Age-specific incidence rates for breast cancer in low-risk and high-risk ethnic populations differ by age at which the incidence maximum is reached. The results show that in many immigrant groups the diagnostic age is earlier than that in natives of Sweden, suggesting that true biological factors underlie the differences. These factors may explain much of the international variation in breast cancer incidence. Identifying these factors should advance understanding of breast cancer etiology and prevention.
Keywords: Ethnic differences, Age-incidence, Risk factors, Environmental effect, Incidence change
Abstract
Background.
Age-specific incidence rates for breast cancer in low-risk and high-risk ethnic populations differ by age at which the incidence maximum is reached: around 50 years in low-risk populations and over 60 years in high-risk populations. The interpretation of these differences remains unsettled, one line primarily referring to biological differences, the second one to cohort effects of rapidly increasing rates in young populations, and the third one to incomplete registration of cancer in the elderly.
Methods.
The nationwide Family-Cancer Database was used to analyze standardized incidence ratios (SIRs) and age at diagnosis of breast cancer in female immigrants to Sweden by their region of origin compared with women native to Sweden matched on birth year and other relevant factors.
Results.
We showed first that the SIRs for breast cancer were lower in many immigrant groups compared with natives of Sweden; women from Turkey had the lowest SIR of 0.45, followed by those from Chile (0.54) and Southeast Asia (0.57). Women from nine regions showed an earlier mean age at diagnosis than their matched Swedish controls, the largest differences being 5.5 years for women from Turkey, 5.1 years for those from Asian Arab and “Other African” countries, 4.3 years for those from Iran, and 4.0 years for those from Iraq.
Conclusions.
The results show that in many immigrant groups, the diagnostic age is earlier (<50 years) than in natives of Sweden (>50 years), suggesting that true biological factors underlie the differences. These factors may explain much of the international variation in breast cancer incidence. Identifying these factors should advance understanding of breast cancer etiology and prevention.
Introduction
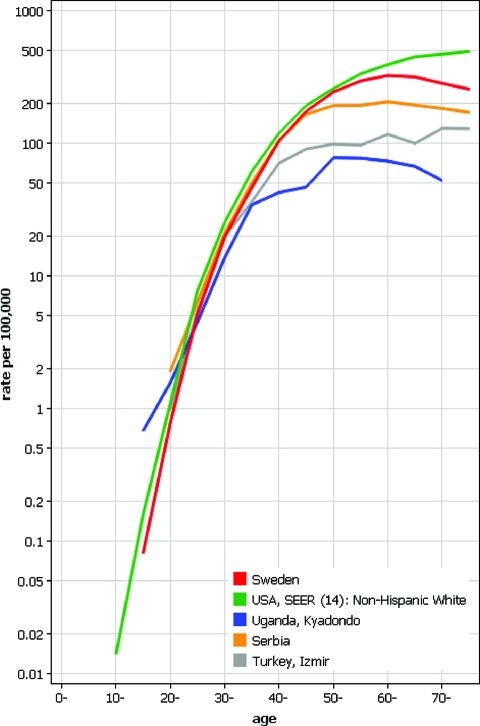
The overall and age-specific incidence for female breast cancer differs between developing and developed countries [1–5]. In Figure 1 we show age-specific rates for breast cancer using the data from Cancer Incidence in Five Continents IX for Sweden and selected countries from which migrants have moved to Sweden. The incidence differences are small until age 35 years when the low-incidence rates diverge and reach a maximum at around age 50 years. The Swedish rate reaches a maximum at the age of 60 years and the white U.S. rates at age >70 years [5]. Mammography has reshaped the age-incidence curves for Sweden and the United States as for these populations age maxima were higher in the premammography era [6, 7]. In the United States, the age-specific rates of breast cancer are almost identical between white and black women until menopause but the white rates are substantially higher toward higher ages [8]. Among the Californian Asian immigrants, postmenopausal breast cancer rates are at a plateau, similar to the Serbian rate in Figure 1 [5]. In South Africa, most white women are postmenopausal at diagnosis compared with women of other races who are premenopausal [9].
Figure 1.
Age-specific incidence of breast cancer in some countries/regions from which women have emigrated to live in Sweden, illustrating differences between high- and low-risk regions, based on Cancer Incidence in Five Continents (logarithmic scale) [5].
In spite of the distinct age-specific incidence rates for breast cancer in low-risk and high-risk regions, the interpretation of these differences has remained controversial [1, 3, 4, 9, 10]. One line of interpretation posits that age at presentation depends on tumor biology, which is assumed to differ between low-risk and high-risk ethnic groups or regions because of genetic and environmental risks factors. Another view considers the early maximal age in developing countries to be a cohort effect of rapidly increasing rates in the young population. Yet another view is doubtful about the level of registration of cancers in the old patients. A solution to this problem may advance understanding of breast cancer etiology and prevention. Probably the only means of finding a solution to this problem would be to study age at diagnosis of breast cancer in relatively recent immigrants who are still at a substantially lower risk than women in the host country.
We analyzed the age at diagnosis of breast cancer in female immigrants to Sweden by their region of origin and compared the results to the age at diagnosis in women from Sweden matched on birth year and other relevant factors. The study is based on the nationwide Family-Cancer Database with 11.8 million individuals, among whom some 15% are foreign born [11]. Sweden offers excellent opportunities to study cancer experience in immigrants because of a uniform cancer registration and health care system and the origin of immigrants from practically all around the world.
Subjects and Methods
The Swedish Family-Cancer Database was first assembled from the national databases in 1996 and since then it has been periodically updated [12]. The database contains data on those born in Sweden since 1932 with their biological parents and additionally data on immigrants are included. This database is the largest in the world on familial cancer and its updated version (2008, VIII), which has been supplied with longitudinal demographic and socioeconomic data from each national census from 1960, 1970, 1980, and 1990, has been used for the present study [11]. Immigrants were defined according to their birth country. First-generation immigrants were defined as those without identified parents in the database. For each female first-generation immigrant, four women from Sweden were selected by matching on birth year, age at first childbirth, parity, and geographical region. To exclude women who immigrated with diagnosed or suspected cancer, cancers in immigrants were included when diagnosed at least 4 years after immigration. The follow-up of the controls was started at the same time as that of the immigrants. The follow-up was stopped for all in the case-control sets when the first of them was diagnosed with cancer, emigrated, or died or when last day of the study, December 31, 2006, was reached, whichever occurred first. Confidence intervals for the difference in mean diagnostic age between immigrants and reference population were based on the t statistic.
The incidence in immigrants was compared with that in natives of Sweden and standardized incidence ratios (SIRs) were calculated, as done previously [13]. The expected numbers were calculated for 5-year age groups, sex, time periods (10-year bands from 1958 to 2006), age at first childbirth (<20, 20–24, 25–29, 30+), and parity (0, 1, 2+) in the native Swedish reference population. The immigrant populations were divided into three groups according to the World Bank income classification from year 2009 (http://siteresources.worldbank.org/DATASTATISTICS/Resources/CLASS.XLS).
Results
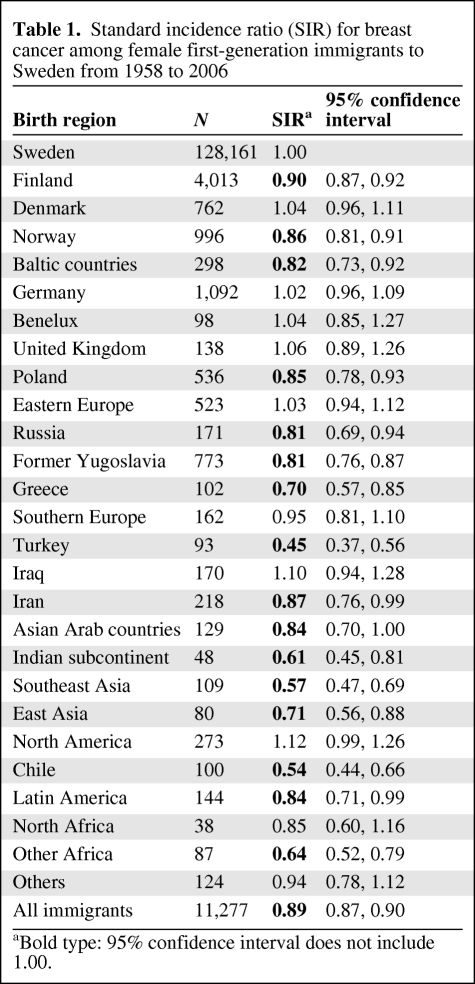
The Family-Cancer Database included 4.3 million women from Sweden and 0.6 million female immigrants. The relative risk of breast cancer in female immigrants compared with that in women from Sweden is shown in Table 1. Women from Finland were by far the largest immigrant group and their risks were lower than those of women from Sweden, SIR 0.90. Among the European immigrants, women from Greece had the lowest risk, 0.70. Among women from non-European countries, those from Turkey had the lowest risk of 0.45, followed by those from Chile (0.54) and Southeast Asia (0.57).
Table 1.
Standard incidence ratio (SIR) for breast cancer among female first-generation immigrants to Sweden from 1958 to 2006
aBold type: 95% confidence interval does not include 1.00.
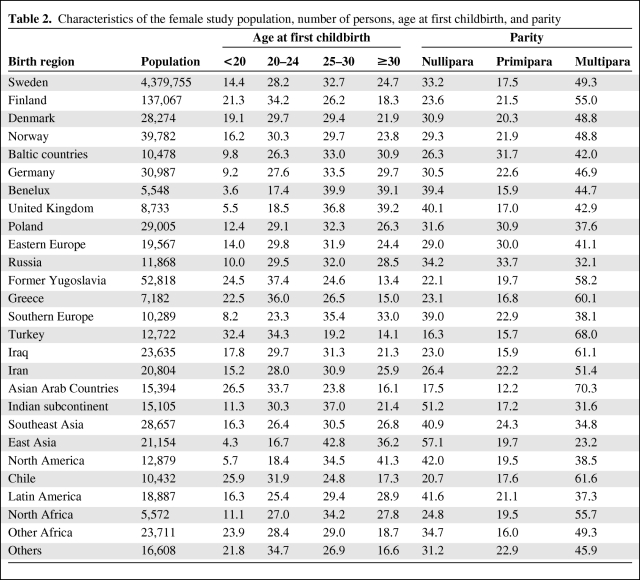
Table 2 shows the number of women by their birth region and the percentage distribution of ages at first childbirth and parity, which were the matching criteria. The reproductive features differed extensively between the immigrant groups. Although only 3.5% of women from the Benelux region had their first child under age 20 years, 32.4% of women from Turkey already had their first child by that age; 41.3% of women from North America had their first child after age 29 years. More than half of the women from the Indian subcontinent were nulliparous and 70.3% of the women from Asian Arab countries were multiparous.
Table 2.
Characteristics of the female study population, number of persons, age at first childbirth, and parity
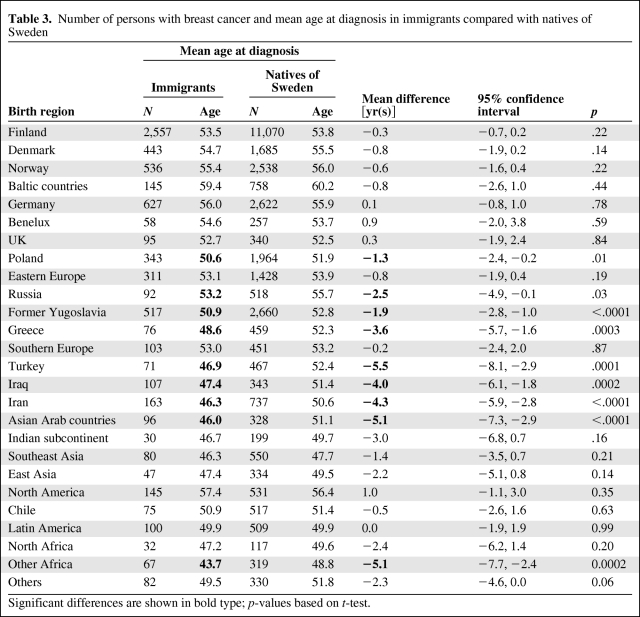
The Family-Cancer Database covered years 1958–2006 from the Swedish Cancer Registry and it included 128,885 cases of breast cancer in the native Swedish population and 11,323 in the first-generation female immigrants. The mean diagnostic age in the immigrant populations and the corresponding Swedish reference populations are shown in Table 3. Women from nine regions showed a younger mean diagnostic age (means and age difference in bold) than their matched Swedish controls, the largest differences being 5.5 years for women from Turkey, 5.1 years for women from both Asian Arab and “Other African” countries, 4.3 years for women from Iran, and 4.0 years for women from Iraq. Among only women from European countries, those from Greece (3.6 years), former Yugoslavia (1.9 years), Russia (2.5 years), and Poland (1.3 years) were diagnosed at a younger age than women from Sweden. No immigrant group had a significantly higher mean age than the women from Sweden. We carried out an additional analysis by classifying parity further, instead of “multipara,” as para 2, 3, and 4+; the results changed only at the decimal level (data not shown).
Table 3.
Number of persons with breast cancer and mean age at diagnosis in immigrants compared with natives of Sweden
Significant differences are shown in bold type; p-values based on t-test.
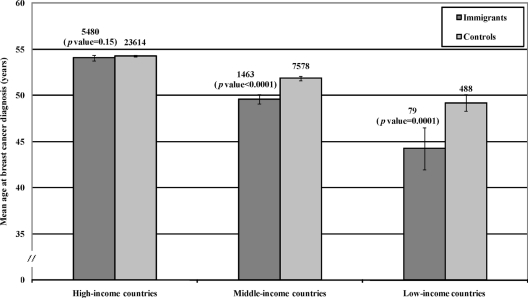
The immigrant populations were divided into three groups according to the income in their native country (Fig. 2). The age at diagnosis differed significantly between immigrants from middle-income countries and natives of Sweden (p < .0001) and low-income countries and natives of Sweden (p = .0001).
Figure 2.
Mean age at breast cancer diagnosis in immigrants, classified according to the income level of the country of origin, compared with natives of Sweden. Case numbers, 95% confidence intervals, and significance levels are shown.
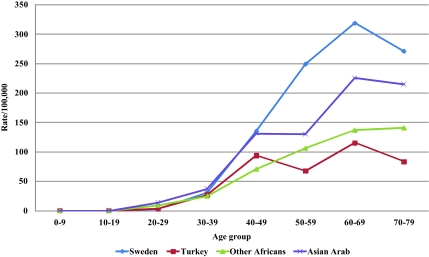
Age-specific incidence rates for the three immigrant groups with the lowest diagnostic age were compared with the Swedish rate as shown in Figure 3. The rates for women from Turkey and Other African countries were well below the Swedish rates, whereas women from Asian Arab countries showed an intermediary incidence curve.
Figure 3.
Age-specific incidence rates of breast cancer among immigrants from Turkey, Asian Arab countries, and Other African countries in Sweden compared with natives of Sweden from 1990 to 2006.
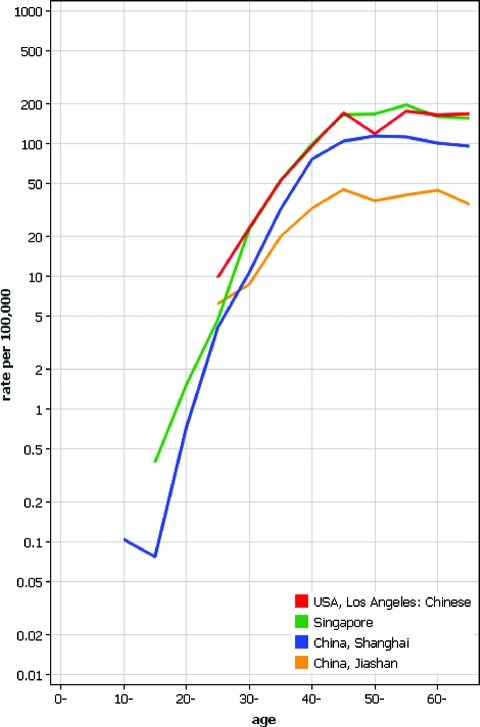
To find evidence for a cohort effect in breast cancer, age-specific incidence rates of diverse Chinese populations were plotted using Cancer Incidence in Five Continents (Fig. 4). If cohort effects were important in shaping the age-specific curves, one would assume that the Chinese populations at various levels of socioeconomic development would show distinct patterns. This was not the case, and the postmenopausal incidence plateau was reached in all populations at age 45–50 years. Note the two lowest curves for Jiashan, a farming and fishing county, and metropolitan Shanghai, located 80 km apart.
Figure 4.
Age-specific incidence of breast cancer among women of Chinese descent in Los Angeles, the United States, and Singapore and those in China, Shanghai, and Jiashan in 1998 to 2002, based on Cancer Incidence in Five Continents IX (logarithmic scale) [5].
Discussion
In the present paper, covering cancers from the Swedish Cancer Registry through year 2006, we showed that the first-generation female immigrants in Sweden have a lower risk of breast cancer than the women from Sweden. The difference in risk was largest for women from developing countries, women from Turkey showing the lowest SIR of 0.45. Remarkably, the age-specific incidence curve for the Turkish immigrants (Fig. 3) was practically superimposable with the Izmir Turkish data (Fig. 1). The SIR of 0.45 for women from Turkey is in line, to the second decimal, with a previous study that we conducted with cancers followed through 1998, with less than one half of the number of cases in immigrants and even relatively fewer cases in non-European immigrants [13]. The difference in risk between the natives of Sweden and the immigrants from developing countries, such as China and India, was not as large as that cited in Cancer Incidence in Five Continents (about fourfold) [5]. The most important reason is that the non-European immigrants in Sweden are still relatively young [11] and, according to Figure 1, the incidence differences between developing and developed countries are mainly due to postmenopausal breast cancer. Other minor reasons may be that the local incidence rates differ because of geographic and socioeconomic factors and the emigrants are likely to be a selected active group of the population [14]. The duration of residence in Sweden has probably narrowed the difference in rates between the immigrants and the natives of Sweden, as has been observed for women of Italian descent living in Australia [15] and women of Asian descent living in California [16].
In a comparison of the age at diagnosis of breast cancer, a number of design features were introduced to guarantee unbiased results. The populations were matched on birth year, region, period, and reproductive factors. The follow-up was started 4 years after immigration to exclude individuals who might have entered Sweden for cancer treatment. The follow-up was terminated whenever any member of the case-control set ceased to be at risk. The results showed that women from nine regions, five from non-European and four from European countries, showed younger mean age of onset than their matched Swedish controls. The largest differences were 5.5 years for women from Turkey and 5.1 years for women from both Asian Arab and Other African countries. The diagnostic age for these women was around 45 years compared with 50 years for the Swedish matched controls. This difference is equal to comparing women with and without family history of breast cancer [17]. Notably, no immigrant group had a significantly higher mean age than the natives of Sweden. These data should settle the debated issue of whether the different age-specific rates between low-risk and high-risk countries are truly biological or whether they are recording artifacts because of rapidly changing incidence or missed elderly patients. Whether these biological effects are related to known or yet unknown risk factors will be discussed below.
Contribution of Known Risk Factors
Among the reproductive variables in Table 2, the countries with low age at breast cancer diagnosis, particularly the Middle East countries, were distinguished by high frequency of multiparity. However, when we divided the parity classes even further, the results remained. Thus, parity is an unlikely explanation to the findings. No data were available on the start of menstruation. According to the literature, however, no large differences have been reported for women from Turkey (12.4–13.3 years) and India (12.4–12.9 years) and female adolescents from Sweden (13 years) [18–20]. A study on London schoolgirls in 1980–1981 found mean ages at menarche as 13.6 years (of European descent), 13.2 years (of Afro-Caribbean descent), and 13.1 years (of Indo-Pakistani descent) [21]. Of course, we do not know how relevant these data are for women who migrated to Sweden some decades ago. Age at menarche is a relatively weak risk factor of breast cancer and the available data on ethnic variation do not support contribution by this factor. Another uncontrolled factor was the length of lactation, which is weakly protective of premenopausal breast cancer in white populations of women who have ever breastfed compared with those who never breastfed any children [22]. A study on women from China found a protective effect on all breast cancers but the shortest significant breastfeeding duration was 6–9 years, which is probably uncommon among immigrants to Sweden [23]. For the relevant birth cohorts of the present study, women born between 1930 and 1950, breastfeeding rates were at a historical low in Sweden around 1970 (2 months of breastfeeding, ∼35% of infants; 6 months, ∼10%) [24]. In 1950 and 1980 the rates were much higher (about 80% and 40%, respectively). Discounting this short period, the Swedish breastfeeding rates have been high in comparison with those of European countries, which does not help to explain the low age at diagnosis for women from Greece, Russia, Poland, and the former Yugoslavia [25]. Historical data from developing countries are limited but they suggest that lactation periods for many infants were extended to 2 years [26]. This trend appears to remain among immigrants to Sweden: 6-month breastfeeding rates were around 75% and not different from natives of Sweden, but 12-month rates were lower for natives of Sweden (18%) than for, for example, women from African (44%) and Middle Eastern (36%) countries [25]. We had no data on whether tumors were found in the national mammographic screening program. The participation of non-Nordic immigrants in this screening has been lower (about 80%) compared with that of natives of Sweden (90%) [27]. However, the difference is not large and the effect, if any, would be opposite to the present findings because the start of screening (between ages 40 and 50 years, depending on regional programs in Sweden) would probably advance the detection of breast cancers [28]. Finally, early age at diagnosis is a feature of familial and heritable cancers but there is no evidence that familial breast cancer or BRCA1/2 mutation carrier frequencies would be higher in non-European ethnic populations [29, 30]; a high familial cluster would not be compatible with the generally low incidence in immigrants.
Conclusions
The present data provide strong support to the notion that the age-incidence relationships for breast cancer are not registration artifacts between high-risk and low-risk populations. It is also unlikely that the differences were only due to cohort effects because they were uniform between the different Chinese populations (Fig. 4) and in many other populations in low-risk countries at various levels of development [10]. Additionally, according to the Danish and Swedish cancer registries (started in 1943 and 1958, respectively), age-specific incidence data have never resembled those from the developing countries, although cohort effects have been observed over the years [31]. In low-risk immigrant populations premenopausal breast cancer is relatively more common than in natives of Sweden. It is not obvious from the discussion in the above paragraph that the identified biological factors modulating risk would explain the differences. Tumor characteristics can potentially be distinct in immigrants from low-risk areas but our preliminary data on histology and survival in breast cancer show only minor differences to the natives of Sweden. Instead, the Western lifestyle, low physical activity, and being overweight may contribute and these should be targeted in prevention [32, 33]. Western lifestyle with changing reproductive patterns and improved access to diagnostics is also contributing to the increasing breast cancer rates in developing countries [34, 35]. Moreover, even studies among Swedish immigrants show that many elderly immigrants, particularly from the Middle East, have become physically inactive with ensuing weight gain, which may unfavorably influence their breast cancer risk in the long run [36–38]. Increasing rates, particularly for postmenopausal breast cancer, have also been noted for women of Asian American/Pacific Islander descent living in the United States and California [8, 39]; in Sweden, trend analysis by individual immigrant groups is not yet possible because the residence time of the low-risk immigrants is too short.
Acknowledgments
This work was supported by the German Cancer Research Center (DKFZ) guest scientist program, the Swedish Council for Working Life and Social Research, the Swedish Cancer Society, and Deutsche Krebshilfe. The used database was created by linking registers maintained at Statistics Sweden and the Swedish Cancer Registry.
Author Contributions
Conception/Design: Kari Hemminki, Seyed Mohsen Mousavi
Provision of study material or patients: Kari Hemminki, Jan Sundquist
Collection and/or assembly of data: Kari Hemminki, Jan Sundquist
Data analysis and interpretation: Seyed Mohsen Mousavi, Andreas Brandt
Manuscript writing: Kari Hemminki, Seyed Mohsen Mousavi, Jan Sundquist, Andreas Brandt
Final approval of manuscript: Kari Hemminki, Seyed Mohsen Mousavi, Jan Sundquist, Andreas Brandt
References
- 1.Tavassoli F, Devilee P, editors. Tumours of the breast and female genital organs. Lyon: IARC Press; 2003. [Google Scholar]
- 2.Mousavi SM, Montazeri A, Mohagheghi MA, et al. Breast cancer in Iran: an epidemiological review. Breast J. 2007;13:383–391. doi: 10.1111/j.1524-4741.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 3.Kwong A, Cheung P, Chan S, et al. Breast cancer in Chinese women younger than age 40: are they different from their older counterparts? World J Surg. 2008;32:2554–2561. doi: 10.1007/s00268-008-9589-6. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Sitas F, Chirenje M, et al. Part I: Cancer in Indigenous Africans–burden, distribution, and trends. Lancet Oncol. 2008;9:683–692. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- 5.Curado M, Edwards B, Shin H, et al., editors. IARC. Cancer Incidence in Five Continents. Vol. IX. Lyon: IARC; 2007. [Google Scholar]
- 6.Hemminki K, Rawal R, Lorenzo Bermejo J. Mammographic screening is dramatically changing age-incidence data for breast cancer. J Clin Oncol. 2004;22:4652–4653. doi: 10.1200/JCO.2004.04.156. [DOI] [PubMed] [Google Scholar]
- 7.Hemminki K, Lorenzo Bermejo J. Effects of screening for breast cancer on its age-incidence relationships and familial risk. Int J Cancer. 2005;117:145–149. doi: 10.1002/ijc.21149. [DOI] [PubMed] [Google Scholar]
- 8.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 9.Vorobiof DA, Sitas F, Vorobiof G. Breast cancer incidence in South Africa. J Clin Oncol. 2001;19:125S–127S. [PubMed] [Google Scholar]
- 10.Mousavi SM, Zheng T, Dastgiri S, et al. Age distribution of breast cancer in the middle East, implications for screening. Breast J. 2009;15:677–679. doi: 10.1111/j.1524-4741.2009.00843.x. [DOI] [PubMed] [Google Scholar]
- 11.Hemminki K, Ji J, Brandt A, et al. The Swedish Family-Cancer Database 2009: prospects for histology-specific and immigrant studies. Int J Cancer. 2009;29 doi: 10.1002/ijc.24795. in press. [DOI] [PubMed] [Google Scholar]
- 12.Hemminki K, Li X, Plna K, et al. The nation-wide Swedish Family-Cancer Database: updated structure and familial rates. Acta Oncol. 2001;40:772–777. doi: 10.1080/02841860152619214. [DOI] [PubMed] [Google Scholar]
- 13.Hemminki K, Li X, Czene K. Cancer risks in first generation immigrants to Sweden. Int J Cancer. 2002;99:218–228. doi: 10.1002/ijc.10322. [DOI] [PubMed] [Google Scholar]
- 14.Parkin DM, Khlat M. Studies of cancer in migrants: rationale and methodology. Eur J Cancer. 1996;32A:761–771. doi: 10.1016/0959-8049(96)00062-7. [DOI] [PubMed] [Google Scholar]
- 15.McMichael AJ, Giles GG. Cancer in migrants to Australia: extending the descriptive epidemiological data. Cancer Res. 1988;48:751–756. [PubMed] [Google Scholar]
- 16.Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 17.Brandt A, Lorenzo Bermejo J, Sundquist J, et al. Breast cancer risk in women who fulfill high-risk criteria: at what age should surveillance start? Breast Cancer Res Treat. 2010;121:133–141. doi: 10.1007/s10549-009-0486-y. [DOI] [PubMed] [Google Scholar]
- 18.Vicdan K, Kukner S, Dabakoglu T, et al. Demographic and epidemiologic features of female adolescents in Turkey. J Adolesc Health. 1996;18:54–58. doi: 10.1016/1054-139x(95)00127-e. [DOI] [PubMed] [Google Scholar]
- 19.Semiz S, Kurt F, Kurt DT, et al. Pubertal development of Turkish children. J Pediatr Endocrinol Metab. 2008;21:951–961. doi: 10.1515/jpem.2008.21.10.951. [DOI] [PubMed] [Google Scholar]
- 20.Proos L. Growth and development of Indian children adopted in Sweden. Indian J Med Res. 2009;130:646–650. [PubMed] [Google Scholar]
- 21.Ulijaszek SJ, Evans E, Miller DS. Age at menarche of European, Afro-Caribbean and Indo-Pakistani schoolgirls living in London. Ann Hum Biol. 1991;18:167–175. doi: 10.1080/03014469100001502. [DOI] [PubMed] [Google Scholar]
- 22.Stuebe AM, Willett WC, et al. Lactation and incidence of premenopausal breast cancer: a longitudinal study. Arch Intern Med. 2009;10;169:1364–1371. doi: 10.1001/archinternmed.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan JM, Yu MC, Ross RK, et al. Risk factors for breast cancer in Chinese women in Shanghai. Cancer Res. 1988;48:1949–1953. [PubMed] [Google Scholar]
- 24.Hofvander Y, Sjolin S. Breast feeding trends and recent information activities in Sweden. Acta Paediatr Scand Suppl. 1979;275:122–125. doi: 10.1111/j.1651-2227.1979.tb06171.x. [DOI] [PubMed] [Google Scholar]
- 25.Wallby T, Hjern A. Region of birth, income and breastfeeding in a Swedish county. Acta Paediatr. 2009;98:1799–1804. doi: 10.1111/j.1651-2227.2009.01455.x. [DOI] [PubMed] [Google Scholar]
- 26.Grummer-Strawn L. The effect of changes population characteristics on breastfeeding trends in fifteen developing countries. Int J Epidemiol. 1996;25:94–102. doi: 10.1093/ije/25.1.94. [DOI] [PubMed] [Google Scholar]
- 27.Lagerlund M, Maxwell AE, Bastani R, et al. Sociodemographic predictors of non-attendance at invitational mammography screening–a population-based register study (Sweden) Cancer Causes Control. 2002;13:73–82. doi: 10.1023/a:1013978421073. [DOI] [PubMed] [Google Scholar]
- 28.IARC. Breast cancer screening. Lyon: IARC Press; 2002. [Google Scholar]
- 29.Brandt A, Bermejo JL, Sundquist J, et al. Age of onset in familial cancer. Ann Oncol. 2008;19:2084–2088. doi: 10.1093/annonc/mdn527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liede A, Narod SA. Hereditary breast and ovarian cancer in Asia: genetic epidemiology of BRCA1 and BRCA2. Hum Mutat. 2002;20:413–424. doi: 10.1002/humu.10154. [DOI] [PubMed] [Google Scholar]
- 31.Rostgaard K, Väth M, Holst H, et al. Age-period-cohort modelling of breast cancer incidence in the Nordic countries. Statist Med. 2001;20:47–61. doi: 10.1002/1097-0258(20010115)20:1<47::aid-sim613>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein L. Identifying population-based approaches to lower breast cancer risk. Oncogene. 2008;27(Suppl 2):S3–S8. doi: 10.1038/onc.2009.348. [DOI] [PubMed] [Google Scholar]
- 33.IARC. Weight Control and Physical Activity. Lyon: IARC; 2002. [Google Scholar]
- 34.El Saghir NS, Khalil MK, Eid T, et al. Trends in epidemiology and management of breast cancer in developing Arab countries: a literature and registry analysis. Int J Surg. 2007;5:225–233. doi: 10.1016/j.ijsu.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Linos E, Spanos D, Rosner BA, et al. Effects of reproductive and demographic changes on breast cancer incidence in China: a modeling analysis. J Natl Cancer Inst. 2008;100:1352–1360. doi: 10.1093/jnci/djn305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wandell PE, Ponzer S, Johansson SE, et al. Country of birth and body mass index: a national study of 2,000 immigrants in Sweden. Eur J Epidemiol. 2004;19:1005–1010. doi: 10.1007/s10654-004-0159-4. [DOI] [PubMed] [Google Scholar]
- 37.Gadd M, Sundquist J, Johansson SE, et al. Do immigrants have an increased prevalence of unhealthy behaviours and risk factors for coronary heart disease? Eur J Cardiovasc Prev Rehabil. 2005;12:535–541. doi: 10.1097/01.hjr.0000174829.25388.ed. [DOI] [PubMed] [Google Scholar]
- 38.Faskunger J, Eriksson U, Johansson SE, et al. Risk of obesity in immigrants compared with natives of Sweden in two deprived neighbourhoods. BMC Public Health. 2009;9:304. doi: 10.1186/1471-2458-9-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deapen D, Liu L, Perkins C, et al. Rapidly rising breast cancer incidence rates among Asian-American women. Int J Cancer. 2002;99:747–750. doi: 10.1002/ijc.10415. [DOI] [PubMed] [Google Scholar]