 scores were significantly higher in the grade 2 and grade 3 groups than in the grade 1 group (p < .001 and p < .0001, respectively); the higher the grade, the higher the HFS-14 score and the greater the QoL impairment (Table 2).
Figure 3.
Comparison of mean HFS-14 scores in patients with various grades of HFS severity according to the NCI-CTCAE (version 3.0).
Tukey's test comparison with grade 1: ****p < .0001; ***p < .001.
Abbreviations: HFS, hand–foot syndrome; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
Table 2.
Variations in HFS-14, SF-12, DLQI, and Skindex-16 scores according to HFS clinical grade
Comparisons versus grade 1 using Tukey's test: **p < .01; ***p < .001; ****p < .0001.
aWilcoxon test.
Abbreviations: DLQI, Dermatology Life Quality Index; HFS, hand–foot syndrome; MCS, mental component summary; PCS, physical component summary; SD, standard deviation; SF-12, short-form 12 item.
Analysis of SF-12, DLQI, and Skindex-16 Scores According to HFS Clinical Grade
The mean DLQI and Skindex-16 scores were significantly higher with higher HFS grades (p < .001 and p < .0001, respectively), whereas the SF-12 PCS and MCS scores did not differ (Table 2). Significantly higher mean DLQI and Skindex-16 scores were observed in patients with grade 2 or grade 3 HFS than in those with grade 1 HFS (Table 2).
Analysis of Correlations Between the HFS-14 Score and the SF-12, DLQI, and Skindex-16 Scores
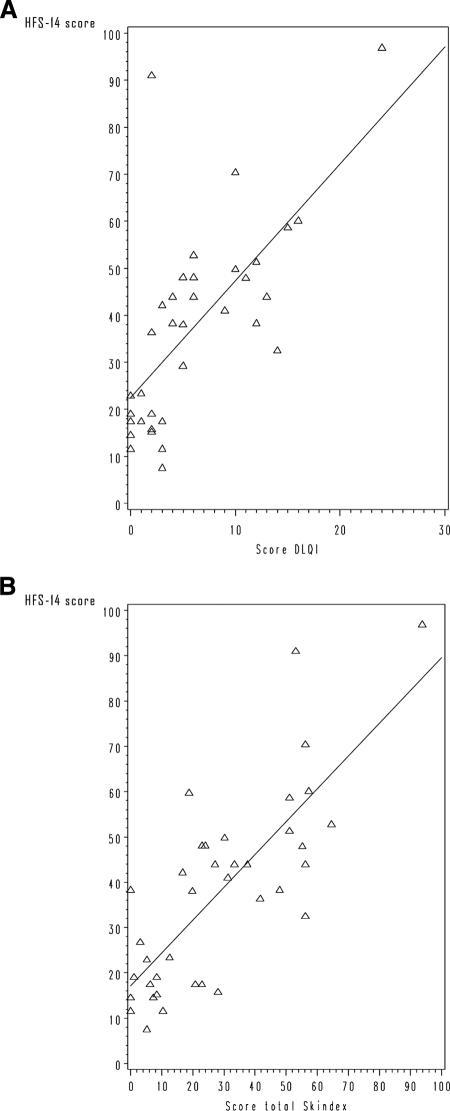
The HFS-14 score was positively correlated with the DLQI and Skindex-16 scores, with highly significant consistency (r = 0.713 and r = 0.735, respectively; p < .0001 for both) (Fig. 4). In contrast, a moderate, but statistically significant, negative correlation was found between the HFS-14 and SF-12 PCS scores (r = −0.467; p < .005). The HFS-14 score was not significantly correlated with the SF-12 MCS score (r = −0.126; p = .446).
Figure 4.
Correlation between HFS-14 score and DLQI and Skindex-16 scores. (A): Scatterplot of HFS-14 and DLQI scores. (B): Scatterplot of HFS-14 and Skindex-16 scores.
Abbreviations: DLQI, Dermatology Life Quality Index; HFS, hand–foot syndrome.
Analysis of the Correlation Between the HFS-14 Score and Pain Intensity
The mean (±SD) VAS score in the overall population (n = 37) was 2.9 (±2.3). A highly significant positive correlation was observed between the HFS-14 score and VAS measurement of pain intensity, with a Spearman's correlation coefficient of 0.681 (p < .0001).
Analysis of the HFS-14, DLQI, and Skindex-16 Scores According to Limb Involvement
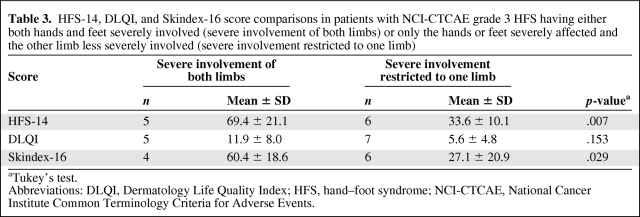
The HFS-14, DLQI, and Skindex-16 score analysis in patients with grade 3 NCI-CTCAE HFS showed significant differences according to the degree of limb involvement (Table 3). In this population, the HFS-14 score was significantly higher in patients having both hands and feet severely involved than in those having severe involvement of either the hands or the feet with the other limb less severely affected (p = .007). Similarly, the Skindex-16 score was significantly higher when HFS involved both hands and feet severely (p = .029). In contrast, in the same population of patients with grade 3 NCI-CTCAE HFS, DLQI scores were not significantly different between patients having severe involvement of both hands and feet and patients having only one limb severely involved (Table 3).
Table 3.
HFS-14, DLQI, and Skindex-16 score comparisons in patients with NCI-CTCAE grade 3 HFS having either both hands and feet severely involved (severe involvement of both limbs) or only the hands or feet severely affected and the other limb less severely involved (severe involvement restricted to one limb)
aTukey's test.
Abbreviations: DLQI, Dermatology Life Quality Index; HFS, hand–foot syndrome; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
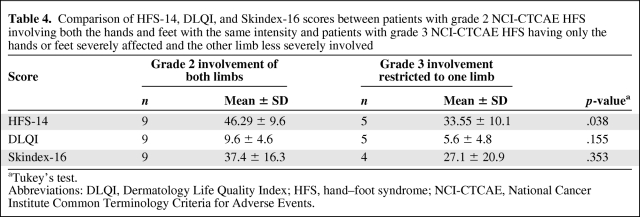
In addition, the HFS-14, DLQI, and Skindex-16 scores were compared between patients with grade 2 NCI-CTCAE HFS involving both hands and feet with the same intensity and patients with grade 3 NCI-CTCAE HFS having only the hands or feet severely affected and the other limb less severely involved (Table 4). The HFS-14 score was significantly higher in patients with grade 2 HFS involving both the hands and the feet with the same intensity than in patients with grade 3 HFS having only the hands or the feet severely affected and the other limb less severely involved (p = .038). In contrast, neither the DLQI scores nor the Skindex-16 scores were different between these two groups of patients (Table 4).
Table 4.
Comparison of HFS-14, DLQI, and Skindex-16 scores between patients with grade 2 NCI-CTCAE HFS involving both the hands and feet with the same intensity and patients with grade 3 NCI-CTCAE HFS having only the hands or feet severely affected and the other limb less severely involved
aTukey's test.
Abbreviations: DLQI, Dermatology Life Quality Index; HFS, hand–foot syndrome; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
Discussion
Using a well-established methodology and according to the principles of good practice, we developed a new HFS-specific scale for assessing QoL in patients suffering from HFS associated with chemotherapy drugs or targeted anticancer therapies. This tool, designed to be self-administered and simple and easy to use, demonstrated excellent internal consistency, with a Cronbach's α >0.9. Comparison of the HFS-14 score according to clinical grade showed that the mean HFS-14 scores was significantly higher with higher grades of HFS, indicating a worsening of QoL impairment with severity of the HFS condition. The HFS-14 questionnaire seems, therefore, to be a valid tool to measure changes in QoL according to differences in HFS severity.
The clinical validity of the HFS-14 scale was clearly demonstrated through correlations of the HFS-14 score with other QoL measures such as the DLQI and Skindex-16 scores, which were also shown to be positively correlated with HFS clinical grade. As expected, the HFS-14 score was strongly and significantly correlated with the DLQI and Skindex-16 scores (r = 0.713 and r = 0.735, respectively; p < .0001) calculated using dermatologic health-related QoL scales, whereas it was only moderately and not at all correlated with SF-12 PCS and MCS scores, respectively, measured with a general health-related QoL questionnaire. A highly significant correlation was also found between the HFS-14 score and VAS pain measurement (r = 0.681; p < .0001), confirming the validity of the HFS-14 scale in measuring QoL in this population, because pain is commonly experienced in patients with HFS of grades 2 and 3 and has a marked impact on their QoL [17].
Whether HFS clinical grading is performed with the most widely used NCI-CTCAE version 3.0 method or with the new CTCAE version 4.0 [17, 20], it does not allow discrimination among patients displaying various degrees of limb involvement. In a population of patients with severe grade 3 NCI-CTCAE HFS, for instance, it does not show differences in QoL impairment between patients with severe involvement of both hands and feet and those having severe involvement of either the hands or the feet with the other limb less severely affected. In contrast, we showed, in our study, that both the HFS-14 and Skindex-16 scales enabled us to make these clinical distinctions. In addition, with the HFS-14 scale, we demonstrated that a patient with HFS lesions of severe grade (NCI-CTCAE grade 3) on one limb only might have a significantly lower HFS-related QoL impairment than a patient with a less severe grade HFS (grade 2) that affected both the lower and upper limbs with the same intensity (p < .05). This further demonstrates that the HFS-14 scale is sufficiently specific to identify differences in functional status and QoL impairment, whatever the NCI-CTCAE grade of HFS severity.
However, our study displays some limitations. The relatively small population sample size could, in particular, explain the large SDs observed for the Skindex-16 and HFS-14 scores. Nevertheless, despite this variability, we observed very significant differences in HFS-14 scores between HFS grades (p < .0001 for the Skindex-16 and HFS-14 scores). Moreover, this is, to our knowledge, the largest population included in a study evaluating the impact of HFS on QoL with specific and validated tools such as the DLQI or Skindex-16. The gender imbalance in our population that resulted from the inclusion of a large number of patients suffering from breast cancer is another limit of this study, which could influence the results.
Published data on the impact of HFS on patient QoL are scarce. One study evaluating the incidence of HFS in gynecologic cancer patients treated with pegylated doxorubicin chemotherapy did not show the HFS condition to disrupt patient-rated QoL [21]. QoL measurement was performed with the Functional Assessment of Cancer Therapy–General (FACT-G) scale, a validated and reliable tool to measure QoL impairment related to cancer [22], which does not, however, specifically assess the impact of chemotherapeutic treatment-related HFS on QoL. In that study, the FACT-G scale failed to show a difference in QoL according to HFS grade [21], which underlines the need for the development of a specific scale. Only one published study assessed the impact of HFS on QoL in 12 cancer patients treated with sorafenib or sunitinib using a dermatologic scale [10]. Results showed a high median QoL impairment in patients suffering from HFS (75% for grade 3 HFS; median Skindex-16 score, 43) and a correlation between the Skindex-16 score and clinical improvement. Our findings are in agreement with these data. Indeed, the Skindex-16 score increased with increasing HFS severity and the median Skindex-16 score was 22.9, which is in accordance with the distribution of the patients according to HFS grade (30% of patients with grade 3 HFS).
Several experts highlighted the importance of properly assessing QoL in combination with therapeutic efficacy to help determine the most effective HFS management strategies in patients treated for cancer [9, 10, 11, 14]. If appropriate management is not rapidly implemented, HFS may worsen and QoL impairment may lead to anticancer treatment modification or discontinuation, with the risk for potentially limiting its efficacy [3, 23]. It seems necessary to develop a health-related QoL scale enabling investigators to independently assess the effects of anticancer treatment and the effects of the cancer itself on QoL. Besides, additional research is needed to determine to what extent HFS prevention and treatment strategies could alter the QoL of cancer patients with HFS [10, 11, 14].
Conclusion
The HFS-14 scale may be a valuable tool for further use in these studies. We demonstrated its validity in specifically assessing the impact of HFS on the health-related QoL of cancer patients treated with chemotherapy drugs or targeted anticancer therapies. As a result of its ability to identify differences in QoL impairment among patients with HFS of same NCI-CTCAE clinical grade, it would allow early fine tuning of the cancer treatment and initiation of HFS symptomatic therapy to avoid further treatment modification. Furthermore, the HFS-14 scale might also be useful for further assessing the clinical efficacy of new symptomatic treatments of HFS. Before that can happen, this new health-related QoL evaluation tool needs to be tested in a longitudinal study that includes a larger and gender-balanced sample population to confirm its validity and assess its ability to measure changes in QoL with time and with the evolution of the HFS condition.
Acknowledgments
This work was supported by Laboratoires Pierre Fabre.
Author Contributions
Conception/Design: Vincent Sibaud, Charles Taïeb, Florence Dalenc, Christine Chevreau
Provision of study material or patients: Vincent Sibaud, Florence Dalenc, Christine Chevreau, Henri Roché, Jean-Pierre Delord, Loïc Mourey, Jean-Louis Lacaze
Collection and/or assembly of data: Vincent Sibaud, Nora Rahhali, Florence Dalenc, Christine Chevreau
Data analysis and interpretation: Vincent Sibaud, Nora Rahhali, Charles Taïeb
Manuscript writing: Vincent Sibaud
Final approval of manuscript: Charles Taïeb
The authors take full responsibility for the content of the paper but thank Marielle Romet, Ph.D. (Santé Active Edition, France, supported by Laboratoires Pierre Fabre), for her medical writing assistance.
References
- 1.Clark AS, Vahdat LT. Chemotherapy-induced palmar-plantar erythrodysesthesia syndrome: Etiology and emerging therapies. Support Cancer Ther. 2004;1:213–218. doi: 10.3816/SCT.2004.n.013. [DOI] [PubMed] [Google Scholar]
- 2.Nagore E, Insa A, Sanmartin O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. Incidence, recognition and management. Am J Clin Dermatol. 2000;1:225–234. doi: 10.2165/00128071-200001040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Webster-Gandy JD, How C, Harrold K. Palmar-plantar erythrodysesthesia (PPE): A literature review with commentary on experience in a cancer centre. Eur J Oncol Nurs. 2007;11:238–246. doi: 10.1016/j.ejon.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Janusch M, Fischer M, Marsch WCh, et al. The hand-foot syndrome—a frequent secondary manifestation in antineoplastic chemotherapy. Eur J Dermatol. 2006;16:494–499. [PubMed] [Google Scholar]
- 5.Baack BR, Burgdorf WH. Chemotherapy-induced acral erythema. J Am Acad Dermatol. 1991;24:457–461. doi: 10.1016/0190-9622(91)70073-b. [DOI] [PubMed] [Google Scholar]
- 6.Robert C, Soria JC, Spatz A, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491–500. doi: 10.1016/S1470-2045(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 7.Sibaud V, Delord JP, Chevreau C. Sorafenib-induced hand-foot skin reaction: A Koebner phenomenon? Target Oncol. 2009;4:307–310. doi: 10.1007/s11523-009-0127-8. [DOI] [PubMed] [Google Scholar]
- 8.Yang CH, Lin WC, Chuang CK, et al. Hand-foot skin reaction in patients treated with sorafenib: A clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol. 2008;158:592–596. doi: 10.1111/j.1365-2133.2007.08357.x. [DOI] [PubMed] [Google Scholar]
- 9.Autier J, Escudier B, Wechsler J, et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144:886–892. doi: 10.1001/archderm.144.7.886. [DOI] [PubMed] [Google Scholar]
- 10.Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19:1955–1961. doi: 10.1093/annonc/mdn389. [DOI] [PubMed] [Google Scholar]
- 11.von Moos R, Thuerlimann BJ, Aapro M, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: Recommendations of an international panel of experts. Eur J Cancer. 2008;44:781–790. doi: 10.1016/j.ejca.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: A brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105–110. doi: 10.1007/BF02737863. [DOI] [PubMed] [Google Scholar]
- 13.Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI) J Investig Dermatol Symp Proc. 2004;9:169–180. doi: 10.1111/j.1087-0024.2004.09113.x. [DOI] [PubMed] [Google Scholar]
- 14.Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. The Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 15.Seidenberg M, Haltiner A, Taylor MA, et al. Development and validation of a multiple ability self-report questionnaire. J Clin Exp Neuropsychol. 1994;16:93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- 16.Wild D, Grove A, Martin M, et al. ISPOR Task Force for Translation and Cultural Adaptation. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: Report of the ISPOR Task Force for Translation and Cultural Adaptation. Value in Health. 2005;8:94–104. doi: 10.1111/j.1524-4733.2005.04054.x. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS March 31, 2003. [accessed August 16, 2011]. Published August 9, 2006. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 18.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 4.03, DCTD, NCI, NIH, DHHS. [accessed August 16, 2011]. Published on June 14, 2010. Available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010–06-14_QuickReference_5x7.pdf.
- 21.von Gruenigen V, Frasure H, Fusco N, et al. A double-blind, randomized trial of pyridoxine versus placebo for the prevention of pegylated liposomal doxorubicin-related hand-foot syndrome in gynecologic oncology patients. Cancer. 2010;116:4735–4743. doi: 10.1002/cncr.25262. [DOI] [PubMed] [Google Scholar]
- 22.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: Properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lokich JJ, Ahlgren JD, Gullo JJ, et al. A prospective randomized comparison of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: A Mid-Atlantic Oncology Program study. J Clin Oncol. 1989;7:425–432. doi: 10.1200/JCO.1989.7.4.425. [DOI] [PubMed] [Google Scholar]