The efficacy and safety results of treatment with low-dose-rate vaginal brachytherapy for grade 3 vaginal intraepithelial neoplasia over a 25-year period at Gustave Roussy Institute are presented. This treatment was found to be both safe and effective.
Keywords: Vaginal neoplasms, Carcinoma in situ, Cervical intraepithelial neoplasia, Brachytherapy
Learning Objectives
After completing this course, the reader will be able to:
Utilize data supporting the efficacy of low-dose definitive brachytherapy to inform clinical decisions about treating women with high-grade vaginal intraepithelial neoplasia.
Implement methods for delivering low-dose definitive brachytherapy that minimize toxicity.
Communicate to patients the type and incidence of toxic events associated with low-dose definitive brachytherapy.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
Treatment of high-grade vaginal intraepithelial neoplasia (VAIN) is controversial and could include surgical excision, topical medication, brachytherapy, or other treatments. We report the results of low-dose-rate (LDR) vaginal brachytherapy for grade 3 VAIN (VAIN-3) over a 25-year period at Gustave Roussy Institute.
Patients and Methods.
We retrospectively reviewed the files of all patients treated at Gustave Roussy Institute for VAIN-3 since 1985. The treatment consisted of LDR brachytherapy using a personalized vaginal mold and delivered 60 Gy to 5 mm below the vaginal mucosa. All patients had at least an annual gynecological examination, including a vaginal smear.
Results.
Twenty-eight patients were eligible. The median follow-up was 41 months. Seven patients had a follow-up <2 years, and the median follow-up for the remaining 21 patients was 79 months. The median age at brachytherapy was 63 years (range, 38–80 years). Twenty-six patients had a history of VAIN recurring after cervical intraepithelial neoplasia and 24 had a previous hysterectomy. The median brachytherapy duration was 4.5 days. Median doses to the International Commission of Radiation Units and Measurements rectum and bladder points were 68 Gy and 45 Gy, respectively. The median prescription volume (60 Gy) was 74 cm3. Only one “in field” recurrence occurred, corresponding to a 5- and 10-year local control rate of 93% (95% confidence interval, 70%–99%). The treatment was well tolerated, with no grade 3 or 4 late toxicity and only one grade 2 digestive toxicity. No second cancers were reported.
Conclusion.
LDR brachytherapy is an effective and safe treatment for vaginal intraepithelial neoplasia.
Introduction
Invasive vaginal carcinoma is rare, accounting for only 1%–4% of all gynecological malignancies [1]. Similarly, vaginal intraepithelial neoplasia (VAIN) is also uncommon. Although its true incidence is difficult to assess, it is believed to be approximately one hundred times less frequent than cervical intraepithelial neoplasia (CIN) [2].
Major risk factors for VAIN are low sociocultural level, alterations on prior Papanicolaou smears, a history of genital warts, hysterectomy at an early age, concomitant or prior CIN, a history of sexually transmissible infections and/or human papillomavirus infection, smoking, immunosuppression, a history of radiotherapy, and a history of exposure to diethylstilbestrol [3, 4]. VAIN is associated with vulvar intraepithelial neoplasia or CIN in 65% and 10% of cases, respectively [4]. The main risk associated with VAIN is the progression to invasive vaginal carcinoma. In a series of 23 untreated patients (grade 1–2, n = 18; grade 3, n = 5) followed for ≤3 years, Aho et al. [5] showed that vaginal carcinoma occurred in two cases (9%): one grade 1 VAIN progressed to stage I invasive carcinoma in 5 years and one grade 3 VAIN progressed to stage I invasive carcinoma in 4 years. Persistence of VAIN occurred in three cases (13%) and regression of VAIN occurred in 18 cases (78%), with the majority (78%) occurring in patients with grade 1–2 VAIN. In that series, the risk for progression to invasive carcinoma was as high as 20% among patients with grade 3 VAIN (VAIN-3), although this estimation was based on a very limited number of patients [5].
Similarly to the management of low-grade CIN, active surveillance is considered the standard treatment for low-grade VAIN. Treatment of high-grade VAIN, either primary or recurrent after CIN treatment, is controversial. Various treatments have been used, including local excision, partial or total colpectomy, vaginal brachytherapy, laser vaporization, chemosurgery, topical 5-fluorouracil (5-FU) administration, systemic interferon therapy, or imiquimod application [3, 6, 7]. None of these options have been properly compared in well-designed clinical trials, and none can therefore be considered standard. After local excision only, the recurrence rate is as high as 20% [4].
Vaginal brachytherapy has been reported as an effective treatment for high-grade VAIN [8–17]. Initially, low-dose-rate (LDR) techniques were described, mostly using vaginal cylinders. Typically, 60 Gy was prescribed to the vaginal mucosa, but a wide dose range, also depending upon the depth of the dose prescription, and various techniques have been reported. Most of these publications are old, used ancient and heterogeneous techniques, lacked adequate follow-up, and reported on a very limited number of patients [8–13]. More recently, high-dose-rate (HDR) techniques were reported, using various doses and fractionation regimens [14–16]. Success rates differed greatly among studies. These results are difficult to interpret because of small patient numbers, short follow-up, and the heterogeneity of brachytherapy techniques and doses/fractionation among and within these studies. A medium-dose-rate (MDR) technique has also been reported [17].
It seems clear that the cure rates achieved using vaginal brachytherapy alone are among the highest of the treatment options for VAIN, but overall, and as a result of the large heterogeneity among studies, it is difficult to draw a conclusion regarding the cure rates and toxicity profiles obtained using different regimens of brachytherapy. Because patients with high-grade VAIN have a long life expectancy, it is important to minimize long-term side effects in order to provide a good quality of life to these patients.
The purpose of this chart review was to evaluate our experience with the use of LDR vaginal brachytherapy for the management of women with high-grade VAIN.
Methods
Patients
A chart review of all patients diagnosed with VAIN-3 in the period 1985–2008 at the Gustave Roussy Institute brachytherapy unit was performed. Twenty-eight patients were identified. For all patients, the diagnosis was based on repeated vaginal smears, with at least one having been performed at our institution, and on vaginal biopsies performed during a colposcopic procedure. Seven patients had a follow-up <2 years after brachytherapy, because they were followed up at a different hospital. No recurrences were recorded in these patients at their last consultation.
Treatments
The brachytherapy procedure and treatment delivery were homogeneous during the entire period. As previously reported, the intracavitary brachytherapy technique at our institution is based on the vaginal mold technique, which has been extensively described elsewhere [18]. Briefly, for each patient, a customized vaginal mold was made based on a vaginal impression. The number and the length of the vaginal catheters depended on the site and length of VAIN extension. Usually, two to three catheters were inserted into the vaginal mold. The distance from the catheter to the mold surface was 2–3 mm. Holes were made in the applicator, allowing daily vaginal irrigation through a specific catheter integrated into the mold and inducing mucosal herniation to prevent mold displacement. At the beginning of each brachytherapy procedure, a careful vaginal examination was performed, including Lugol staining in order to confirm the location and extension of disease. If necessary, silver seeds were inserted into the vaginal mucosa to signal the caudal portion of the disease. The mold was then carefully inserted into the vagina. In two patients who had not had a previous hysterectomy, the mold was inserted after dilation of the cervix channel and introduction of a semiflexible tandem catheter into the uterine cavity. This procedure was performed under either general or local anesthesia. Because the mold naturally expands the vaginal mucosa, no vaginal packing was required. Brachytherapy was delivered using LDR cesium-137 sources with afterloading carriers. Patients were treated 24 hours a day, with treatment stopped only for patient care, usually around 50 minutes per day. Sixty Grays were prescribed to a distance of 5 mm below the vaginal surface, from the vaginal cuff to 1 cm below the most caudal lesion. There was no constraint on the vaginal dose surface. The dose rate was in the range of 0.4–0.6 Gy/hour, that is, 10–15 Gy/day. Doses to International Commission of Radiation Units and Measurements (ICRU) points were calculated using x-rays. During the entire procedure, patients had to remain hospitalized. A fiber-free diet and thromboprophylaxis were provided. Vaginal irrigations were performed twice daily. The sources were withdrawn after the required time and the applicators removed without anesthesia.
Data Collection and Follow-Up
Follow-up was scheduled at 6 weeks after completion of brachytherapy. After that, all patients had at least an annual gynecological examination, including a vaginal smear. During clinics, all information on late effects was systematically recorded. Although cervicovaginal cytology after radiotherapy does not have high sensitivity, it is still considered a reliable test for the diagnosis of local recurrence [19].The following data were retrospectively collected for each patient: patient demographics, clinical description of VAIN, previous treatments for VAIN, history of cervical or uterine carcinoma or CIN, history of conization or hysterectomy, detailed description of brachytherapy, recurrence of VAIN or invasive vaginal carcinoma, and vital status. Morbidity scoring was performed retrospectively during the chart review using the Common Terminology Criteria for Adverse Events, Version 4.0.
Statistical Analysis
Continuous variables are presented as medians and ranges, and dichotomous variables are presented as percentages. A Kaplan–Meier estimate of the 5- and 10-year local control rate was calculated using Rothman's confidence interval formula to take variable follow-up into account. No prognostic statistical analysis was performed because of the very low rate of local failure, which precluded the identification of any prognostic factor.
Results
Patient Characteristics
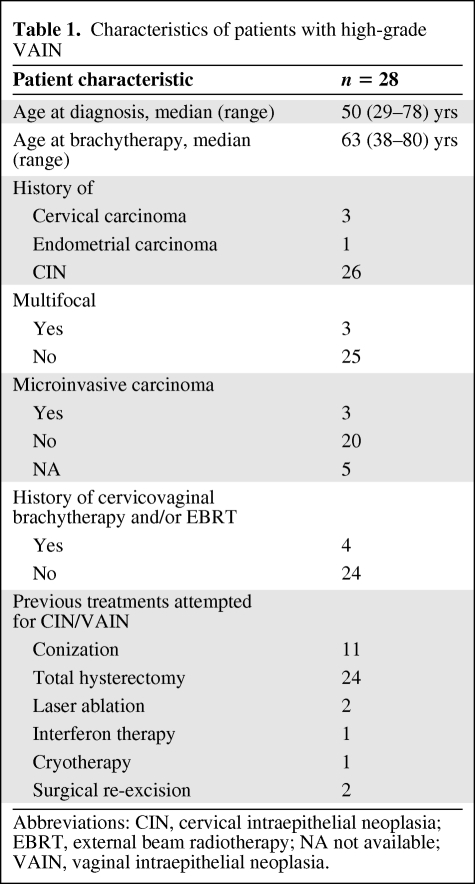
Twenty-eight patients treated with LDR vaginal brachytherapy for VAIN-3 in 1983–2008 were included in this analysis. The median follow-up was 41 months (range, 0–284 months). Seven patients had a follow-up <2 years because they were referred to our institution for the procedure and were followed up elsewhere. However, for the 21 remaining patients, the median follow-up was 79 months. The median age at diagnosis was 50 years (range, 29–78 years), and the median age at brachytherapy was 63 years (range, 38–80 years). Twenty-six patients had a history of VAIN recurring after CIN. All patients presented asymptomatically. Twenty-four patients had had a previous hysterectomy, predominantly for CIN (n = 20) or cervical or endometrial cancer (n = 3 and n = 1, respectively). VAIN was located in the upper third of the vagina in all patients. Multifocality was found in three patients, and microinvasion was found in three patients. Previous treatment for CIN/VAIN included conization in 11 patients, hysterectomy in 24 patients, partial colpectomy in two patients, laser ablation in two patients, and cryotherapy and interferon therapy in one patient each. Four patients had a history of curative cervicovaginal brachytherapy with or without external beam radiotherapy (EBRT). Table 1 summarizes the patient characteristics.
Table 1.
Characteristics of patients with high-grade VAIN
Abbreviations: CIN, cervical intraepithelial neoplasia; EBRT, external beam radiotherapy; NA not available; VAIN, vaginal intraepithelial neoplasia.
Brachytherapy Characteristics
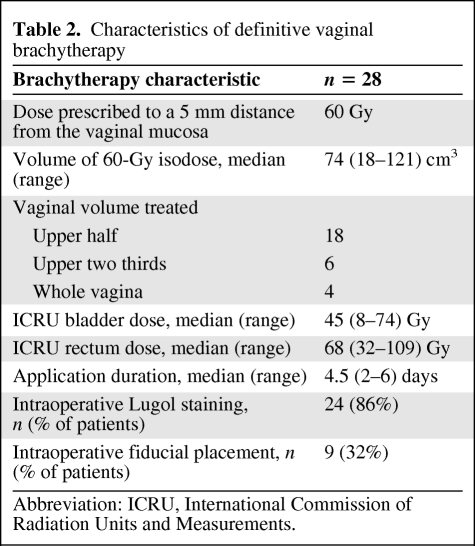
The median time from hysterectomy to brachytherapy was 33 months (range, 2–452 months). Brachytherapy delivered at 60 Gy with a median duration of 4.5 days (range, 2–6 days). Brachytherapy was delivered in two 30-Gy steps in one patient who had a previous history of EBRT and brachytherapy 24 years earlier. Median doses to ICRU rectum and bladder points were 68 Gy (range, 32–109 Gy) and 45 Gy (range, 8–74 Gy), respectively. The median prescription volume (60 Gy) was 74 cm3 (range, 18–121 cm3); the upper half of the vagina was the treated volume in 18 patients and more than the upper half was treated in 10 patients. Intraoperative Lugol staining was performed in 24 patients (86%). Table 2 summarizes the treatment characteristics.
Table 2.
Characteristics of definitive vaginal brachytherapy
Abbreviation: ICRU, International Commission of Radiation Units and Measurements.
Outcome
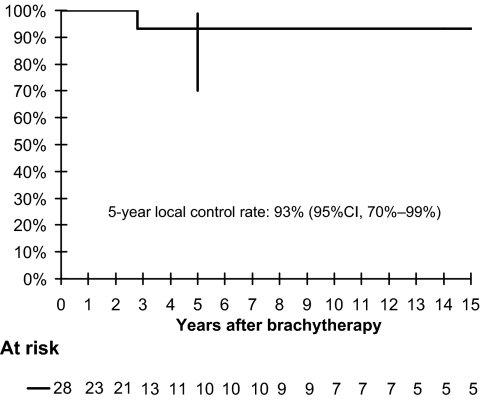
The local control curve is showed in Figure 1. The 5- and 10-year local control rates were 93% (95% confidence interval, 70%–99%). Altogether, one “in field” recurrence occurred in a patient who had a history of recurrent VAIN 24 years after treatment for invasive cervical squamous cell carcinoma with surgery, radiotherapy, chemotherapy, and brachytherapy (this patient had also had unsuccessful interferon and laser therapy for VAIN). Another patient recurred with vulvar intraepithelial neoplasia. This relapse was not considered a local failure, because the vulva was not included in the treated volume during vaginal brachytherapy.
Figure 1.
Kaplan–Meier curve for local control.
Abbreviation: CI, confidence interval.
Tolerance
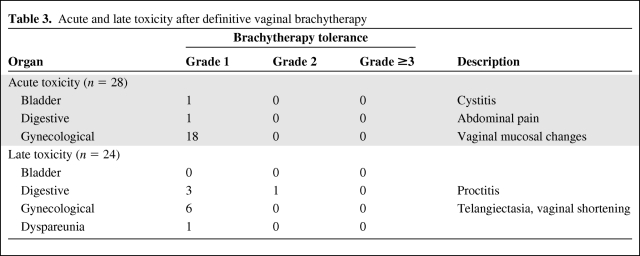
Acute tolerance was recorded for all 28 patients, but late toxicity was recorded only for patients with a follow-up >1 year (n = 24). Table 3 summarizes the acute and late toxicities. Overall, the treatment was very well tolerated and no limiting toxicities were encountered. Acute toxicities consisted of grade 1 cystitis in one patient and grade 1 abdominal pain in one patient. Eighteen patients had mild vaginal mucosal changes in the days following brachytherapy; all had resolved by the time of review 6 weeks later. No deep vein thrombosis resulting from supine nursing occurred.
Table 3.
Acute and late toxicity after definitive vaginal brachytherapy
There was no grade 3 or 4 late toxicity. One case of grade 2 gastrointestinal toxicity (rectal bleeding treated medically, ICRU rectal point receiving 75 Gy), six cases of grade 1 gynecological toxicity (vaginal sclerosis, telangectasia), one case of grade 1 sexual toxicity, and three cases of grade 1 digestive toxicity were reported. No second cancer was reported. No late grade ≥2 toxicity was seen in the four patients previously irradiated for invasive cancer or dysplasia.
Discussion
LDR brachytherapy is an effective and safe treatment for VAIN. The major interests of our series are the long clinical and cytological follow-up, the relatively high number of patients, and the use of a homogeneous treatment procedure over time, which allow drawing conclusions about efficacy and toxicity. In our series, only one relapse was seen in 28 patients after a median follow-up of 41 months. As mentioned before, the relapse occurred in a heavily pretreated patient, with the brachytherapy procedure performed in two 30-Gy sessions using a very limited irradiated volume (18 cm3). These two factors, along with the biologically resistant nature of VAIN-3 in this patient, could explain why brachytherapy was ineffective. Seven years after the unsuccessful brachytherapy, she was alive without any sign of invasive vaginal cancer, which underlines the uncertainty of the natural history of VAIN and the controversies in choosing the optimal time for treatment and treatment technique.
The vast majority of studies on vaginal brachytherapy for high-grade VAIN report recurrence rates in the range of 0%–14% [13–17]. This high cure rate is similar to that seen in our series and in the other largest series of vaginal LDR brachytherapy reported by Perez et al. [13]. Only one recurrence in 20 patients was recorded after a median follow-up of 10 years. Graham et al. [17] reported the results of their MDR technique with a follow-up of 77 months and noted three local relapses in 22 patients. The HDR series by Ogino et al. [14] reported no recurrence in 14 patients after a mean follow-up of 90.5 months. Teruya et al. [16] also reported no recurrence in 13 patients with a median follow-up of 127 months. After a median follow-up of 46 months, MacLeod et al. [15] reported one VAIN persistence and one invasive relapse among 14 patients treated with HDR. Overall, the success rates achieved using definitive brachytherapy are high and homogeneous across published series. The remaining variability in the success rate might be explained by differences in treatment; for instance, the prescription point (vaginal mucosa or 5 mm deeper) and prescribed dose, population (only VAIN-3 or all grades of VAIN), or duration of follow-up.
The long-term toxicity profile of LDR vaginal brachytherapy for high-grade VAIN is very favorable, because no grade 3 or 4 early or late toxicity was recorded in our series. This is consistent with the report by Perez et al. [13], who noted one grade 3 urinary complication (bladder neck stenosis requiring surgery) among 40 patients with stage 0 or 1 vaginal cancer. This compares favorably with HDR and MDR vaginal brachytherapy published series. Indeed, in the MDR series by Graham et al. [17], five grade 3 and one grade 4 toxicities were reported. Ogino et al. [14] reported three cases of rectal bleeding and two cases of moderate-to-severe vaginal adhesions in 20 patients treated with HDR vaginal brachytherapy for CIN-3 or VAIN-3. Vaginal toxicity was greater when the entire vagina was treated. Teruya et al. [16] reported three cases of rectal bleeding or macroscopic hematuria in 13 patients after vaginal HDR treatment for VAIN after hysterectomy for CIN. This toxicity appeared only in the patients for whom brachytherapy was delivered to 10 mm below the vaginal mucosa (n = 3 patients) and not for those in whom it was delivered to 5 mm (n = 10 patients) below the vaginal mucosa [16]. MacLeod et al. [15] reported two cases of grade 3 late vaginal toxicity (atrophy and stenosis) in 14 patients treated with HDR for high-grade VAIN. In their series, the whole residual vagina was treated.
The favorable toxicity profile achieved in our series can be explained by different reasons. First, the upper half of the vagina, not the whole vagina, was treated in the majority of patients, because VAIN is predominantly located in the upper third of the vagina. Second, the use of LDR brachytherapy might relatively spare normal tissues. Third, the dose was prescribed to 5 mm below the vaginal surface (and not to the vaginal mucosa or 10 mm below the vaginal mucosa, which leads to either recurrence or toxicity). Last, the use of the mold technique, expanding the vagina without the use of packing, allowed lowering the dose received by the healthy parts of the vaginal walls. This toxicity profile might even be further improved by individualization of the prescription depth according to findings using vaginal ultrasound, as previously described by Onsrud et al. [20].
Other treatment options for VAIN-3 include surgery, topical 5-FU, laser therapy, interferon therapy, and imiquimod. There is no comparative study testing which treatment is the best, so no treatment can be considered standard, although local excision or upper colpectomy are the most frequently used treatments in the largest published series [2, 6]. Topical 5-FU is considered the least active treatment. It is often poorly tolerated, because it induces a hypersensitivity reaction, vaginal burning, and vulvar irritation. Recurrence rates among patients with VAIN treated using topical 5-FU, laser therapy, and partial colpectomy were 59%, 38%, and 0%, respectively, according to Dodge et al. [4]. In a study reported by Rome and England [6], the respective recurrence rates after local excision, laser ablation, and topical 5-FU were 31%, 31%, and 25% in 132 patients. Altogether, relapse rates seemed lower, or at least comparable, after vaginal brachytherapy than after other treatment modalities.
No invasive cancer was found in our series. It is important that VAIN-3 patients treated with a conservative approach are followed up carefully and for a long time. This allows early detection of recurrence, either intraepithelial or invasive. Second cancers are always a concern with radiotherapy, especially for diseases with a high cure rate and long life expectancy, but none has been reported in all the retrospective series of vaginal brachytherapy. This could be related to the small irradiated volume. Major potential shortcomings of brachytherapy are that, unlike surgery, it does not allow a detailed pathological review to be performed, therefore leading to undertreatment of patients with microinvasive disease. Indeed, rates of occult invasive vaginal cancer could be as high as 12%–28% according to surgical series [21, 22]. Besides, surgery after radiotherapy is more complex, leading to a higher rate of perioperative complications. On the other hand, surgery can lead to overtreatment because the rate of negative pathology after upper colpectomy for VAIN is estimated at around 20% [20].
To conclude, the outcome of this series of patients treated for VAIN-3 shows that vaginal LDR brachytherapy is a safe and very effective treatment. It should be offered to and discussed with patients, along with other treatment options. In the end, the choice of a treatment option for patients with VAIN-3 relies on the number of lesions, their location, the length of the vagina, whether the patient is sexually active, her menopausal status, previous radiation therapy, and physician experience and preference. And also, and very importantly, for a condition for which no standard treatment exists, the choice of treatment modality depends on patient preference.
Author Contributions
Conception/Design: Pierre Blanchard, Christine Haie-Meder
Provision of study material or patients: Pierre Blanchard, Laurie Monnier, Isabelle Dumas, Philippe Morice, Patricia Pautier, Pierre Duvillard, Fares Azoury, Ranaud Mazeron, Christine Haie-Meder
Collection and/or assembly of data: Pierre Blanchard, Laurie Monnier
Data analysis and interpretation: Pierre Blanchard, Philippe Morice, Christine Haie-Meder
Manuscript writing: Pierre Blanchard, Christine Haie-Meder
Final approval of manuscript: Pierre Blanchard, Laurie Monnier, Isabelle Dumas, Philippe Morice, Patricia Pautier, Pierre Duvillard, Fares Azoury, Ranaud Mazeron, Christine Haie-Meder
References
- 1.Duong TH, Flowers LC. Vulvo-vaginal cancers: Risks, evaluation, prevention and early detection. Obstet Gynecol Clin North Am. 2007;34:783–802. doi: 10.1016/j.ogc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Sillman FH, Fruchter RG, Chen YS, et al. Vaginal intraepithelial neoplasia: Risk factors for persistence, recurrence, and invasion and its management. Am J Obstet Gynecol. 1997;176:93–99. doi: 10.1016/s0002-9378(97)80018-x. [DOI] [PubMed] [Google Scholar]
- 3.Brinton LA, Nasca PC, Mallin K, et al. Case-control study of in situ and invasive carcinoma of the vagina. Gynecol Oncol. 1990;38:49–54. doi: 10.1016/0090-8258(90)90010-i. [DOI] [PubMed] [Google Scholar]
- 4.Dodge JA, Eltabbakh GH, Mount SL, et al. Clinical features and risk of recurrence among patients with vaginal intraepithelial neoplasia. Gynecol Oncol. 2001;83:363–369. doi: 10.1006/gyno.2001.6401. [DOI] [PubMed] [Google Scholar]
- 5.Aho M, Vesterinen E, Meyer B, et al. Natural history of vaginal intraepithelial neoplasia. Cancer. 1991;68:195–197. doi: 10.1002/1097-0142(19910701)68:1<195::aid-cncr2820680135>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Rome RM, England PG. Management of vaginal intraepithelial neoplasia: A series of 132 cases with long-term follow-up. Int J Gynecol Cancer. 2000;10:382–390. doi: 10.1046/j.1525-1438.2000.010005382.x. [DOI] [PubMed] [Google Scholar]
- 7.Diakomanolis E, Haidopoulos D, Stefanidis K. Treatment of high-grade vaginal intraepithelial neoplasia with imiquimod cream. N Engl J Med. 2002;347:374. doi: 10.1056/NEJM200208013470521. [DOI] [PubMed] [Google Scholar]
- 8.Benedet JL, Sanders BH. Carcinoma in situ of the vagina. Am J Obstet Gynecol. 1984;148:695–700. doi: 10.1016/0002-9378(84)90776-2. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez-Linares W, Puthawala A, Nolan JF, et al. Carcinoma in situ of the vagina: Past and present management. Obstet Gynecol. 1980;56:356–360. [PubMed] [Google Scholar]
- 10.Punnonen R, Grönroos M, Meurman L, et al. Diagnosis and treatment of primary vaginal carcinoma in situ and dysplasia. Acta Obstet Gynecol Scand. 1981;60:513–514. doi: 10.3109/00016348109155472. [DOI] [PubMed] [Google Scholar]
- 11.Woodman CB, Mould JJ, Jordan JA. Radiotherapy in the management of vaginal intraepithelial neoplasia after hysterectomy. Br J Obstet Gynaecol. 1988;95:976–979. doi: 10.1111/j.1471-0528.1988.tb06500.x. [DOI] [PubMed] [Google Scholar]
- 12.Murta EF, Neves Junior MA, Sempionato LR, et al. Vaginal intraepithelial neoplasia: Clinical-therapeutic analysis of 33 cases. Arch Gynecol Obstet. 2005;272:261–264. doi: 10.1007/s00404-005-0022-1. [DOI] [PubMed] [Google Scholar]
- 13.Perez CA, Grigsby PW, Garipagaoglu M, et al. Factors affecting long-term outcome of irradiation in carcinoma of the vagina. Int J Radiat Oncol Biol Phys. 1999;44:37–45. doi: 10.1016/s0360-3016(98)00530-6. [DOI] [PubMed] [Google Scholar]
- 14.Ogino I, Kitamura T, Okajima H, et al. High-dose-rate intracavitary brachytherapy in the management of cervical and vaginal intraepithelial neoplasia. Int J Radiat Oncol Biol Phys. 1998;40:881–887. doi: 10.1016/s0360-3016(97)00924-3. [DOI] [PubMed] [Google Scholar]
- 15.MacLeod C, Fowler A, Dalrymple C, et al. High-dose-rate brachytherapy in the management of high-grade intraepithelial neoplasia of the vagina. Gynecol Oncol. 1997;65:74–77. doi: 10.1006/gyno.1996.4608. [DOI] [PubMed] [Google Scholar]
- 16.Teruya Y, Sakumoto K, Moromizato H, et al. High dose-rate intracavitary brachytherapy for carcinoma in situ of the vagina occurring after hysterectomy: A rational prescription of radiation dose. Am J Obstet Gynecol. 2002;187:360–364. doi: 10.1067/mob.2002.123202. [DOI] [PubMed] [Google Scholar]
- 17.Graham K, Wright K, Cadwallader B, et al. 20-year retrospective review of medium dose rate intracavitary brachytherapy in VAIN3. Gynecol Oncol. 2007;106:105–111. doi: 10.1016/j.ygyno.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Magné N, Chargari C, SanFilippo N, et al. Technical aspects and perspectives of the vaginal mold applicator for brachytherapy of gynecologic malignancies. Brachytherapy. 2010;9:274–277. doi: 10.1016/j.brachy.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Shield PW, Wright RG, Free K, et al. The accuracy of cervicovaginal cytology in the detection of recurrent cervical carcinoma following radiotherapy. Gynecol Oncol. 1991;41:223–229. doi: 10.1016/0090-8258(91)90313-t. [DOI] [PubMed] [Google Scholar]
- 20.Onsrud M, Strickert T, Marthinsen AB. Late reactions after postoperative high-dose-rate intravaginal brachytherapy for endometrial cancer: A comparison of standardized and individualized target volumes. Int J Radiat Oncol Biol Phys. 2001;49:749–755. doi: 10.1016/s0360-3016(00)01464-4. [DOI] [PubMed] [Google Scholar]
- 21.Indermaur MD, Martino MA, Fiorica JV, et al. Upper vaginectomy for the treatment of vaginal intraepithelial neoplasia. Am J Obstet Gynecol. 2005;193:577–580. doi: 10.1016/j.ajog.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman MS, DeCesare SL, Roberts WS, et al. Upper vaginectomy for in situ and occult, superficially invasive carcinoma of the vagina. Am J Obstet Gynecol. 1992;166:30–33. doi: 10.1016/0002-9378(92)91823-s. [DOI] [PubMed] [Google Scholar]