The study defines prognostic factors for breast cancer patients with bone-only metastases and examines progression-free and overall survival times in patients with hormone receptor–positive disease and bone-only metastases treated with different therapies.
Keywords: Bone metastases, Breast cancer, Prognostic factor
Abstract
Purpose.
Limited information is available about the optimal management and clinical outcome of bone-only metastases in breast cancer patients. The objective of this study was to define prognostic factors for patients with bone-only metastases. Our second objective was to compare progression-free survival (PFS) and overall survival (OS) between patients with hormone receptor (HR)+ tumors and bone-only metastases who received combinatory therapy (chemotherapy followed by endocrine therapy, or endocrine therapy combined with molecular targeted therapy) and those treated with endocrine or chemotherapy alone.
Patients and Methods.
We retrospectively identified 351 breast cancer patients diagnosed with bone-only metastasis in 1997–2008 at our institution.
Results.
Patients with metastasis detected at the time of their primary breast cancer diagnosis (rather than at recurrence), a single metastasis, or asymptomatic bone disease had a longer PFS interval, and patients with a performance status of 0–1, a single metastasis, or asymptomatic bone disease had a longer OS time. Among patients with HR+ human epidermal growth factor receptor (HER)-2− disease, combinatory therapy was associated with longer PFS and OS times than with endocrine therapy. In multivariate analyses, combinatory therapy was not associated with longer PFS or OS times than with endocrine therapy. Among patients with HER-2+ disease, trastuzumab led to a longer PFS interval but no difference in the OS time.
Conclusion.
Our results indicate that, for HR+ disease, a prospective trial of chemotherapy followed by endocrine therapy is warranted to determine whether it prolongs survival more than endocrine therapy alone in patients with bone-only metastases.
Introduction
Bone metastases are common in patients with advanced breast cancer. In a retrospective study of patients with metastatic breast cancer, bone was the most common site of metastatic disease (70% of patients) [1]. Bone-only metastatic breast cancer was first described in the 1970s and 1980s [2–8]. Bone-only metastasis has been reported to occur in 17%–37% of women with metastatic breast cancer [4, 6, 9]. Its prognosis seems to be better than that of visceral metastasis. The median survival times from the diagnosis of metastatic disease to death have been reported as 22 months overall, 26 months in patients with bone metastases only, 21 months in patients with bone and visceral metastases, and 18 months in patients with visceral metastases only [10]. Additional previous studies have also shown that patients with bone-only metastatic disease survive longer than patients with visceral metastases, with median survival times for patients with bone metastases in the range of 24–36 months [6, 9–12]. The more indolent disease course may be the result of hormone-responsive disease—many patients with bone-only metastases have hormone receptor (HR)+ disease, which can be treated with endocrine (hormonal) therapy. Supportive care with bisphosphonates may also contribute to the more indolent disease course [12].
Little information is available regarding the optimal management and clinical outcome of bone-only metastases in breast cancer patients. Current options for these patients may include endocrine therapy, chemotherapy, a combination of the two, molecular targeted therapy, and i.v. bisphosphonate therapy. External radiotherapy and orthopedic interventions, when appropriate, to prevent or correct pathological fractures are also an integral part of management. Perez et al. [11] suggested that, for patients with bone metastases, efforts should be made to select the least aggressive therapy to avoid excessive toxicity; however, this approach has not been evaluated for effectiveness in a clinical trial setting. For example, many investigators have suggested using endocrine therapy, which is less toxic than chemotherapy, to treat patients with HR+ disease that is not life threatening [13]. It is unknown which treatment approach (endocrine therapy alone, chemotherapy alone, combinatory therapy) prolongs survival in patients with bone-only metastasis. Moreover, little is known about the prognostic factors that may predict better or worse outcomes among patients with bone-only metastases.
With these gaps in mind, the first objective of our study was to define prognostic factors in breast cancer patients with bone-only metastases. Our second objective was to compare progression-free survival (PFS) and overall survival (OS) times among patients with bone-only metastatic disease treated with combinatory therapy, endocrine therapy alone, and chemotherapy alone.
Patients and Methods
We retrospectively identified patients diagnosed with bone metastasis at the time of initial staging or who developed bone metastasis as the first recurrence site during follow-up. Patients diagnosed from January 1, 1997 to December 31, 2008 at The University of Texas MD Anderson Cancer Center were included in this analysis, and their records were retrieved from the Department of Breast Medical Oncology database. Of the 2,254 patients diagnosed with bone metastases with or without nonskeletal metastases from breast cancer, 756 were diagnosed with bone-only metastases. Of the 756 patients with bone-only metastases, 405 were excluded because the patients did not undergo follow-up in our hospital (n = 140), there was no medical record in the Breast Medical Oncology files (n = 42), the patients had treatment at another hospital before coming to our institution (n = 208), or the patients had another malignant tumor along with breast cancer (n = 15). Thus, data for 351 patients were evaluated. This study was approved by MD Anderson Cancer Center's institutional review board, which waived the need for written informed consent because of the retrospective nature of the study.
Definition of Bone-Only Metastases
We defined patients with bone-only metastases as patients with bone metastasis demonstrated by appropriate imaging and/or biopsy and without nonskeletal distant metastases at the time of their initial diagnosis of metastatic breast cancer. We defined metastasis at presentation as bone-only metastasis detected at the time of the patient's initial diagnosis of breast cancer, and we defined metastasis at recurrence as bone-only metastasis detected after the completion of definitive curative management of the primary breast tumor and neoadjuvant and/or adjuvant systemic treatment. We defined a single metastasis as one bone metastasis based on imaging reports from a bone scan and/or positron emission tomography/computed tomography imaging, and we defined multiple metastases as two or more metastatic sites. We defined combinatory therapy as chemotherapy followed by endocrine therapy before progression of disease or as endocrine therapy combined concurrently with molecular targeted therapy.
Staging and Pathology Review
Primary breast cancer was staged according to the sixth edition of the American Joint Committee on Cancer's AJCC Cancer Staging Manual [14]. Metastatic bone disease was confirmed by histopathological analysis if specimens were available. Primary tumors were graded using the modified Black's nuclear grading system [15] and histologically classified using the World Health Organization criteria [16]. A patient was considered to have human epidermal growth factor receptor (HER)-2+ disease if the primary tumor or a metastatic tumor had a score of 3+ on HER-2 immunohistochemical analysis or if fluorescence in situ hybridization revealed amplification of the HER2 gene. A patient was considered to have HR+ disease if ≥10% of the tumor cells stained positive for estrogen receptor (ER) or progesterone receptor on immunohistochemical analysis.
Statistical Methods
Means and standard deviations were used to summarize age at diagnosis. Frequencies and proportions were used to present the categorical clinical characteristics. Pearson's χ2 tests and Fisher's exact tests were used to test association of treatments and categorical clinical characteristics. Analysis of variance was used to determine differences in the mean age among patients in various treatment groups. PFS was defined as the time interval from diagnosis of metastases to progression, death, or the last follow-up date, whichever occurred first. Patients who were alive without progression at the last follow-up were censored in the PFS analyses. OS was defined as the length of time from diagnosis of metastases to death or to the last follow-up date if patients were alive at the last follow-up. Patients who were alive at the last follow-up were censored in the OS analyses. PFS and OS were estimated by the Kaplan–Meier product-limit method. Kaplan–Meier curves were used to present PFS and OS durations over time for patients in each group. Univariate and multivariate Cox proportional hazards regression models were used to assess the effect of treatment and other predictive factors. The analyses were performed using SAS 9.1 for Windows (Copyright © 2002–2003 by the SAS Institute Inc., Cary, NC) and the plots were generated by S-PLUS 8.0 (Copyright © 1988, 2007 by Insightful Corp., Seattle, WA).
Results
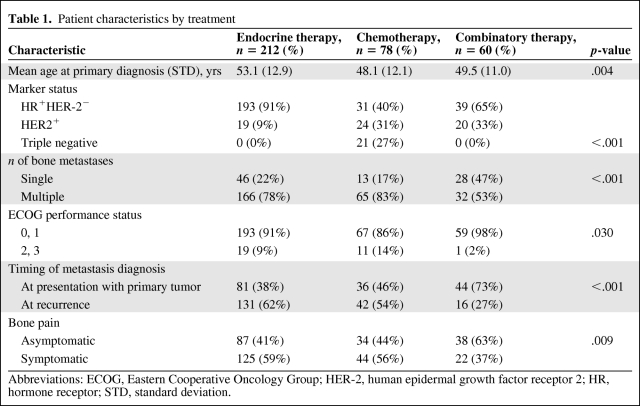
Three hundred fifty-one patients whose clinical records were available for review and who were diagnosed with bone metastasis at initial staging or who later developed bone metastasis as the first metastatic site were included in this study (Table 1). Among them, 116 patients were diagnosed by biopsy. Of the 87 patients with a single bone metastasis, 46 patients were confirmed to have metastasis by biopsy. A total of 182 patients experienced disease progression and died, 111 patients experienced disease progression but were alive at last follow-up, four patients died without disease progression, and 54 patients were alive without progression at last follow-up. The median OS time was 51.9 months (95% confidence interval [CI], 46.8–57.5 months). The median PFS interval was 16.3 months (95% CI, 13.6–17.7 months). The median follow-up time was 33 months (range, 4–143 months).
Table 1.
Patient characteristics by treatment
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HER-2, human epidermal growth factor receptor 2; HR, hormone receptor; STD, standard deviation.
Patient characteristics are shown in Table 1. One patient did not receive endocrine therapy or chemotherapy. We excluded this patient to analyze treatment effect. Comparing the endocrine therapy, chemotherapy, and combinatory therapy groups, there were significant differences in the percentages of patients based on marker status, number of bone metastases, Eastern Cooperative Oncology Group (ECOG) performance status score, timing of metastasis diagnosis, and presence of bone pain (p < .001, p < .001, p = .030, p < .001, and p = .009, respectively). Patients who received endocrine therapy were older than patients who received chemotherapy or combined treatment (p = .004).
Prognostic Factors
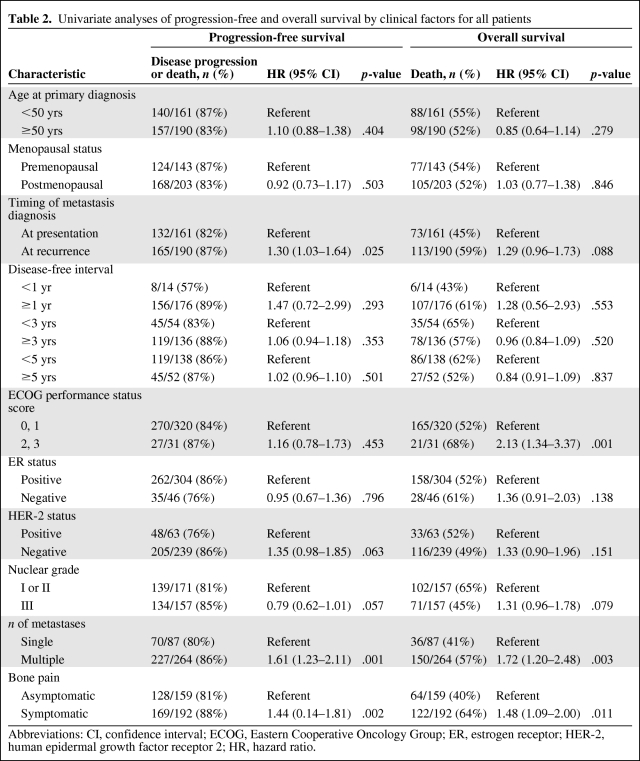
Table 2 shows the univariate analyses of PFS and OS by clinical factors among all patients. Patients with metastasis at presentation, a single bone metastasis, or asymptomatic bone disease had a longer PFS interval than patients with metastasis at recurrence, multiple metastases, or symptomatic bone disease. Patients with an ECOG performance status score of 0 or 1, a single metastasis, or asymptomatic bone disease had a longer OS time than patients with a performance status score of 2 or 3, multiple sites of bone metastasis, or symptomatic bone disease.
Table 2.
Univariate analyses of progression-free and overall survival by clinical factors for all patients
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER-2, human epidermal growth factor receptor 2; HR, hazard ratio.
Combinatory Therapy
In our study, 60 patients received combinatory therapy. Of these patients, 51 had chemotherapy followed by endocrine therapy. The median duration of chemotherapy was 5.5 months (range, 1.0–24.0 months). Thirty-three patients received an anthracycline regimen, 37 patients received taxanes, 13 patients received chemotherapy with trastuzumab, and seven patients received high-dose chemotherapy. Of the 18 patients with HER-2+ tumors, 12 received chemotherapy followed by endocrine therapy plus trastuzumab. Nine of the 60 patients received endocrine therapy combined with molecular targeted therapy; two of those patients received endocrine therapy combined with trastuzumab, three received endocrine therapy combined with imatinib, and four received endocrine therapy combined with gefitinib.
Breast Cancer Subtypes and Treatment Outcome
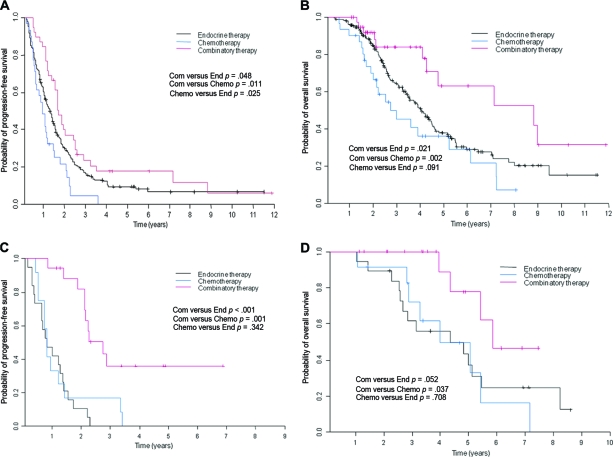
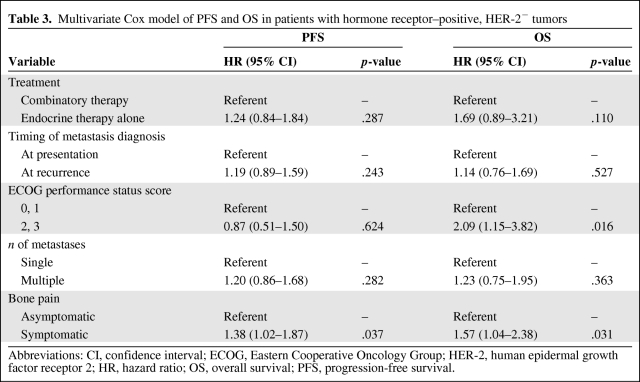
Among patients with HR+HER-2− tumors, combinatory therapy was associated with a longer PFS interval than with endocrine therapy (hazard ratio [HR], 0.68; p = .048) and with chemotherapy (HR, 0.49; p = .011) (Fig. 1A). Moreover, combinatory therapy was associated with a longer OS time than with endocrine therapy (HR, 0.48; p = .021) and chemotherapy (HR, 0.32; p = .002) (Fig. 1B). In the multivariate Cox model of PFS and OS in patients with HR+HER-2− tumors (Table 3), patients who received combinatory therapy appeared to have a longer survival time, but treatment was not significantly associated with PFS (HR, 1.24; p = .287) or with OS (HR, 1.69; p = .110) times after adjustments were made for detection of metastasis at presentation versus at recurrence, performance status (0 or 1 versus 2 or 3), number of bone metastases (single versus multiple), and presence of bone pain.
Figure 1.
Kaplan–Meier curves. (A): Progression-free survival by treatment in patients with HR+HER-2− tumors. (B): Overall survival by treatment in patients with HR+HER-2− tumors. (C): Progression-free survival by treatment in patients with HR+HER-2+ tumors. (D): Overall survival by treatment in patients with HR+HER-2+ tumors.
Abbreviations: Chemo, chemotherapy; Com, combinatory therapy; End, endocrine therapy; HER-2, human epidermal growth factor receptor 2; HR, hormone receptor.
Table 3.
Multivariate Cox model of PFS and OS in patients with hormone receptor–positive, HER-2− tumors
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HER-2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Among patients with HR+HER-2+ tumors, combinatory therapy was associated with a longer PFS interval than with endocrine therapy (HR, 0.17; p < .001) and with chemotherapy (HR, 0.24; p = .001) (Fig. 1C). Combinatory therapy was also statistically significantly associated with a longer OS time than with chemotherapy (HR, 0.28; p = .037) but not with endocrine therapy (HR, 0.33; p = .052) (Fig. 1D).
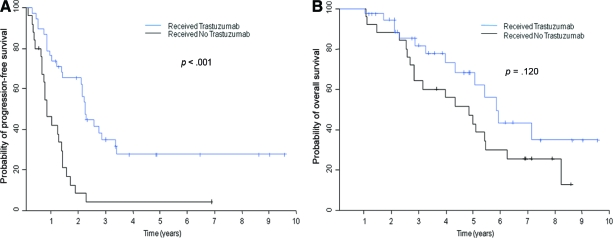
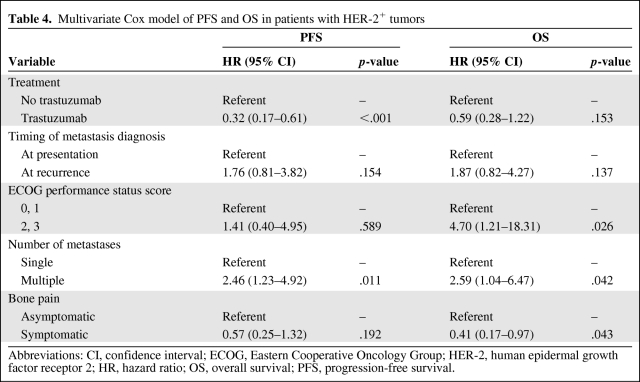
In addition, among 63 patients with HER-2+HR+ or HER-2+HR− tumors, 36 received trastuzumab therapy. Patients who received trastuzumab had a longer PFS time than patients who did not receive trastuzumab (HR, 0.31; p < .001) (Fig. 2A). Patients who received trastuzumab tended to have a longer OS time, but treatment was not significantly associated with OS (HR, 0.58; p = .120) (Fig. 2B). In the multivariate Cox model of PFS and OS in patients with HER-2+ tumors, patients who received trastuzumab therapy tended to have a long survival duration, and trastuzumab was significantly associated with a longer PFS time (HR, 0.32; p < .001), but trastuzumab was not significantly associated with OS (HR, 0.59; p = .153) after adjustments were made for detection of metastasis at presentation versus at recurrence, performance status (0 or 1 versus 2 or 3), number of bone metastases (single versus multiple), and presence or absence of bone pain (Table 4).
Figure 2.
Kaplan–Meier curves of progression-free survival (A) and overall survival (B) for patients with human epidermal growth factor receptor 2–positive tumors by whether they received trastuzumab.
Table 4.
Multivariate Cox model of PFS and OS in patients with HER-2+ tumors
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HER-2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Discussion
To our knowledge, this is the largest study documenting treatment and patient outcomes in breast cancer patients with bone-only metastases. We found that patients with bone-only metastases had a long PFS interval (median, 16.3 months) and OS time (median, 51.9 months). Good prognostic factors for survival in patients with bone-only metastases were a single metastasis, asymptomatic bone disease, metastasis at presentation (rather than at recurrence), and a good performance status.
Among the HR+HER-2− group and the HR+HER-2+ group, patients who received combinatory therapy had longer survival than those who received chemotherapy or endocrine therapy alone on univariate analysis, but on multivariate analysis there was no statistically significant difference in terms of the PFS or OS time in patients treated with combinatory therapy compared with those who received endocrine therapy alone. However, this may be because of the limited number of patients in our study. In addition, among those in the HER-2+ group, patients who received trastuzumab tended to have a longer PFS interval than those who did not receive trastuzumab.
To our knowledge, all studies reported to date have shown a relatively long survival duration in the subgroup of patients with bone-only metastases, with median survival times in the range of 24–36 months [6, 9–12]. Cazzaniga et al. [17] reported that patients with bone-only metastases had a longer OS time than those with both bone and nonskeletal metastases. In their study, the 2-year probabilities for survival according to the presence of nonskeletal metastases and their time of appearance were 0.74 (95% CI, 0.67–0.79) for patients with bone-only metastases, 0.38 (95% CI, 0.25–0.51) for patients with nonskeletal metastases occurring before bone metastases, and 0.56 (95% CI, 0.46–0.66) for patients with concomitant nonskeletal and bone metastases (p < .0001), after a median follow-up of 28 months (range, 2–43 months). In our study, the median OS time of >4 years for patients with bone-only metastases was longer than that observed in previously reported studies. Improvements in the management of metastatic disease in the bone can be attributed to new and more specific diagnostic methodology, the use of orthopedic surgery, advances in radiation therapy and chemotherapy, the use of radiolabeled drugs, and new-generation bisphosphonates.
Previous studies have reported factors that affect prognosis in bone metastases. Cazzaniga et al. [17] reported that the principal prognostic factors that correlated with OS after the appearance of bone metastases were tumor grade, histological type, HR status, and occurrence of skeletal-related events. Coleman et al. [12] reported that important prognostic factors for survival after the development of bone metastasis from breast cancer were the histopathological grade of the primary tumor, ER status, presence of skeletal metastasis at initial breast cancer diagnosis, disease-free interval, and age. Koizumi et al. [18] found that ER status, progesterone receptor status, disease-free interval (bone metastasis–free interval), the first site of nonskeletal metastasis, and the number of bone lesions (solitary versus multiple) were independent prognostic factors. Major et al. [19] reported that the hydroxyproline–creatinine ratio and a positive progesterone receptor status were the only variables to significantly correlate with OS. Most of the previous studies included not only patients with bone-only metastases but also patients with bone and nonskeletal metastases. In our study, asymptomatic bone disease was among the good prognostic factors for survival. Asymptomatic patients may appear to have longer survival time than symptomatic patients because of lead-time bias (the effect of an early diagnosis).
Previous recommendations specified that endocrine therapy was the preferred treatment to control symptoms, prevent serious complications, and prolong survival while preserving quality of life for patients with HR+HER-2− disease and bone-only metastases [8, 13]. However, our retrospective study suggests that combinatory therapy resulted in longer survival than with endocrine therapy or with chemotherapy alone. In multivariate analyses, the differences in the PFS and OS times between patients who received combinatory therapy and those who received endocrine therapy alone did not reach statistical significance. This loss of statistical significance is because the group treated with combinatory therapy included more patients with performance status scores of 0 or 1, a single metastasis, metastasis at presentation, and no bone pain than the group that received only endocrine therapy. In addition, among patients with HR+HER-2+ tumors, combinatory therapy that included trastuzumab tended to be associated with longer survival time than with endocrine therapy or with chemotherapy alone.
Treatment of bone-only metastases was reported in 1986 by Scheid et al. [6], who noted objective responses to doxorubicin-containing combinatory chemotherapy (with cyclophosphamide, 5-fluorouracil, ftorafur, or vincristine) in 59% of patients: complete responses in 7% and partial responses in 52%. The median OS duration was 28 months, and the median PFS interval was 14 months. Vredenburgh et al. [20] reported a randomized trial of high-dose chemotherapy versus observation in women with bone-only metastases; patients who received chemotherapy had a significantly longer event-free survival interval with no difference in OS. Our study included seven patients treated with high-dose chemotherapy. Ueno et al. [21] reported complete responses lasting nearly 9 years for two of six patients with bone-only metastatic breast cancer who received bone-targeted radiation therapy using 166Ho-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethylene-phosphonate.
Among patients with HER-2+ tumors in our study, those who received trastuzumab tended to have longer survival than those who did not receive trastuzumab. Previous studies have reported that trastuzumab prolongs survival in patients with HER-2+ metastatic tumors, including bone metastases [22–26]. However, no papers have reported that trastuzumab is effective against bone-only metastases. In our study, the number of patients with HER-2+ disease treated with trastuzumab was too small to enable meaningful conclusions. Our results indicate that trastuzumab may be an effective treatment for bone-only metastases, although further study is needed to establish which systemic treatments are most effective.
Our study had some limitations. First, this was a retrospective evaluation of data collected from patients' charts; therefore, this study suffers from the bias associated with any retrospective study, such as inherent selection bias. The combinatory therapy received by patients included various drugs, doses, and schedules. Patients were not randomized to different treatments; there were significant differences between treatment groups in the number of bone metastases and ECOG performance status scores. Further, there were a substantial number of inevaluable patients resulting from loss to follow-up and treatment at other hospitals. We considered disease HR+ if ≥10% of the tumor cells stained positively for ER or progesterone receptor on immunohistochemical analysis. The current American Society of Clinical Oncology guideline recommends 1% tumor cell staining for determination of ER positivity; however, our data were collected prior to publication of the guideline in 2010. Finally we could not address whether metastatic new sites of bone metastases or subsequent nonbone distant disease sites had an impact on outcome.
In summary, we identified good prognostic factors for survival in patients with bone-only metastasis from breast cancer. For patients with HR+ tumors and bone-only metastases, we recommend that the standard therapy remain endocrine therapy alone. However, investigation of chemotherapy followed by endocrine therapy is needed to determine whether it prolongs survival over that seen with endocrine therapy alone. Although our results can only be considered hypothesis-generating, this hypothesis is worth exploring. Which treatments most effectively prolong survival for patients with bone-only metastasis are still unknown pending a prospective study to confirm our observations.
Acknowledgments
This research was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant, CA016672, and by the Nellie B. Connally Breast Cancer Research Fund.
Author Contributions
Conception/Design: Richard L. Theriault, Naoki Niikura, Naoto T. Ueno, Gabriel N. Hortobagyi
Administrative support: Richard L. Theriault, Naoto T. Ueno, Gabriel N. Hortobagyi, Yutaka Tokuda
Provision of study material or patients: Richard L. Theriault, Naoki Niikura, Naoto T. Ueno, Gabriel N. Hortobagyi
Collection and/or assembly of data: Naoki Niikura, Naoki Hayashi
Data analysis and interpretation: Richard L. Theriault, Naoki Niikura, Naoto T. Ueno, Shana L. Palla, Jun Liu
Manuscript writing: Richard L. Theriault, Naoki Niikura, Naoto T. Ueno, Gabriel N. Hortobagyi, Yutaka Tokuda, Naoki Hayashi, Shana L. Palla, Jun Liu
Final approval of manuscript: Richard L. Theriault, Naoki Niikura, Naoto T. Ueno, Gabriel N. Hortobagyi, Yutaka Tokuda, Naoki Hayashi, Shana L. Palla, Jun Liu
References
- 1.Manders K, van de Poll-Franse LV, Creemers GJ, et al. Clinical management of women with metastatic breast cancer: A descriptive study according to age group. BMC Cancer. 2006;6:179. doi: 10.1186/1471-2407-6-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smalley RV, Scogna DM, Malmud LS. Advanced breast cancer with bone-only metastases: A chemotherapeutically responsive pattern of metastases. Am J Clin Oncol. 1982;5:161–166. doi: 10.1097/00000421-198204000-00063. [DOI] [PubMed] [Google Scholar]
- 3.Swenerton KD, Legha SS, Smith T, et al. Prognostic factors in metastatic breast cancer treated with combination chemotherapy. Cancer Res. 1979;39:1552–1562. [PubMed] [Google Scholar]
- 4.Sherry MM, Greco FA, Johnson DH, et al. Metastatic breast cancer confined to the skeletal system. An indolent disease. Am J Med. 1986;81:381–386. doi: 10.1016/0002-9343(86)90286-x. [DOI] [PubMed] [Google Scholar]
- 5.Sherry MM, Greco FA, Johnson DH, et al. Breast cancer with skeletal metastases at initial diagnosis. Distinctive clinical characteristics and favorable prognosis. Cancer. 1986;58:178–182. doi: 10.1002/1097-0142(19860701)58:1<178::aid-cncr2820580130>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Scheid V, Buzdar AU, Smith TL, et al. Clinical course of breast cancer patients with osseous metastasis treated with combination chemotherapy. Cancer. 1986;58:2589–2593. doi: 10.1002/1097-0142(19861215)58:12<2589::aid-cncr2820581206>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Chiedozi LC. Prognostic significance of exclusive skeletal metastases in stage IV primary carcinoma of the breast. Surg Gynecol Obstet. 1988;167:303–306. [PubMed] [Google Scholar]
- 8.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plunkett TA, Smith P, Rubens RD. Risk of complications from bone metastases in breast cancer: Implications for management. Eur J Cancer. 2000;36:476–482. doi: 10.1016/s0959-8049(99)00331-7. [DOI] [PubMed] [Google Scholar]
- 10.Domchek SM, Younger J, Finkelstein DM, et al. Predictors of skeletal complications in patients with metastatic breast carcinoma. Cancer. 2000;89:363–368. doi: 10.1002/1097-0142(20000715)89:2<363::aid-cncr22>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Perez JE, Machiavelli M, Leone BA, et al. Bone-only versus visceral-only metastatic pattern in breast cancer: Analysis of 150 patients. A GOCS study. Grupo Oncologico Cooperativo del Sur. Am J Clin Oncol. 1990;13:294–298. doi: 10.1097/00000421-199008000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336–340. doi: 10.1038/bjc.1998.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hortobagyi GN. Treatment of breast cancer. N Engl J Med. 1998;339:974–984. doi: 10.1056/NEJM199810013391407. [DOI] [PubMed] [Google Scholar]
- 14.Singletary SE, Allred C, Ashley P, et al. Staging system for breast cancer: Revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 15.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105:97–102. [PubMed] [Google Scholar]
- 16.The World Health Organization. Histological typing of breast tumors. Neoplasma. 1983;30:113–123. [PubMed] [Google Scholar]
- 17.Cazzaniga ME, Dogliotti L, Cascinu S, et al. Diagnosis, management and clinical outcome of bone metastases in breast cancer patients: Results from a prospective, multicenter study. Oncology. 2006;71:374–381. doi: 10.1159/000107772. [DOI] [PubMed] [Google Scholar]
- 18.Koizumi M, Yoshimoto M, Kasumi F, et al. Comparison between solitary and multiple skeletal metastatic lesions of breast cancer patients. Ann Oncol. 2003;14:1234–1240. doi: 10.1093/annonc/mdg348. [DOI] [PubMed] [Google Scholar]
- 19.Major PP, Cook RJ, Lipton A, et al. Natural history of malignant bone disease in breast cancer and the use of cumulative mean functions to measure skeletal morbidity. BMC Cancer. 2009;9:272. doi: 10.1186/1471-2407-9-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vredenburgh JJ, Madan B, Coniglio D, et al. A randomized phase III comparative trial of immediate consolidation with high-dose chemotherapy and autologous peripheral blood progenitor cell support compared to observation with delayed consolidation in women with metastatic breast cancer and only bone metastases following intensive induction chemotherapy. Bone Marrow Transplant. 2006;37:1009–1015. doi: 10.1038/sj.bmt.1705367. [DOI] [PubMed] [Google Scholar]
- 21.Ueno NT, de Souza JA, Booser D, et al. Pilot study of targeted skeletal radiation therapy for bone-only metastatic breast cancer. Clin Breast Cancer. 2009;9:173–177. doi: 10.3816/CBC.2009.n.028. [DOI] [PubMed] [Google Scholar]
- 22.Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 23.Tokuda Y, Suzuki Y, Ohta M, et al. Compassionate use of humanized anti-HER2/neu protein, trastuzumab for metastatic breast cancer in Japan. Breast Cancer. 2001;8:310–315. doi: 10.1007/BF02967530. [DOI] [PubMed] [Google Scholar]
- 24.Hortobagyi GN. Overview of treatment results with trastuzumab (Herceptin) in metastatic breast cancer. Semin Oncol. 2001;28(suppl 18):43–47. [PubMed] [Google Scholar]
- 25.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 26.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]