A systematic review and meta-analysis was performed to compare treatment effectiveness and adverse effects in cancer patients receiving chemotherapy with palonosetron to prevent chemotherapy-induced nausea and vomiting (CINV). The use of palonosetron should be considered an integral part of adjuvant therapy for prevention of the acute, delayed, and overall phases of chemotherapy-induced nausea and vomiting.
Abstract
Objectives.
We performed a systematic review and meta-analysis to compare treatment effectiveness and adverse effects in cancer patients receiving chemotherapy with palonosetron to prevent chemotherapy-induced nausea and vomiting (CINV).
Methods.
We identified randomized controlled clinical trials (RCT) comparing palonosetron with first-generation 5-HT3RA in the prevention of CINV in cancer patients. Meta-analyses were performed on homogeneous studies. Fixed or random-effects models were used to combine data.
Results.
Eight eligible trials were identified, reporting outcomes on 3,592 patients. Meta-analyses showed statistically significant differences in favor of palonosetron compared with first-generation 5-HT3RA in prevention of acute CINV (p = .0003), delayed CINV (p < .00001), and overall phase of CINV (p < .00001). Subgroup analyses showed statistically significant differences in favor of both 0.25 mg and 0.75 mg of palonosetron in prevention of all phases of CINV. There were no statistically significant differences between 0.25 and 0.75 mg of palonosetron. Compared with the first-generation 5-HT3RA, 0.75 mg of palonosetron showed a statistically significant difference in the occurrence of constipation (p = .04).
Interpretation.
The use of palonosetron should be considered an integral part of adjuvant therapy for prevention of the acute, delayed, and overall phases of CINV. The 0.25 mg intravenous palonosetron dose is as effective as the 0.75 mg intravenous palonosetron dose. However, 0.75 mg intravenous palonosetron causes constipation more frequently than the first-generation 5-HT3RA.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a serious problem associated with poor health-related quality of life [1], especially in patients who experience more than two episodes [2]. As such, it might reduce therapeutic compliance [3]. Patients surveyed state that nausea and vomiting are the most feared effects of chemotherapy [4].
CINV can be divided into an acute phase, defined as vomiting that occurs within 24 hours after chemotherapy, delayed CINV that takes place 24–120 hours after chemotherapy, and anticipatory CINV, which occurs before chemotherapy. Without adequate antiemetic prophylaxis, more than 90% of patients who receive highly emetogenic chemotherapy (HEC) will experience emesis [5]. Characteristics that increase the risk of CINV include female gender, younger age, previous exposure to chemotherapy, history of alcohol abstention, and presence of nausea and vomiting with prior chemotherapy [6].
The 5-hydroxytryptamine3 (5-HT3) receptor plays a pivotal role in the process of emesis. Chemotherapy initiates the release of serotonin from enterochromaffin cells in the small intestine, activating 5-HT3 receptors located on vagal afferents, and thus causing emesis [7]. The efficacy and low incidence of side effects have established the 5-HT3 receptor antagonists (5-HT3RA) as first-line treatments for preventing acute CINV. Complete response of first-generation 5-HT3RA as monotherapy for prophylaxis against acute CINV has ranged from 41% to 71% [8–14], but for prophylaxis against delayed CINV it has ranged from 27% to 43% [8, 9, 11, 15]. Combining first-generation 5-HT3RA with corticosteroids, such as dexamethasone, can bring acute and delayed CINV response rates to 68%–90% [9–14] and 47%–63.8% [8, 9, 11, 14, 15], respectively. However, meta-analysis shows that adding a first-generation 5-HT3RA to dexamethasone is no more effective than dexamethasone for preventing delayed emesis [16].
Guidelines recommend a three-drug regimen of dexamethasone, a 5-HT3RA, and aprepitant/fosaprepitant for the prevention of acute CINV associated with HEC, and a three- or two-drug regimen of dexamethasone and a 5-HT3RA (with or without aprepitant/fosaprepitant) for the prevention of acute CINV associated with moderately emetogenic chemotherapy (MEC) [5, 17–19]. Meta-analysis confirms that there is no difference in efficacy between first-generation 5-HT3RAs (ondansetron, granisetron, dolasetron, and tropisetron) for prevention of acute CINV [20].
Palonosetron is a second-generation 5-HT3RA characterized by much higher binding affinity (Pki = 10.45 for palonosetron, 8.39 for ondansetron, 8.91 for granisetron, and 7.60 for dolasetron) and longer serum half-life compared with first-generation 5-HT3RAs (40 hours for palonosetron, 4 hours for ondansetron, 9 hours for granisetron, and 7.3 hours for dolasetron) [21]. In contrast to ondansetron and granisetron, which bond to one site on the 5-HT3 receptors and act in synergy with competing antagonistic agents, palonosetron binds to two sites on the 5-HT3 receptor, and also has a positive synergistic effect. Moreover, prolonged inhibition of Ca2+ has been observed in palonosetron [22]. With these unique characteristics, palonosetron has been demonstrated to be more effective than first-generation 5-HT3RAs in the prevention of the delayed and overall phases of CINV [23, 24]. However, the advantage of palonosetron in prevention of acute CINV and the optimal dosage remain controversial. Whether adding a corticosteroid to palonosetron is beneficial is also unclear. We performed a systematic review and meta-analysis to evaluate the effectiveness and adverse effects of palonosetron in the prevention of CINV.
Methods
Trial Identification
We searched for RCTs in the English or Chinese literature that compared palonosetron with first-generation 5-HT3RA in (a) MEDLINE (1950 through March 2010), (b) EMBASE (1966 through March 2010), (c) Chinese VIP database (1989 through March 2010), (d) Evidence-based Medicine Review, (e) Cochrane Central Register of Controlled Trials, (f) Current Controlled Trials, (g) The National Research Register, and (9) Clinicaltrials (clinicaltrials.gov). Search terms included the combination of palonosetron with “chemotherapy induced nausea and vomiting,” “chemotherapy-induced nausea or vomiting,” and “CINV.” We excluded RCTs of pediatric patients.
Outcomes
The primary outcome was complete response (CR) of the acute, delayed, and overall phases of CIVN after chemotherapy. Complete response was defined as no emetic episodes and no rescue medication. Overall phase was defined as 0–120 hours after chemotherapy. Secondary outcomes included adverse effects of palonosetron.
Statistical Analysis
For nonheterogenerous trials, we performed meta-analysis with Review Manager (Revman 4.2) using fixed or random effects models. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. A heterogeneity test p > .05 was interpreted as signifying a low level of heterogeneity suitable for meta-analysis.
Methodological Quality of Included Studies
We evaluated the methodological quality of included studies by assessing methods of randomization, allocation, concealment, and blinding.
Subgroup Analyses
We performed subgroup analyses by using different dosages of palonosetron (0.25 or 0.75 mg), and by either combining or not combining them with glucocorticoid. We also evaluated the effectiveness of palonosetron in differently emetogenic chemotherapy.
Results
Results of the Search Strategy
Of 235 references, 8 RCTs involving 3,592 patients were identified as eligible for inclusion in this review. We excluded 5 RCTs [25–29]. The reasons for exclusion are listed in Table 1.
Table 1.
Exclusion reasons of excluded studies
Characteristics of Included Trials
Four studies evaluated the effectiveness of palonosetron in HEC [30–33] and 2 in MEC [23, 24]. Two studies included both HEC and MEC regimens [34, 35]. Two studies were published in Chinese [31, 34]; the rest were published in English. The female-to-male ratio varied from 0.58 to 4.58. Two studies did not mention chemotherapy-naive patients [31, 34]; one included more than 90% chemotherapy-naive patients [32].
Quality of Included Studies
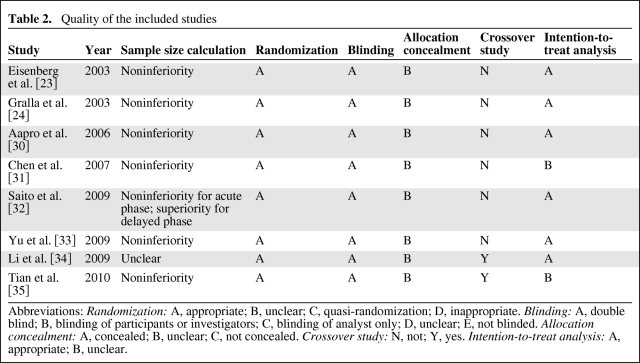
Of the eight RCTs in the review, one failed to mention sample size calculation [34]. Six of the RCTs were designed as noninferiority trials, except for one study that used a superiority test for calculation of delayed CINV prevention [32]. All included studies were double-blinded trials with appropriate randomization methods. None mentioned allocation concealment. Two of the eight studies used a crossover design [34, 35]. We used the results for cycle 1 of one study [35]. “Intention to treat” was mentioned in six of the eight studies. The characteristics of included studies are summarized in Table 2.
Table 2.
Quality of the included studies
Abbreviations: Randomization: A, appropriate; B, unclear; C, quasi-randomization; D, inappropriate. Blinding: A, double blind; B, blinding of participants or investigators; C, blinding of analyst only; D, unclear; E, not blinded. Allocation concealment: A, concealed; B, unclear; C, not concealed. Crossover study: N, not; Y, yes. Intention-to-treat analysis: A, appropriate; B, unclear.
Results of Included Studies
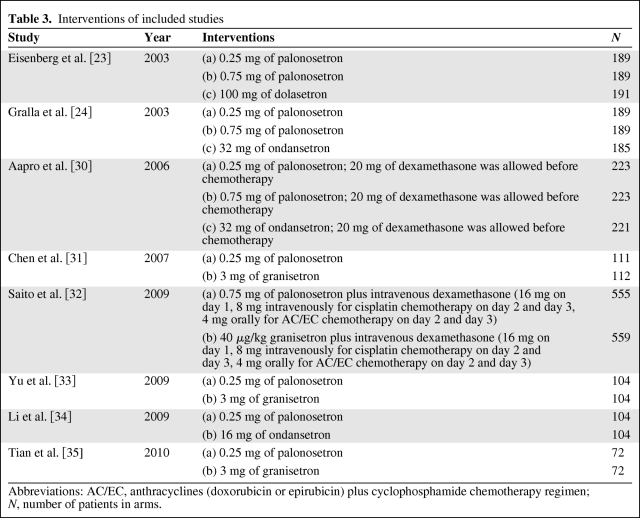
All included studies compared intravenous palonosetron with first-generation 5-HT3RA (one compared dolasetron, three compared ondansetron, and four compared granisetron).Three studies evaluated the effectiveness of both 0.25 and 0.75 mg of palonosetron [23, 24, 30]. Four studies evaluated the effectiveness of 0.25 mg of palonosetron [31, 33–35] and one evaluated 0.75 mg of palonosetron [32]. Corticosteroids were used before chemotherapy in two studies [30, 32]. Saito et al. investigated palonosetron combined with dexamethasone [32]. Intravenous dexamethasone (20 mg) before chemotherapy was permitted but not required in another study [30]. Yu et al. evaluated the effectiveness of 0.25 mg of palonosetron in the prevention of CINV in 0–24, 24–48, 48–72, 72–96, and 96–120 hours [33]; thus, the CR rates of delayed and overall phases were unavailable. The most common adverse events were headache and constipation. These interventions are summarized in Table 3.
Table 3.
Interventions of included studies
Abbreviations: AC/EC, anthracyclines (doxorubicin or epirubicin) plus cyclophosphamide chemotherapy regimen; N, number of patients in arms.
Results of Meta-analyses
Primary Outcome
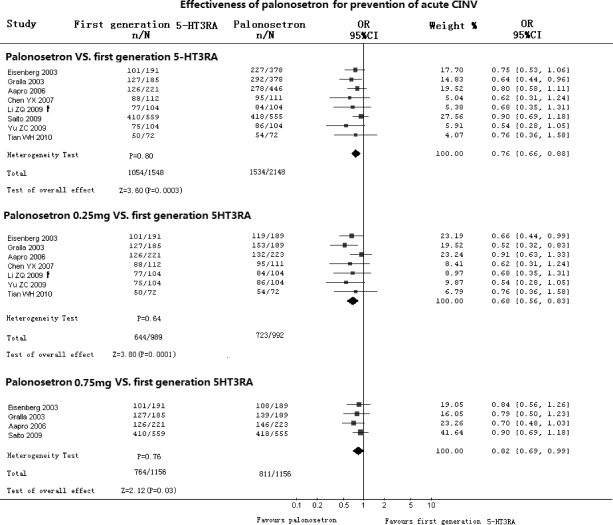
Effectiveness of Palonosetron Compared with First-Generation 5-HT3RA in Prevention of Acute CINV (Fig. 1)
Figure 1.
Effectiveness of palonosetron compared with first-generation 5-HT3RA in prevention of acute CINV. Abbreviations: CI, confidence interval; CINV, chemotherapy-induced nausea and vomiting; CR, complete response; n, number of CRs; N, number of groups; OR, odds ratio; †, from crossover data.
All eight RCTs compared palonosetron with first-generation 5-HT3RA for prevention of acute CINV. There was no heterogeneity between included studies (p = .80). Meta-analysis that included 3,592 patients with 3,696 cycles showed that palonosetron reduced the risk of acute CINV by 24% (OR, 0.76; 95% CI, 0.66–0.88, p = .0003). Subgroup analysis showed that there were statistically significant differences in favor of both 0.25 mg of palonosetron (OR, 0.68; 95% CI, 0.56–0.83; p = .0001) and 0.75 mg of palonosetron (OR, 0.82; 95% CI, 0.69–0.99; p = .03).
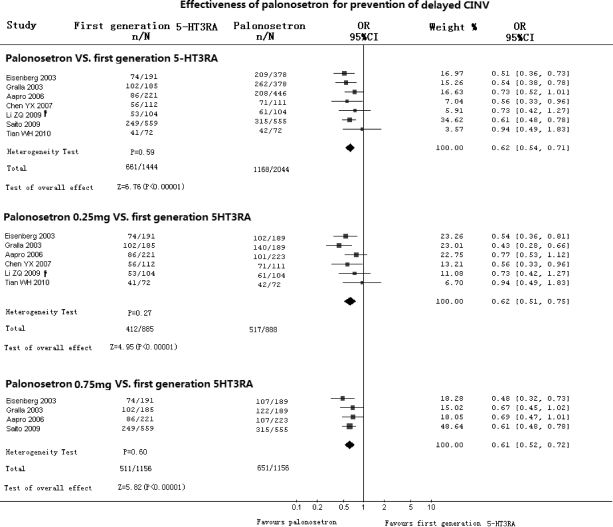
Effectiveness of Palonosetron Compared with First-Generation 5-HT3RA in Prevention of Delayed CINV (Fig. 2)
Figure 2.
Effectiveness of palonosetron compared with first-generation 5-HT3RA in prevention of delayed CINV. Abbreviations: CI, confidence interval; CINV, chemotherapy-induced nausea and vomiting; CR, complete response; n, number of CRs; N, number of groups; OR, odds ratio; †, from crossover data.
Seven RCTs with 3,384 patients (3,488 cycles) compared palonosetron with first-generation 5-HT3RA in prevention of delayed CINV. The results showed no heterogeneity (p = .59) in any included studies (OR, 0.62; 95% CI, 0.54–0.71) in favor of palonosetron (p < .00001). Subgroup analyses indicated statistically significant differences in favor of both 0.25 mg of palonosetron (OR, 0.62; 95% CI, 0.51–0.75; p < .00001) and 0.75 mg of palonosetron (OR, 0.61; 95% CI, 0.52–0.72; p < .00001).
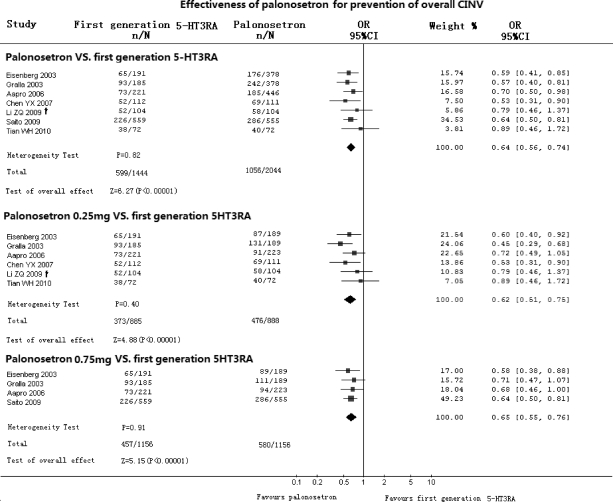
Effectiveness of Palonosetron Compared with First-Generation 5-HT3RA in Prevention of the Overall Phase of CINV (Fig. 3)
Figure 3.
Effectiveness of palonosetron compared with first-generation 5-HT3RA in prevention of overall phase of CINV. Abbreviations: CI, confidence interval; CINV, chemotherapy-induced nausea and vomiting; CR, complete response; n, number of CRs; N, number of groups; OR, odds ratio; †, from crossover data.
Seven RCTs compared palonosetron with first-generation 5-HT3RA in prevention of the overall phase of CINV. Meta-analysis showed an OR of 0.64 (95% CI, 0.56–0.74) in favor of palonosetron (p < .00001). Subgroup analysis showed statistically significant differences in favor of both 0.25 mg of palonosetron (OR, 0.62; 95% CI, 0.51–0.75; p < .00001) and 0.75 mg (OR, 0.65; 95% CI, 0.55–0.76; p < .00001).
Effectiveness of 0.25 mg of Palonosetron Compared with 0.75 mg of Palonosetron
Meta-analysis included three studies (n = 1,202) showed no statistically significant differences between 0.25 and 0.75 mg of palonosetron in terms of preventing acute CINV (OR, 1.09; 95% CI, 0.85–1.38; p = .50), delayed CINV (OR, 1.05; 95% CI, 0.83–1.32; p = .68), or overall phase CINV (OR, 1.11; 95% CI, 0.88–1.40; p = .38).
Effectiveness of Palonosetron Plus Dexamethasone Compared with First-Generation 5-HT3RA Plus Dexamethasone
We extracted data from two studies on HEC that compared palonosetron plus dexamethasone with first-generation 5-HT3RA plus dexamethasone [30, 32]; one study allowed dexamethasone before chemotherapy by choice [30]. Meta-analyses of 1,561 patients showed a trend in favor of palonosetron plus dexamethasone in prevention of acute CINV (OR, 0.84; 95% CI, 0.67–1.05; p = .36). For prevention of delayed CINV and overall phase of CINV, the meta-analyses showed that palonosetron plus dexamethasone significantly reduced risk of CINV by 40% and 38% (p < .00001), respectively.
Effectiveness of Palonosetron Compared with First-Generation 5-HT3RA for Prevention of CINV in Differently Emetogenic Chemotherapy
Results of meta-analyses for MEC [23, 24] showed that there were statistically significant differences in favor of palonosetron in the prevention of acute CINV (OR, 0.70; 95% CI, 0.54–0.91; p = .008), delayed CINV (p < .00001), and overall phase CINV (p < .0001).
Results of the meta-analyses for HEC [30–33] showed that there were statistically significant differences in favor of palonosetron for prevention of acute CINV (OR, 0.79; 95% CI, 0.64–0.96; p = .02), delayed CINV (p < .00001), and overall phase CINV (p < .00001). Meta-analysis of two studies [31, 33] showed a significant difference in favor of 0.25 mg of palonosetron in prevention of acute CINV in HEC (OR, 0.58; 95% CI, 0.36–0.93; p = .02).
Analyses of Adverse Effects
Constipation
Seven RCTs reported constipation as an adverse event. Meta-analysis showed that palonosetron increased the risk of constipation by 39% (OR, 1.39; 95% CI, 1.08–1.78; p = .01). Subgroup analyses showed significant differences between 0.75 mg of palonosetron and first-generation 5-HT3RA (p = .04), but not between 0.25 mg of palonosetron and first-generation 5-HT3RA (p = .20).
Headache
Data showed no significant difference between palonosetron and first-generation 5-HT3RA on the occurrence of headache (OR, 1.16; 95% CI, 0.89–1.53; p = .27). Subgroup analyses also showed no significant differences between first-generation 5-HT3RA and palonosetron (0.25 and 0.75 mg).
Discussion
Guidelines indicate that all 5-HT3RAs (ondansetron, granisetron, dolasetron, and palonosetron) have similar effectiveness for control of acute emesis [5]. In our analyses, most of the RCTs indicated that the second-generation 5-HT3RA, palonosetron, has significant advantages in the prevention of the delayed and overall phases of CINV. All but one RCT [24] showed that palonosetron was not superior to first-generation 5-HT3RA for prevention of acute CINV. Our meta-analysis, however, showed that palonosetron was more effective than the first-generation 5-HT3RA in prevention of acute CINV. We noticed that all the RCTs on prevention of acute CINV were designed for noninferior tests. That might make the sample sizes insufficient to determine differences. Our meta-analysis also demonstrated the superiority of 0.25 mg of palonosetron over first-generation 5-HT3RA in the prevention of acute CINV in HEC. Because of its synergism with other agents, dexamethasone has been prescribed with 5-HT3RA to prevent acute CINV. Our analysis showed a trend in favor of palonosetron plus dexamethasone in prevention of acute CINV in HEC, but it did not reach statistical significance (p = .36).
The advantage of palonosetron in prevention of delayed CINV was of value for at least two important reasons. First, a high percentage of patients experienced emesis despite prophylaxis. Second, adding a first-generation 5-HT3RA to dexamethasone was not superior to dexamethasone in prevention of delayed CINV. Two RCTs included dexamethasone in the interventions. One compared palonosetron plus dexamethasone with the first-generation 5-HT3RA plus dexamethasone. Dexamethasone was used before chemotherapy, and on days 2 and 3 [32]. The other study allowed for, but did not require, use of dexamethasone before chemotherapy [30]. Our subgroup analyses proved that palonosetron plus dexamethasone was superior to the first-generation 5-HT3RA plus dexamethasone in prevention of the delayed and overall phases of CINV. In a double-blinded RCT [36], 0.25 mg of intravenous palonosetron plus 8 mg of dexamethasone were given as prophylaxis before chemotherapy, with 4 mg of dexamethasone twice a day orally or placebo administered on days 2 and 3. In this trial of 300 patients, the CR rate was similar in the overall period. The authors suggested a reduction in dexamethasone. However, acute and delayed CINV might have overlapped. CR rate in the overall phase was probably a more useful endpoint [37].
In our analyses, both 0.25 and 0.75 mg of palonosetron were superior to first-generation 5-HT3RA in the overall phase of CINV. A phase II study found that there was no difference between 10 and 30 μg/kg palonosetron in the prevention of all phases of CINV [38]. The doses 0.25 and 0.75 mg of palonosetron were commonly used. Our subgroup analyses showed that both palonosetron doses were more efficient than the first-generation 5-HT3RA, and that 0.25 mg of palonosetron was as effective as 0.75 mg of palonosetron.
Constipation and headache were the most common adverse effects. Our meta-analyses showed that patients treated with palonosetron experienced more constipation than those who received the first-generation 5-HT3RA (p = .01), but there was no significant difference in headaches.
Further subgroup analyses showed no significant difference in constipation between 0.25 mg of palonosetron and the first-generation 5-HT3RA, whereas 0.75 mg of palonosetron caused more constipation than the first-generation 5-HT3RA (p = .04), indicating that a higher dose of palonosetron might cause more constipation. The reason might rely on that 5-HT3RA prolongs the whole gut transit time [39] and inhibits postprandial colonic motor function [40]. However, in a recent study [41], patients treated with palonosetron took adequate caloric intake. The reasons might be that 5-HT3RA did not change the volume of meal tolerated, fasting, or postprandial gastric volumes [42], nor did it change gastric emptying [43].
In summary, the results of our meta-analyses indicate that palonosetron is an effective and safe drug for prevention of CINV. Both 0.25 and 0.75 mg of intravenous palonosetron are more efficacious than first-generation 5-HT3RA in prevention of the acute, delayed, and overall phases of CINV. The 0.25 mg dose of palonosetron is preferred. In the prevention of acute CINV in HEC, 0.75 mg of palonosetron plus dexamethasone is as effective as the first-generation 5-HT3RA, but palonosetron at that dose may increase the risk of constipation. Intravenous palonosetron plus dexamethasone is more efficacious than the first-generation 5-HT3RA plus dexamethasone in prevention of the delayed and overall phases of CINV.
This study has some limitations. We did not evaluate the effectiveness of 0.25 mg of palonosetron plus dexamethasone because of limited data. In addition, we searched studies published only in English and Chinese. Although 0.25 mg of palonosetron is preferred for prevention of CINV, a direct comparison between 0.25 mg of palonosetron plus dexamethasone and the first-generation 5-HT3RA plus dexamethasone is needed.
Acknowledgments
Zhou Likun, Jing Xiang, and Ba Yi contributed equally to this work.
Author Contributions
Conception/Design: Zhou Likun, Ba Yi
Data analysis and interpretation: Zhou Likun, Jing Xiang, Duan Xin, Zheng Liu Tao
Manuscript writing: Zhou Likun, Jing Xiang, Ba Yi, Duan Xin, Zheng Liu Tao
Final approval of manuscript: Zhou Likun, Jing Xiang, Ba Yi
References
- 1.Lindley CM, Hirsch JD, O'Neill CV, et al. Quality of life consequences of chemotherapy-induced emesis. Qual Life Res. 1992;1:331–340. doi: 10.1007/BF00434947. [DOI] [PubMed] [Google Scholar]
- 2.Osoba D, Zee B, Warr D, et al. Effect of postchemotherapy nausea and vomiting on health-related quality of life. The Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. Support Care Cancer. 1997;5:307–313. doi: 10.1007/s005200050078. [DOI] [PubMed] [Google Scholar]
- 3.Laszlo J, Lucas VS., Jr Emesis as a critical problem in chemotherapy. N Engl J Med. 1981;305:948–949. doi: 10.1056/NEJM198110153051609. [DOI] [PubMed] [Google Scholar]
- 4.Coates A, Abraham S, Kaye SB, et al. On the receiving end: patient perception of the side effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983;19:203–208. doi: 10.1016/0277-5379(83)90418-2. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology–Antiemesis—v. 4.2009. [accessed March 2010]. Available at: http://www.nccn.org.
- 6.Gralla RJ, Tyson LB, Kris MG, et al. The management of chemotherapy-induced nausea and vomiting. Med Clin North Am. 1987;71:289–301. doi: 10.1016/s0025-7125(16)30871-9. [DOI] [PubMed] [Google Scholar]
- 7.Hasler WL. Serotonin receptor physiology: relation to emesis. Dig Dis Sci. 1999;44(8 suppl):108S–113S. [PubMed] [Google Scholar]
- 8.Chevallier B, Marty M, Paillarse JM. Methylprednisolone enhances the efficacy of ondansetron in acute and delayed cisplatin-induced emesis over at least three cycles. Ondansetron Study Group. Br J Cancer. 1994;70:1171–1175. doi: 10.1038/bjc.1994.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Italian Group for Antiemetic Research. Dexamethasone, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. N Engl J Med. 1995;332:1–5. doi: 10.1056/NEJM199501053320101. [DOI] [PubMed] [Google Scholar]
- 10.Kleisbauer JP, Garcia-Giron C, Antimi M, et al. Granisetron plus methylprednisolone for the control of high-dose cisplatin-induced emesis. Anticancer Drugs. 1998;9:387–392. doi: 10.1097/00001813-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-del-Muro X, Vadell C, Perez Manga G, et al. Randomised double-blind study comparing tropisetron alone and in combination with dexamethasone in the prevention of acute and delayed cisplatin-induced emesis. Eur J Cancer. 1998;34:193–195. doi: 10.1016/s0959-8049(97)00367-5. [DOI] [PubMed] [Google Scholar]
- 12.Janinis J, Giannakakis T, Athanasiades A, et al. A randomized open-label parallel-group study comparing ondansetron with ondansetron plus dexamethasone in patients with metastatic breast cancer receiving high-dose epirubicin. A Hellenic Cooperative Oncology Group study. Tumori. 2000;86:37–41. doi: 10.1177/030089160008600107. [DOI] [PubMed] [Google Scholar]
- 13.Fauser AA, Pizzocaro G, Schueller J, et al. A double-blind, randomised, parallel study comparing intravenous dolasetron plus dexamethasone and intravenous dolasetron alone for the management of fractionated cisplatin-related nausea and vomiting. Support Care Cancer. 2000;8:49–54. doi: 10.1007/s005209900090. [DOI] [PubMed] [Google Scholar]
- 14.Villalon A, Chan V. Multicenter, randomized trial of ramosetron plus dexamethasone versus ramosetron alone in controlling cisplatin-induced emesis. Support Care Cancer. 2004;12:58–63. doi: 10.1007/s00520-003-0528-7. [DOI] [PubMed] [Google Scholar]
- 15.Gebbia V, Testa A, Valenza R, et al. Oral granisetron with or without methylprednisolone versus metoclopramide plus methylprednisolone in the management of delayed nausea and vomiting induced by cisplatin-based chemotherapy. A prospective randomized trial. Cancer. 1995;76:1821–1828. doi: 10.1002/1097-0142(19951115)76:10<1821::aid-cncr2820761022>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 16.Jordan K, Hinke A, Grothey A, et al. A meta-analysis comparing the efficacy of four 5-HT3-receptor antagonists for acute chemotherapy induced emesis. Support Care Cancer. 2007;15:1023–1033. doi: 10.1007/s00520-006-0186-7. [DOI] [PubMed] [Google Scholar]
- 17.Herrstedt J, Roila F. ESMO Guidelines Working Group. Chemotherapy-induced nausea and vomiting: ESMO clinical recommendations for prophylaxis. Ann Oncol. 2008;19(suppl 2):ii110–112. doi: 10.1093/annonc/mdn105. [DOI] [PubMed] [Google Scholar]
- 18.Herrstedt J. Antiemetics: an update and the MASCC guidelines applied in clinical practice. Nat Clin Pract Oncol. 2008;5:32–43. doi: 10.1038/ncponc1021. [DOI] [PubMed] [Google Scholar]
- 19.American Society of Clinical Oncology. Kris MG, Hesketh PJ, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. 2006;24:2932–2947. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]
- 20.Jordan K, Hinke A, Grothey A, et al. Meta-analysis comparing the efficacy of four 5-HT3-receptor antagonists for acute chemotherapy-induced emesis. Support Care Cancer. 2007;15:1023–1033. doi: 10.1007/s00520-006-0186-7. [DOI] [PubMed] [Google Scholar]
- 21.Navari RM. Palonosetron: a second-generation 5-hydroxytryptamine receptor antagonist. Future Oncol. 2006;2:591–602. doi: 10.2217/14796694.2.5.591. [DOI] [PubMed] [Google Scholar]
- 22.Rojas C, Stathis M, Thomas AG, et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008;107:469–478. doi: 10.1213/ane.0b013e318172fa74. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg P, Figueroa-Vadillo J, Zamora R, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist. Results of a Phase III, single-dose trial versus dolasetron. Cancer. 2003;98:2473–2482. doi: 10.1002/cncr.11817. [DOI] [PubMed] [Google Scholar]
- 24.Gralla R, Lichinitser M, Van der Vegt S, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14:1570–1577. doi: 10.1093/annonc/mdg417. [DOI] [PubMed] [Google Scholar]
- 25.Aapro MS, Macciocchi A, Gridelli C. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting in elderly patients. J Support Oncol. 2005;3:369–374. [PubMed] [Google Scholar]
- 26.Liu P, Feng FY, Wang SJ, et al. Clinical research of palonosetron hydrochloride injection for prevention chemotherapy-induced nausea and vomiting [abstract]. Medical Frontier Forum and the Tenth National Conference on Cancer Pharmacology and Chemotherapy; June 16–20, 2007; Tsingtao. [Google Scholar]
- 27.Kadota R., Shen V., Messinger Y. Safety, pharmacokinetics, and efficacy of palonosetron in pediatric patients: A multicenter, stratified, double-blind, phase 3, randomized study. J Clin Oncol. 2007;25(suppl 18):9570. [Google Scholar]
- 28.Sepúlveda-Vildósola AC, Betanzos-Cabrera Y, Lastiri GG, et al. Palonosetron hydrochloride is an effective and safe option to prevent chemotherapy-induced nausea and vomiting in children. Arch Med Res. 2008;39:601–606. doi: 10.1016/j.arcmed.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Herrington JD, Jaskiewicz AD, Song J. Randomized, placebo-controlled, pilot study evaluating aprepitant single dose plus palonosetron and dexamethasone for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. Cancer. 2008;112:2080–2087. doi: 10.1002/cncr.23364. [DOI] [PubMed] [Google Scholar]
- 30.Aapro MS, Grunberg SM, Manikhas GM, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17:1441–1449. doi: 10.1093/annonc/mdl137. [DOI] [PubMed] [Google Scholar]
- 31.Cheng YX, Qin SK, Cheng Y, et al. A multicenter double-blind, randomized control clinical trial of palonosetron hydrochloride injection to prevent chemotherapy-induced nausea and vomiting. Chin Clin Oncol. 2007;12:161–165. [Google Scholar]
- 32.Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10:115–124. doi: 10.1016/S1470-2045(08)70313-9. [DOI] [PubMed] [Google Scholar]
- 33.Yu ZC, Liu WC, Wang L, et al. The efficacy and safety of palonosetron compared with granisetron in preventing highly emetogenic chemotherapy-induced vomiting in the Chinese cancer patients: a phase II, multicenter, randomized, double-blind, parallel, comparative clinical trial. Support Care Cancer. 2009;17:99–102. doi: 10.1007/s00520-008-0503-4. [DOI] [PubMed] [Google Scholar]
- 34.Li ZQ, Xu JM, Liu DQ, et al. Phase II trial of hydrochloride in prevention of moderately to severely emetogenic chemotherapy induced nausea and vomiting. Chin Clin Oncol. 2009;14:487–490. [Google Scholar]
- 35.Tian W, Wang Z, Zhou J, et al. Randomized, double-blind, crossover study of palonosetron compared with granisetron for the prevention of chemotherapy-induced nausea and vomiting in a Chinese population. Med Oncol. 2010 Jan 5; doi: 10.1007/s12032-009-9398-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Aapro M, Fabi A, Nolè F, et al. Double-blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol. 2010;21:1083–1088. doi: 10.1093/annonc/mdp584. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Roddy JVF, Berger M. Palonosetron hydrochloride in the treatment of chemotherapy-induced nausea and vomiting. Clin Med: Ther. 2009;1:1145–1158. [Google Scholar]
- 38.Eisenberg P, MacKintosh FR, Ritch P, et al. Efficacy, safety and pharmacokinetics of palonosetron in patients receiving highly emetogenic cisplatin-based chemotherapy: a dose-ranging clinical study. Ann Oncol. 2004;15:330–337. doi: 10.1093/annonc/mdh047. [DOI] [PubMed] [Google Scholar]
- 39.Gore S, Gilmore IT, Haigh CG, et al. Colonic transit in man is slowed by ondansetron (GR38032F), a selective 5-hydroxytryptamine receptor (type 3) antagonist. Aliment Pharmacol Ther. 1990;4:139–144. doi: 10.1111/j.1365-2036.1990.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 40.Scolapio JS, Camilleri M, von der Ohe MR, et al. Ascending colon response to feeding: evidence for a 5-hydroxytryptamine-3 mechanism. Scand J Gastroenterol. 1995;30:562–567. doi: 10.3109/00365529509089790. [DOI] [PubMed] [Google Scholar]
- 41.Lorusso V, Spedicato A, Petrucelli L, et al. Single dose of palonosetron plus dexamethasone to control nausea, vomiting and to warrant an adequate food intake in patients treated with highly emetogenic chemotherapy (HEC) Support Care Cancer. 2009;17:1469–1473. doi: 10.1007/s00520-009-0611-9. [DOI] [PubMed] [Google Scholar]
- 42.Kuo B, Camilleri M, Burton D, et al. Effects of 5-HT(3) antagonism on postprandial gastric volume and symptoms in humans. Aliment Pharmacol Ther. 2002;16:225–233. doi: 10.1046/j.1365-2036.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen OH, Hvid-Jacobsen K, Lund P, et al. Gastric emptying and subjective symptoms of nausea: lack of effects of a 5-hydroxytryptamine-3 antagonist ondansetron on gastric emptying in patients with gastric stasis syndrome. Digestion. 1990;46:89–96. doi: 10.1159/000200337. [DOI] [PubMed] [Google Scholar]