The results of a trial evaluating a regimen of clofarabine and Ara-C in patients with relapsed or refractory AML and those with a known history of cardiovascular disease for whom there was a concern about further anthracycline use are reported.
Keywords: Nucleoside analogs, Clofarabine, Acute myeloid leukemia, Cytarabine
Abstract
Purpose.
To determine the efficacy and safety of clofarabine and cytarabine (Ara-C) in adult patients with relapsed or refractory acute myeloid leukemia (AML) and in elderly patients with untreated AML and heart disease.
Patients and Methods.
Patients with relapsed/refractory AML and older patients for whom there was a concern over toxicity from additional anthracyclines received 5 days of clofarabine, 40 mg/m2 per day i.v. over 1 hour, followed 4 hours later by Ara-C, 1,000 mg/m2 per day i.v. over 2 hours.
Results.
Thirty patients were enrolled. The median age was 67 years (range, 38–82 years) and 18 (60%) had received at least one prior therapy. Eleven (37%) patients had a history of cardiovascular disease and were considered to be at high risk for anthracycline toxicity. High-risk cytogenetic abnormalities were present in 14 (47%) patients. The overall response rate (complete remission [CR] plus partial remission) was 53%, including a CR in 14 patients (47%). Responses were observed in all cytogenetic risk groups and in patients who had received up to five prior therapies. The median disease-free survival interval was 9.5 months. The 30-day mortality rate was 20% (de novo AML, 8%; relapsed/refractory AML, 28%). Of the 14 patients achieving a CR, half were able to proceed to curative hematopoietic stem cell transplantation.
Conclusions.
Clofarabine in combination with Ara-C is effective in both untreated and previously treated patients with AML. In addition, it represents a useful remission induction strategy to serve as a bridge to transplantation in older patients with AML.
Introduction
Acute myeloid leukemia (AML) is recognized as being a disease of the elderly, and the median age at diagnosis is 67 years [1]. Elderly patients, defined as >60 years of age, have lower complete remission (CR) rates and lower disease-free survival (DFS) rates than younger AML patients [2]. Five-year overall survival (OS) rates are reported as 18.4% for patients 55–64 years of age, 8% for patients 65–74 years of age, and 1.7% for patients aged ≥75 years [1]. Although the reasons for the differences in survival between older and younger patients can be partially explained by the presence of comorbid conditions, it is also recognized that the biology of AML is different in older patients. For example, older patients with AML have a higher incidence of unfavorable karyotypes, a higher rate of primary drug resistance, and a greater history of pre-existing but often unrecognized myelodysplastic syndrome (MDS) [3–9].
Relapsed/refractory AML patients also have a poor prognosis, with CR rates of 1%–30% except when allogeneic hematopoietic stem cell transplantation (HSCT) is a viable option [10]. Higher response rates are seen, however, in relapsed/refractory AML patients who have longer first remission durations (>12 months) [11]. Although retrospective modeling studies have demonstrated the prognostic value of selected parameters, such as remission interval, cytogenetics, age, previous HSCT, etc., in both first and second relapse AML patients, responses with salvage therapies remain poor [10, 12]. For these reasons, existing chemotherapy regimens fail to produce durable remissions or long-term survival in both older and relapsed/refractory patients with AML. Therefore, new treatment options are urgently needed for this patient group.
Attempts to improve clinical results observed with the standard “7 + 3” regimen, including modifications of the dose and intensity of Ara-C, different anthracyclines, different doses of anthracycline, and the use of gemtuzumab ozogamicin (withdrawn from the U.S. market on June 21, 2010), have not significantly improved the long-term prognosis for these patients [13–18]. Recent efforts have focused on the role of reduced intensity conditioning (RIC) regimens for allogeneic HSCT to consolidate initial responses in patients with adverse risk factors to produce durable remissions [19, 20]. The lower incidence of toxicities associated with RIC regimens offers a potentially curative treatment option to elderly patients with AML who otherwise would not be candidates.
Of the nontransplant options, clofarabine, a new generation deoxyadenosine analog that was rationally designed to resist inactivation by deamination and phosphorolysis, has demonstrated greater potency and less neurotoxicity [21–23]. When first studied, single-agent clofarabine yielded impressive responses in pediatric patients with relapsed acute lymphoid leukemia [24]. A number of studies from the MD Anderson Cancer Center (MDACC) similarly demonstrated encouraging response rates with clofarabine-based combinations in both newly diagnosed and relapsed/refractory AML patients, including many with unfavorable cytogenetics and prior MDS [25–27]. Response rates of 46%–60% have been reported, with an acceptable safety profile and low induction mortality [27]. Recently, the U.K. National Cancer Research Institute Group demonstrated a CR rate of 48% in elderly AML patients who would otherwise have been considered unsuitable for intensive chemotherapy [28]. Additionally, clofarabine was evaluated in the de novo setting with considerable efficacy. A recent study by investigators from the MDACC in untreated elderly AML patients with unfavorable prognostic factors, including unfavorable karyotypes, yielded an overall response rate (ORR) of 46% [29].
Our trial was designed to evaluate a regimen of clofarabine and Ara-C similar to that published by the MDACC [25–27], and included patients with relapsed or refractory AML and those with a known history of cardiovascular disease for whom there was a concern about further anthracycline use. Herein, we report the results of our study.
Materials and Methods
Eligibility Criteria
Eligible patients were recruited and enrolled in this phase II, single-institution study from June 2005 through October 2006 at Baylor University Medical Center, a tertiary care referral center in Dallas, Texas. Patients with relapsed/refractory AML and older patients for whom there was a concern over potential toxicity from further anthracycline treatment because of a known cardiovascular disease history (myocardial infarction [MI], myocardial stenting) were enrolled. Additional eligibility criteria included: age ≥18 years; histologically confirmed disease (standard or poor cytogenetic risk AML according to the Southwestern Oncology Group criteria in first relapse or primary refractory status; untreated high-risk MDS defined as >10% blasts; chronic myelogenous leukemia in accelerated phase or blast crisis failing imatinib therapy; untreated AML in selected elderly patients who are at high risk for anthracycline toxicity); an Eastern Cooperative Oncology Group performance status score of 0–2; and adequate organ function studies (serum creatinine <2 mg/dl; liver function tests: alanine aminotransferase and aspartate aminotransferase ≤5× the upper limit of normal, bilirubin <2 mg/dl). Exclusion criteria (selected) were as follows: eligibility to receive curative allogeneic transplant; current concomitant chemotherapy, radiation therapy, or immunotherapy; use of investigational agents within 30 days; active heart disease, including MI within the preceding 3 months; and a history of severe coronary artery disease or arrhythmias other than atrial flutter or fibrillation requiring medication, or uncontrolled congestive cardiac failure. Approval for the study was obtained from the institutional review board of the Baylor University Medical Center (Dallas, TX). Informed consent was obtained according to institutional guidelines. The study was conducted in accordance with the Declaration of Helsinki.
Assessments
The pretreatment evaluation in all patients included a medical history, physical examination, CBC with manual differential and platelet count, comprehensive metabolic panel (CMP), coagulation profile (including prothrombin time, partial thromboplastin time, fibrinogen, fibrin-degradation products, and D-dimer), electrocardiogram (EKG), multigated acquisition scan, and bone marrow aspiration (flow cytometry and cytogenetic analysis, including conventional karyotyping). Some patients had additional analyses, including fluorescence in situ hybridization for common abnormalities (del 5, del 7, 11q23, 20q-) and polymerase chain reaction for FMS-like tyrosine kinase 3 (FLT3) mutations and internal tandem duplications (ITDs). Follow-up studies included a CBC with manual differential and CMP three times per week until endpoints were reached. Bone marrow aspiration and biopsy were performed on day 14 and monthly thereafter if remission was achieved. If no remission was documented on day 14, a bone marrow biopsy was repeated on day 28. All patients who achieved a CR or partial remission (PR) had their response confirmed 4 weeks after the first documentation of response, by repeating the same blood and marrow studies used to establish the response.
Treatment Schedule
Clofarabine was supplied as a 20 mg/20 ml vial (Clolar®; Genzyme Corporation, Cambridge, MA). Clofarabine (40 mg/m2 per day) was administered daily as a 1-hour i.v. infusion. Four hours after completion of the clofarabine infusion, Ara-C (1,000 mg/m2 per day) was administered as a 2-hour i.v. infusion. This schedule was repeated daily for five consecutive days, which constituted one cycle of therapy. This was followed by supportive care until hematologic recovery. Reinduction therapy with the same schedule of clofarabine and Ara-C was recommended in patients who achieved a hematologic response, but failed to achieve a CR. Choice of consolidation therapy was at the discretion of the treating physician and was permitted after 4 weeks from the first dose in patients who had achieved hematologic recovery. The maximal allowed number of study treatment cycles was four. Supportive measures during induction were antiemetic prophylaxis with i.v. dexamethasone (10 mg daily) and 5-HT3 antagonists on each day of chemotherapy. Patients were vigorously hydrated to prevent tumor lysis syndrome with i.v. fluids (150 ml/m2 per hour) and bumetanide (2–4 mg i.v. push daily or twice daily as needed to maintain initial weight within 1 kg of their starting weight). After the initial chemotherapy phase, i.v. fluid support was returned to maintenance level. Anti-infective prophylaxis was administered at the onset of neutropenia and included levofloxacin (500 mg/day, i.v. or oral), acyclovir (500 mg, i.v. every 12 hours), and either caspofungin (50 mg/day) or voriconazole (200 mg twice daily). Parenteral nutrition was permitted. Routine use of growth factors was not permitted; however, use of these agents was permitted according to the American Society of Clinical Oncology guidelines to treat neutropenia and potential life-threatening infection [30].
Endpoints
The endpoints of this study included disease response measured as the ORR (CR + PR) and toxicity assessments and quantification. In addition, we evaluated the ability of patients to proceed to subsequent HSCT.
Response Criteria and Toxicity Definitions
Response to treatment was determined by the investigator. Response rates were defined based on the revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, and Treatment Outcomes for Reporting Standards for Therapeutic Trials in Acute myeloid Leukemia [31]. CR was defined as normalization of marrow blasts (<5%), recovery of normal hematopoiesis (absolute neutrophil count >1 × 109/l, platelet count ≥100 × 109/l), and absence of peripheral blood blasts, independent of transfusions and growth factor support. PR was defined as blood count recovery as for CR with the exception of leukemic marrow blasts in the range of 6%–25% or a ≥50% decrease in bone marrow blasts. Treatment failure was defined as a <25% change in marrow blasts within 30 days of starting therapy. Pathologic review was performed by an independent, blinded hematopathologist using both standard histopathologic and multiparameter flow cytometric criteria. Cytogenetic studies were performed by a specialty reference laboratory (Veripath, Dallas, TX) using standard karyotyping.
Adverse events occurring on study were reported using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Statistical Considerations
The primary endpoint of this study was the ORR. Summary statistics were used to describe response rates. The proportion of confirmed responses was estimated by the number of patients who achieved a CR or PR, defined as two consecutive evaluations at least 4 weeks apart, divided by the number of eligible patients enrolled in the study. Kaplan–Meier estimates were used to describe time-to-event outcomes such as DFS and OS. The study was considered successful if the CR rate, ORR, and induction mortality rate were comparable with those reported by the MDACC (mean, 46%, 55%, and 10%, respectively) [25–27]. Safety was monitored by an independent data safety monitoring board.
Results
Patient Disposition and Demographics
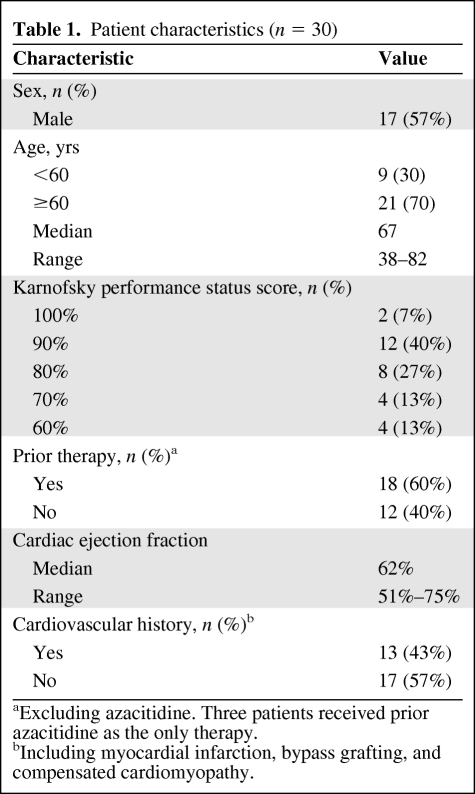
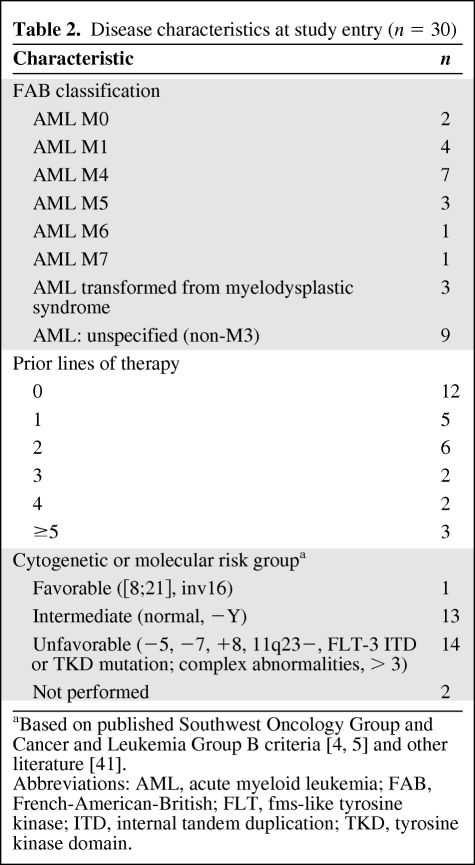
From June 2005 to October 2006, 30 patients were enrolled and received treatment. All patients had a diagnosis of AML. Baseline patient characteristics are summarized in Table 1. Seventy percent of patients were aged ≥60 years and 30% were <60 years of age. The median age was 67 years (range, 38–82 years). Approximately one third of the enrolled patients had not received prior treatment for AML (12 patients) and two thirds had received prior lines of treatment as follows: 1, n = 5; 2, n = 6; 3, n = 2; 4, n = 2; and ≥5, n = 3. For this study, receipt of azacitidine was not included as a prior line of therapy. The most common prior lines of treatment included 7 + 3, n = 18; high-dose Ara-C, n = 6; etoposide plus cyclophosphamide, n = 4; gemtuzumab ozogamicin, n = 3; fludarabine, cytarabine, granulocyte colony-stimulating factor [G-CSF] (FLAG), n = 2; 5 + 2, n = 1; and etoposide plus mitoxantrone, n = 1. Three patients had undergone prior autologous HSCT and one patient had received a previous nonmyeloablative allogeneic HSCT. Cardiovascular history (including MI, bypass grafting, or compensated cardiomyopathy) was noted in 11 of 30 patients (37%) at baseline. Approximately half of the patients (n = 14) had baseline cytogenetic or molecular abnormalities of chromosomes 5 and 7, trisomy 8, deletions of 11q23, and FLT3 abnormalities (either tyrosine kinase domain [TKD] or ITD). Disease characteristics at study entry are summarized in Table 2.
Table 1.
Patient characteristics (n = 30)
aExcluding azacitidine. Three patients received prior azacitidine as the only therapy.
bIncluding myocardial infarction, bypass grafting, and compensated cardiomyopathy.
Table 2.
Disease characteristics at study entry (n = 30)
Treatment Exposure
Thirty patients received at least 1 day of planned therapy, although only 29 patients completed at least one full cycle of treatment (5 days). A second cycle of clofarabine and Ara-C therapy was given to five patients in CR as remission consolidation. Seven patients (23%) proceeded to autologous or allogeneic HSCT, four of whom were de novo AML patients and three who had relapsed/refractory AML. The median age for patients proceeding to HSCT was 65 years (range, 53 to 71 years). Consolidation therapy (for those patients not described above) was left to the discretion of the treating physician.
Response and Outcome
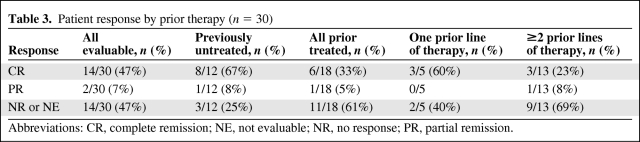
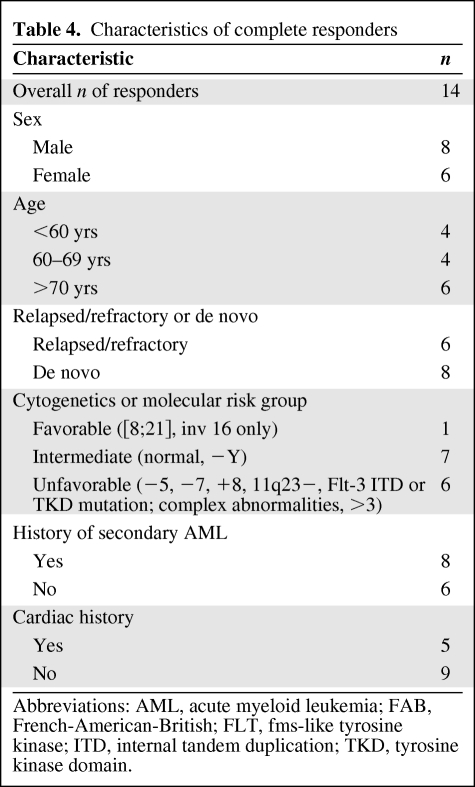
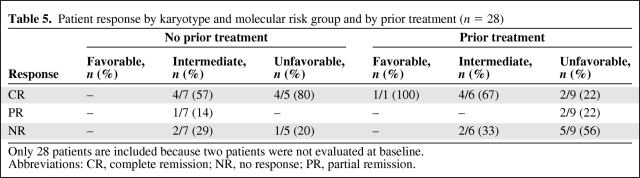
The ORR was 53%, including a CR in 14 of 30 (47%) patients and a PR in two of 30 (7%) patients. As expected, the ORR was higher in treatment-naïve patients (75%) than in those who had received at least one prior therapy (39%). CR was observed more commonly in previously untreated patients (67%) than in previously treated patients (33%). Table 3 describes patient outcomes according to prior treatment and number of lines of therapy. Seven of the 14 patients who achieved a CR had unfavorable cytogenetics, and four achieved cytogenetic remission. The ORR and CR rate in heavily treated (two or more lines of prior therapy) patients were 38% and 23%, respectively. CR was achieved in five of the 11 of patients with a cardiovascular history; three had de novo AML and two had relapsed/refractory AML. Table 4 depicts the characteristics of the complete responders in this study. Responses were characterized according to cytogenetic risk group and are shown in Table 5, stratified by prior treatment.
Table 3.
Patient response by prior therapy (n = 30)
Abbreviations: CR, complete remission; NE, not evaluable; NR, no response; PR, partial remission.
Table 4.
Characteristics of complete responders
Abbreviations: AML, acute myeloid leukemia; FAB, French-American-British; FLT, fms-like tyrosine kinase; ITD, internal tandem duplication; TKD, tyrosine kinase domain.
Table 5.
Patient response by karyotype and molecular risk group and by prior treatment (n = 28)
Only 28 patients are included because two patients were not evaluated at baseline.
Abbreviations: CR, complete remission; NR, no response; PR, partial remission.
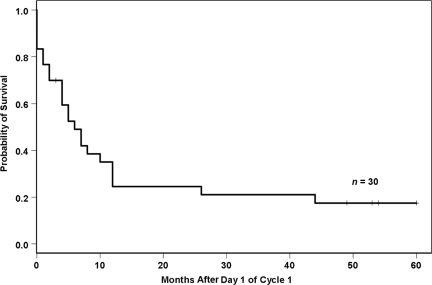
The Kaplan–Meier estimate was used to calculate the overall DFS rate for the 14 patients who achieved a CR. The estimated DFS rates were 79% and 42%, respectively, for 100 days and 1 year. In this subgroup of patients, the median DFS interval was 9.5 months. The median response duration was 9.5 months (range, 2–60 months). The Kaplan–Meier estimate for OS is shown in Figure 1. The median OS duration was 6 months (range, 0–60 months). Although one patient was lost to follow-up, the median duration of follow-up for the remaining 29 patients was 6 months (range, 0–60 months).
Figure 1.
Overall survival.
Early study deaths occurred in six patients who died before their disease status could be evaluated. These early deaths were a result of rapid disease progression (n = 2) and sepsis/multiorgan failure (n = 4), including one patient with myelomonocytic disease (WBC >100 × 109/l) who died after 1 day of treatment as a result of rapid disease progression not impacted by the first dose of clofarabine. The OS rates were 70% and 35%, respectively, for 100 days and 1 year. Mortality was primarily a result of multiorgan failure and disease progression/relapse. Seven of the 14 CR patients received a transplant, five of whom were still alive and in remission as of last follow-up. The median duration of follow-up for those five patients was 54 months (range, 49–60 months).
Safety
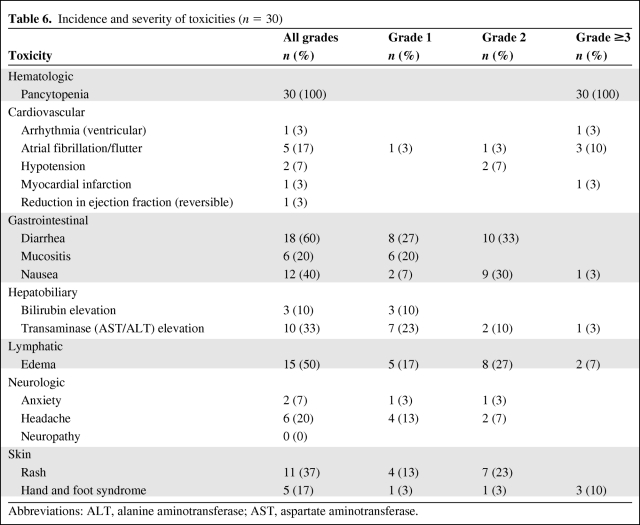
The observed treatment-related adverse events are summarized in Table 6. Grade 3 myelosuppression and grade 4 neutropenia were observed in all patients. Nonhematologic toxicities known to occur with this combination regimen and observed in >10% of patients included diarrhea (60%), skin rashes (53%; including palmoplantar erythrodysesthesia or hand–foot syndrome, 17%), nausea (40%), elevated transaminase levels (33%), and mucositis and headache (20% each). The only cardiac event that occurred in >10% of patients was atrial fibrillation/atrial flutter, which was transient and fully reversible with either diuretics or β-blocker administration in all patients. These cardiovascular events correlated temporally with the period of aggressive hydration and resolved with diuresis, and were therefore considered to be possibly caused by volume overload. There were no instances of creatinine phosphokinase-MB or troponin elevation, EKG changes to suggest ischemia, or other clinical sequelae. The incidence of clinically significant third spacing syndrome was 7%, presumably as a result of aggressive prehydration and steroid administration. There were no cardiac events directly attributable to clofarabine administration. No patients required dose reduction. One patient discontinued therapy on the second day of treatment because of early death from disease progression.
Table 6.
Incidence and severity of toxicities (n = 30)
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
The 30-day mortality rate was 20%—8% in untreated patients and 28% in relapsed/refractory patients—however, two of these deaths were a result of rapid disease progression. Toxic deaths (septicemia with multiorgan failure) occurred in 13% of patients.
HSCT
Of the 14 patients who achieved a CR, seven (50%) proceeded to HSCT. Four of these transplant patients (57.1%) were >60 years of age. Additionally, the majority (four of seven) had normal cytogenetics, whereas unfavorable cytogenetics (n = 2) and favorable cytogenetics (n = 1) were also noted. Two patients underwent autologous HSCT (4 months and 5.5. months following completion of clofarabine and Ara-C) and five underwent allogeneic HSCT. Of the allogeneic HSCT patients, four received myeloablative conditioning and one received RIC regimens. The duration of CR achieved with clofarabine and Ara-C was sufficient to enable patients to proceed to HSCT. The median time interval between the last dose of clofarabine and Ara-C and the date of transplant was 2.5 months (range, 1.5–5.2 months), enabling completion of HSCT logistics including donor search and acquisition and insurance approval.
Discussion
The outcomes of standard cytotoxic chemotherapy in older patients with AML remain dismal, with no regimen providing durable remissions and long-term survival in older patients with AML, or in patients with relapsed or refractory disease. CR rates after conventional induction chemotherapy progressively decline after the age of 60 [2]. In the Cancer and Leukemia Group B 82525 trial, in which patients were randomized to three consolidation arms, only 29% of patients aged ≥60 years were able to complete four cycles of high-dose cytarabine, and in those who did, the 4-year DFS rate was <16% and the OS rate was 9% [32]. Furthermore, elderly patients are unable to tolerate intensive chemotherapy, and as such alternatives are required.
Clofarabine is a promising agent in the treatment of leukemia, both in terms of its enhanced cytotoxicity, resulting from prolonged intracellular clofarabine triphosphate retention, and also its appealing toxicity profile. It can be administered effectively either as a single agent or in combination with other chemotherapeutic agents, including anthracyclines, antimetabolites, topoisomerase I and topoisomerase II inhibitors, and alkylating agents [25–27, 29, 33]. When clofarabine was administered in combination with Ara-C, one of the principal backbone agents of standard AML treatment in phase II studies, the accumulation of clofarabine triphosphate increased levels of intracellular ara-cytosine triphosphate in some patients [25].
Clofarabine has been studied in a number of phase II studies in both the setting of de novo disease in high-risk and standard-risk patients and relapsed/refractory AML patients [28–33]. The earliest phase I studies evaluated clofarabine in combination with Ara-C in relapsed and refractory acute leukemia patients [25]. In that setting, a CR rate of 28% was observed in AML patients. More recent phase II studies have evaluated clofarabine alone [28, 29], in combination with Ara-C as initial induction therapy [26, 27], and in combination with Ara-C and an anthracycline [33] as salvage therapy. Faderl and colleagues evaluated clofarabine and Ara-C as induction therapy for previously untreated AML in patients aged ≥50 years [27]. In that setting, the ORRs were 60% for all treated patients (CR, 52%; CRp, 8%), and 63% for patients aged ≥60 years. Death during induction occurred in 7% of patients, all elderly. The median duration of remission for patients in CR was 8.1 months. A second study from the MDACC group demonstrated similar responses [26]. In a randomized trial comparing clofarabine with clofarabine combined with Ara-C, the ORR for the combination was 67% (CR, 63%), significantly higher than with clofarabine alone. That study was associated with a much higher induction death rate of 21%.
The two most recent publications evaluating clofarabine have focused on clofarabine monotherapy in untreated older patients with high-risk disease and those with underlying conditions that would preclude treatment with an anthracycline agent [28, 29]. In these high-risk populations, for which there are limited treatment options, the ORR was in the range of 46%–48% (CR, 32%–38%) and 30-day mortality rate was in the range of 10%–18%.
The purpose of this phase II study was to confirm the preliminary results published by Faderl and colleagues [34]. This study started enrollment in June 2005, prior to the publication of several other studies on the efficacy and safety of clofarabine in combination with Ara-C. Our study enrolled older adults with relapsed/refractory AML in addition to previously untreated AML patients who were at high risk for anthracycline toxicity because of underlying cardiac risk factors. Because the primary objective of the study was to confirm the results of the aforementioned studies [25–27], we used similar dosing regimen of clofarabine and Ara-C. Based on the results we documented, we were able to confirm the efficacy and safety profile of the combination of clofarabine and Ara-C. In the 30 patients we treated, the ORR was comparable, at 53%, including a CR in 47% of patients. This was an intent-to-treat analysis and included the six patients with early deaths, who were not evaluable for response and therefore classified as failures. We did analyze patients according to receipt of prior therapy and, as anticipated, the ORR was higher among patients who had not received prior therapy (75%) than among those who had received prior therapy (39%). The number of patients with high-risk cytogenetic abnormalities was relatively low, so comparisons are subject to interpretation bias. Regardless, responses were seen in all risk categories.
One of the main challenges with conventional chemotherapy in a predominantly elderly population is reducing induction death rates. Many studies have been performed in a largely elderly population documenting induction death rates of 10%–22% [34–37]. In the earlier clofarabine studies, the induction mortality rate was in the range of 7%–21%. In our study, the mortality rate during induction was 20%. The 30-day mortality rate differed according to prior treatment, with an 8% induction mortality rate in de novo patients and a 28% induction mortality rate in relapsed/refractory patients, which is comparable with the results of Faderl et al. [25–27].
The overall toxicity profile of clofarabine and Ara-C was also similar to that seen in other phase II studies published in the literature [25–28, 29]. The most common toxicities were gastrointestinal (diarrhea, nausea, vomiting), transient and reversible increases in liver transaminases, elevations in serum creatinine, and palmoplantar erythrodysesthesia. The frequency of these toxicities was similar to that of the toxicities reported in other phase II studies [25–27, 29].
One of the secondary objectives of this study was to evaluate a nonanthracycline-based induction regimen in patients who are at high risk for anthracycline toxicity because of a prior cardiac history. Although a formal assessment of cardiovascular risk was not performed, we did include patients with compensated cardiovascular disease as candidates for treatment with the clofarabine and Ara-C combination. There was no cardiac toxicity demonstrated, with the exception of atrial fibrillation, which was transient and reversible in all cases. Although no definite claims of superiority over other regimens can be made, it was demonstrated that compensated cardiovascular disease is not a contraindication to treatment with this combination, as long as aggressive supportive care is provided.
The other point of interest to us in performing this study was the feasibility of using a clofarabine and Ara-C–based regimen to induce remission and serve as a bridge to transplant, specifically for those patients with relapsed/refractory disease. In the relapsed and refractory population, one of the only curative modalities is HSCT. Depending upon the duration of the initial response, it may become difficult to achieve a second CR of sufficient duration to permit a transplant search to occur. In this study, we observed that although the overall rate of patients proceeding to HSCT was low (23%), if a CR was achieved, 50% of those patients were able to proceed to either autologous or allogeneic HSCT. The median age of the seven HSCT patients was 65 years. For the seven patients who achieved a CR but did not receive a transplant, the lack of availability of a matched donor, advanced age, comorbidities, and/or patient preference dictated therapy. In 2010, the use of RIC HSCT was increasing in frequency, particularly for hematologic malignancies. For elderly patients, this offers a potentially curative treatment option that is not otherwise available if a standard myeloablative regimen is used. The clofarabine and Ara-C combination used in our phase II study served as a bridge to transplant in a manner similar to that observed with other clofarabine-based regimens [38, 39].
There are limitations to our small phase II trial. First is the small patient sample size, an inherent difficulty with a single-center study. The second limitation was a lack of formal assessment of cardiac comorbidities, other than patient history. We recognize that many trials include patients with compensated cardiovascular disease, but feel that clofarabine–Ara-C is another treatment option for this group of patients based on the lack of any serious irreversible cardiac outcomes.
Conclusions
The overall results of this study suggest, from efficacy and safety endpoints, that the combination of clofarabine and Ara-C is a feasible option in untreated patients with an acceptable mortality rate and offers a new treatment option in patients who have relapsed and refractory disease. Data from an ongoing randomized phase III study using this combination in the relapsed and refractory AML setting are needed to confirm the efficacy of this regimen [40].
Acknowledgments
The authors would like to thank Khalid Mamlouk and Cheryl Edinbyrd for helping to prepare the manuscript; Ryan Woelfel and Sandi Li for data collection and study coordination; and Genzyme for financial support of editorial assistance in the preparation of the manuscript.
Presented at the 43rd Annual Meeting of the American Society of Clinical Oncology.
Author Contributions
Conception/Design: Edward Agura, Barry Cooper, Houston Holmes, Estil Vance, Robert Brian Berryman, Christopher Maisel, Joseph Fay
Provision of study material or patients: Edward Agura, Barry Cooper, Houston Holmes, Estil Vance, Robert Brian Berryman, Christopher Maisel, Joseph Fay
Collection and/or assembly of data: Edward Agura, Barry Cooper, Houston Holmes, Estil Vance, Robert Brian Berryman, Christopher Maisel, Sandy Li, Giovanna Saracino, Mirjana Tadic-Ovcina, Joseph Fay
Data analysis and interpretation: Edward Agura, Barry Cooper, Houston Holmes, Estil Vance, Robert Brian Berryman, Christopher Maisel, Sandy Li, Giovanna Saracino, Mirjana Tadic-Ovcina, Joseph Fay
Manuscript writing: Edward Agura, Barry Cooper, Houston Holmes, Estil Vance, Robert Brian Berryman, Christopher Maisel, Sandy Li, Giovanna Saracino, Mirjana Tadic-Ovcina, Joseph Fay
Final approval of manuscript: Edward Agura, Barry Cooper, Houston Holmes, Estil Vance, Robert Brian Berryman, Christopher Maisel, Sandy Li, Giovanna Saracino, Mirjana Tadic-Ovcina, Joseph Fay
The authors take full responsibility for the content of the paper but thank Helen Louis Leather (Genzyme) for her assistance.
References
- 1.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute: Bethesda, MD; 1975–2006. [accessed March 1, 2010]. Available at http://seer.cancer.gov/csr/1975_2006/ Based on November 2008 SEER data submission, posted to the SEER web site 2009. [Google Scholar]
- 2.Pinto A, Zagonel V, Ferrara F. Acute myeloid leukemia in the elderly: Biology and therapeutic strategies. Crit Rev Oncol Hematol. 2001;39:275–287. doi: 10.1016/s1040-8428(00)00122-0. [DOI] [PubMed] [Google Scholar]
- 3.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: Analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 4.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 5.Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 6.Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): Analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 7.Baer MR, George SL, Dodge RK, et al. Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: Cancer and Leukemia Group B Study 9720. Blood. 2002;100:1224–1232. [PubMed] [Google Scholar]
- 8.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 9.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giles F, O'Brien S, Cortes J, et al. Outcome of patients with acute myelogenous leukemia after second salvage therapy. Cancer. 2005;104:547–554. doi: 10.1002/cncr.21187. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H, Gandhi V, Cortes J, et al. Phase 2 clinical and pharmacologic study of clofarabine in patients with refractory or relapsed acute leukemia. Blood. 2003;102:2379–2386. doi: 10.1182/blood-2003-03-0925. [DOI] [PubMed] [Google Scholar]
- 12.Breems DA, Van Putten WLJ, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–1978. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Weick JK, Kopecky KJ, Appelbaum FR, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: A Southwest Oncology Group study. Blood. 1996;88:2841–2851. [PubMed] [Google Scholar]
- 14.Bishop JF, Matthews JP, Young GA, et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood. 1996;87:1710–1717. [PubMed] [Google Scholar]
- 15.Amadori S, Suciu S, Stasi R, et al. Gemtuzumab ozogamicin (Mylotarg) as a single agent treatment for frail patients 61 years of age and older with acute myeloid myeloma: Final results of AML-15B, a phase 2 study of the European Organisation for Research and Treatment of Cancer and Gruppo Italiano Malattie Ematologiche dell'Adulto Leukemia Groups. Leukemia. 2005;19:1768–1773. doi: 10.1038/sj.leu.2403901. [DOI] [PubMed] [Google Scholar]
- 16.Estey EH, Thall PF, Cortes JE, et al. Comparison of idarubicin + ara-C-, fludarabine + ara-C-, and topotecan + ara-C-based regimens in treatment of newly diagnosed acute myeloid leukemia, refractory anemia with excess blasts in transformation, or refractory anemia with excess blasts. Blood. 2001;98:3575–3583. doi: 10.1182/blood.v98.13.3575. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Löwenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 19.Huisman C, Meijer E, Petersen EJ, et al. Hematopoietic stem cell transplantation after reduced intensity conditioning in acute myelogenous leukemia patients older than 40 years. Biol Blood Marrow Transplant. 2008;14:181–186. doi: 10.1016/j.bbmt.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Blaise D, Vey N, Faucher C, et al. Current status of reduced-intensity-conditioning allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2007;92:533–541. doi: 10.3324/haematol.10867. [DOI] [PubMed] [Google Scholar]
- 21.Gandhi V, Kantarjian H, Faderl S, et al. Pharmacokinetics and pharmacodynamics of plasma clofarabine and cellular clofarabine triphosphate in patients with acute leukemias. Clin Cancer Res. 2003;9:6335–6342. [PubMed] [Google Scholar]
- 22.Montgomery JA, Shortnacy-Fowler AT, Clayton SD, et al. Synthesis and biologic activity of 2′-fluoro-2-halo derivatives of 9-beta-D-arabinofuranosyladenine. J Med Chem. 1992;35:397–401. doi: 10.1021/jm00080a029. [DOI] [PubMed] [Google Scholar]
- 23.Xie C, Plunkett W. Metabolism and actions of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)-adenine in human lymphoblastoid cells. Cancer Res. 1995;55:2847–2852. [PubMed] [Google Scholar]
- 24.Jeha S, Gandhi V, Chan KW, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–789. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 25.Faderl S, Gandhi V, O'Brien S, et al. Results of a phase 1–2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105:940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- 26.Faderl S, Ravandi F, Huang X, et al. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faderl S, Verstovsek S, Cortes J, et al. Clofarabine and cytarabine combination as induction therapy for acute myeloid leukemia (AML) in patients 50 years of age or older. Blood. 2006;108:45–51. doi: 10.1182/blood-2005-08-3294. [DOI] [PubMed] [Google Scholar]
- 28.Burnett AK, Russell NH, Kell J, et al. European development of clofarabine as treatment for older patients with acute myeloid leukemia considered unsuitable for intensive chemotherapy. J Clin Oncol. 2010;28:2389–2395. doi: 10.1200/JCO.2009.26.4242. [DOI] [PubMed] [Google Scholar]
- 29.Kantarjian HM, Erba HP, Claxton D, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28:549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 30.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 31.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 32.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 33.Faderl S, Ferrajoli A, Wierda W, et al. Clofarabine combinations as acute myeloid leukemia salvage therapy. Cancer. 2008;113:2090–2096. doi: 10.1002/cncr.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faderl S, Gandhi V, Garcia-Manero G, et al. Clofarabine is active in combination with cytarabine (ara-C) in adult patients in first relapse and primary refractory acute leukemia and high-risk myelodysplastic syndrome (MDS) [abstract 2271] Blood. 2003;102:615a. [Google Scholar]
- 35.Anderson JE, Kopecky KJ, Willman CL, et al. Outcome after induction chemotherapy for older patients with acute myeloid leukemia is not improved with mitoxantrone and etoposide compared to cytarabine and daunorubicin: A Southwest Oncology Group study. Blood. 2002;100:3869–3876. doi: 10.1182/blood-2001-12-0354. [DOI] [PubMed] [Google Scholar]
- 36.Juliusson G, Höglund M, Karlsson K, et al. Increased remissions from one course for intermediate-dose cytarabine arabinoside and idarubicin in elderly acute myeloid leukemia when combined with cladribine. A randomised population based phase II study. Br J Haematol. 2003;123:810–818. doi: 10.1046/j.1365-2141.2003.04702.x. [DOI] [PubMed] [Google Scholar]
- 37.Rowe JM, Neuberg D, Friedenberg W, et al. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: A trial by the Eastern Cooperative Oncology Group. Blood. 2004;103:479–485. doi: 10.1182/blood-2003-05-1686. [DOI] [PubMed] [Google Scholar]
- 38.Becker PS, Estey E, Petersdorf S, et al. G-CSF priming, clofarabine and high dose cytarabine (GCLAC) for relapsed or refractory acute myeloid leukemia (AML) Blood. 2009;114:2068. [Google Scholar]
- 39.Locke FL, Artz A, Rich E, et al. Feasibility of clofarabine cytoreduction before allogeneic transplant conditioning for refractory AML. Bone Marrow Transplant. 2010;45:1692–1698. doi: 10.1038/bmt.2010.32. [DOI] [PubMed] [Google Scholar]
- 40.National Institutes of Health. A Study of Clofarabine and Cytarabine for Older Patients With Relapsed or Refractory Acute Myelogenous Leukemia (AML) (CLASSIC I) [accessed March 13, 2010]. Available at http://clinicaltrials.gov/ct2/show/NCT00317642.
- 41.Yanada M, Matsuo K, Suzuki T, Kiyoi H, Naoe T. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: A meta-analysis. Leukemia. 2005;19:1345–1349. doi: 10.1038/sj.leu.2403838. [DOI] [PubMed] [Google Scholar]