The combination of high doses of methotrexate (MTX) and cytarabine (araC) is the standard chemotherapy for patients with primary CNS lymphoma (PCNSL). The addition of an alkylating agent could improve MTX-araC efficacy because it is active against quiescent G0 cells and increases antimetabolites cytotoxicity. MTX, araC, and thiotepa and MTX-araC combinations showed similar tolerability, whereas araC dose reduction was associated with a remarkably lower efficacy, hiding any potential benefit of thiotepa. Four doses of araC 2 g/m2 per course are recommended in patients with primary CNS lymphoma.
Keywords: Primary central nervous system lymphoma, Methotrexate, Cytarabine, IELSG prognostic score, Chemotherapy, Thiotepa
Abstract
Background.
The combination of high doses of methotrexate (MTX) and cytarabine (araC) is the standard chemotherapy for patients with primary CNS lymphoma (PCNSL). The addition of an alkylating agent could improve MTX-araC efficacy because it is active against quiescent G0 cells and increases antimetabolites cytotoxicity. A pilot experience with high doses of MTX, araC, and thiotepa (MAT regimen) was performed to investigate feasibility and efficacy of adding an alkylating agent. With respect to MTX-araC combination, araC dose was halved to minimize toxicity. Herein, we report tolerability, activity, and efficacy of MAT regimen and compare these results to those previously reported with MTX/ara-C combination.
Methods.
Twenty HIV-negative patients with PCNSL treated with MAT regimen and whole-brain irradiation and selected according to eligibility criteria of the International Extranodal Lymphoma Study Group (IELSG) #20 trial were analyzed.
Results.
Patient characteristics of MAT and MTX-araC series were similar. G4 hematologic toxicity was common after MAT chemotherapy, with dose reductions in 60% of patients, infections in 20%, G4 non-hematologic toxicity in 15%, and one (5%) toxic death. Response after chemotherapy was complete in four patients (clinical response rate, 20%; 95% confidence interval, 3%–37%) and partial in three (overall response rate, 35%; 95% confidence interval, 15%–55%). Fifteen patients experienced failure and 16 died (median follow-up, 26 months), with a 2-year overall survival of 24% ± 9%.
Conclusions.
MAT and MTX-araC combinations showed similar tolerability, whereas araC dose reduction was associated with a remarkably lower efficacy, hiding any potential benefit of thiotepa. Four doses of araC 2 g/m2 per course are recommended in patients with PCNSL.
Introduction
Therapeutic progress in primary central nervous system lymphomas (PCNSL) is modest because of three main aspects: the rarity of these tumors that hampers the conduction of randomized trials, the limited molecular and biologic knowledge, and the difficulties in conducting prospective trials testing new potentially active drugs in patients with relapsed/refractory disease. In this context, a recently reported international randomized trial (the IELSG #20 trial) [1], the only one with completed accrual, demonstrated that, in patients ≤75 years old with PCNSL, the combination of high doses of methotrexate (MTX) and cytarabine (araC) results in consistently better outcome and acceptable toxicity over high-dose MTX (HD-MTX) alone. MTX-araC followed by whole-brain radiotherapy (WBRT) has been associated with a 5-year overall survival (OS) of 45%, with a plateau in survival curve after the third year [1]. Thus, MTX-araC combination should be considered as the standard chemotherapy regimen for patients with PCNSL because it is supported by the best level of evidence available in this field [2, 3].
Despite the benefit of the addition of HD-araC, current results in PCNSL patients remain unsatisfactory. According to the worldwide used therapeutic strategies for aggressive lymphomas, it is unthinkable to treat PCNSL exclusively with antimetabolites, and the identification of other drugs active against different phases of the tumor cell cycle is an unmet need. Some alkylating agents (i.e., temozolomide, ifosfamide, thiotepa, and nitrosoureas) are interesting candidates because they are able to cross the blood-brain barrier, exhibit antilymphoma activity, are active against phase-G0 cells, and increase cytotoxicity of antimetabolites. The risk/benefit ratio of the addition of one of these agents to the MTX-araC combination deserves to be investigated to improve chemotherapy efficacy in PCNSL patients.
In the time interval between the conclusion of the accrual of the IELSG #20 trial (accrual completed by December 2007) and the start of a new randomized trial, participating centers treated patients with PCNSL with a combination of HD-MTX, HD-araC, and thiotepa (MAT regimen) to investigate the role of this alkylating agent. To reduce potential toxicity, araC dose was decreased from 2 to 1 g/m2, and feasibility, tolerability, activity, and efficacy were monitored. This paper reports the results of this pilot clinical experience, and comparison with the outcome of MTX-araC standard combination reported in the IELSG #20 trial [1] seems to suggest that araC dose reduction may have a negative effect on activity and efficacy.
Patients and Methods
Study Group
HIV-negative patients with PCNSL diagnosed during 2008 at five Italian Centers and one Swiss Center were treated with MAT chemotherapy and WBRT. Patients were selected following the same eligibility criteria of the IELSG #20 trial [1]: (a) diagnosis of non-Hodgkin lymphoma performed on stereotactic or surgical biopsy, cerebrospinal fluid (CSF) cytology examination, or vitrectomy; (b) disease exclusively localized in the CNS, cranial nerves, or eyes; (c) no previous treatment apart from steroids; (d) at least one measurable lesion; (e) age 18–75 years; (f) Eastern Cooperative Oncology Group (ECOG) performance status ≤3; (g) adequate bone marrow, renal, cardiac, and hepatic function. Patients with HBsAg positivity, hepatitis C virus (HCV) seropositivity, other malignancies, and pregnant or lactating status were not considered. Written informed consent was obtained from each patient once eligibility was confirmed and after patient received a complete illustration of treatment modalities, acute and late side effects, and efficacy perspectives. The study was approved by the Ethics Committee of the San Raffaele Scientific Institute, Milan.
MAT Regimen
Considered patients were treated with four courses of MTX 3.5 g/m2 day 1; araC 1 g/m2, 1-hour infusion, twice a day (every 12 hours), days 2 and 3; and thiotepa 30 mg/m2 day 4; recycling every 3 weeks. The first 0.5 g/m2 of MTX were administered in 15 minutes followed by a 3-hour infusion of 3 g/m2. Patients received adequate pre- and post-MTX hydration, urinary alkalinization, and folinic rescue [1]. Intrathecal chemotherapy was not included in the chemotherapy regimens. Dexamethasone dose depended on clinical requirements. rHuG-CSF support from day 7 to day 12 of every course in association with antimicrobial prophylaxis was strongly recommended. Cytostatic dose reductions were determined according to grade and type of toxicity. Dose intensity was estimated as previously reported [4]. Patients in complete remission (CR), partial response (PR), or stable disease (SD) after two chemotherapy courses received two more courses of the same regimen. Patients who achieved CR, PR, or SD after the fourth course were referred to WBRT; patients who experienced progressive disease (PD) at any time were referred to salvage therapy according to physician's preferences.
Radiation Therapy
Complementary WBRT started within 4 weeks from the last chemotherapy course. Photons of 4–10 MeV, 180 cGy per day, 5 weekly fractions were used. Whole brain was irradiated by two opposite lateral fields including the first two segments of cervical spinal cord and the posterior two thirds of the orbits, which had to be shielded after 30 Gy (after 36 Gy in the case of intraocular disease). Tumor bed was irradiated by 2–4 isocentric fields based on tumor location; in the case of multifocal lesions, the boost volume included each single lesion. Radiation dose was chosen according to response after chemotherapy: patients in CR were treated with 36 Gy WBRT; patients in PR were treated with 36 Gy WBRT plus a tumor-bed boost of 9 Gy; patients with SD or PD were treated with WBRT 40 Gy plus a 9 Gy boost.
Clinical Evaluation and Staging
Staging work-up and pretreatment tests included physical examination, biochemical serum profile, HIV, hepatitis B virus and HCV serological evaluation, echocardiography, thorax-abdomen CT scan, whole-brain MRI, bone marrow biopsy, ophthalmologic evaluation, and CSF examination. Risk groups were defined according to the IELSG score [5]. Patients in whom lumbar puncture was contraindicated were considered as having an unfavorable feature for CSF protein level variable.
Toxicity and Response Assessment
Treatment side effects were assessed separately for each chemotherapy course and graded according to the NCI-NCIC CTC version 3.0 [6]. The worst toxicity per organ, per patient, was considered for analyses. Response to treatment was assessed on contrast-enhanced brain MRI performed immediately before chemotherapy and repeated after the second and fourth courses and after WBRT. Response definition was based on changes in tumor size of enhanced lesions on T1-weighted MRI, and following the NCI standardized response criteria [7]. In brief, CR was defined as the complete disappearance of all evidence of lymphoma; PR was defined as ≥50% decrease in tumor size; PD was defined as ≥25% increase in tumor size or the appearance of any new tumor lesion; SD was defined as situations that did not meet any of the previous criteria. In cases with concomitant positive CSF, cytology examination was performed after the second and fourth courses of chemotherapy and after treatment completion; a reduction of >50% of cell number was considered PR, whereas a lower reduction was considered SD. The maximum response recorded from treatment start was considered for activity analyses. The duration of response was measured from the date of maximum response (CR or PR) to the date of objective progression or last date of follow-up in the absence of progression.
After the end of treatment, the disease was assessed every 3 months for the first 3 years, every 6 months during the fourth and fifth years, and every year thereafter. After progression, patients were followed every 3 months for survival, and they returned to the previous follow-up schedule in the case of a second remission.
Statistical Considerations
Survival curves were generated using the Kaplan-Meier method. OS was calculated from the first chemotherapy course date to death or to the last date of follow-up; failure-free survival (FFS) was calculated from the first chemotherapy course date to relapse, progression, or death, or to the last date of follow-up. A death for any cause without relapse/progression was considered as an event in FFS analysis. Survival rates were reported as 3-year FFS and OS ± standard error. All the probability values were two-sided. All analyses were carried out using the Statistica 4.0 statistical package for Windows (Statsoft Inc., 1993, Tulsa, OK, USA).
Results
Patients' Characteristics
Twenty patients with PCNSL were treated between January and December 2008. Distribution of patients' characteristics in this series was similar to those of patients treated with MTX-araC combination in the IELSG #20 trial [1] (Table 1). Increased lactate dehydrogenase serum levels and positive CSF cytology were more common among patients treated with MAT, whereas involvement of deep regions of the CNS and high CSF protein level were more common in patients treated in the IELSG #20 trial.
Table 1.
Patient characteristics and distribution of lymphoma categories
aSeries treated with MTX-araC conventional combination reported in ref [1].
bTissue sample for diagnosis was obtained by surgical partial resection in 5 patients and by stereotactic biopsy in 15.
Chemotherapy Feasibility and Tolerability
Fifty-five (69%) of the 80 planned courses were actually delivered: 7 patients received four courses, 5 patients received three courses, 4 received two courses, and 4 patients received a single course. Causes of chemotherapy interruption were PD in 11 patients, toxicity in 1, and physician's preference in 1. As expected, hematologic toxicity was common (Table 2). As main complications, febrile neutropenia was observed in three patients and Staphilococcus heamoliticus septicemia and Clostridium difficile diarrhea were observed in one. There was a single toxic death: a 64-year-old woman in partial lymphoma response after the second MAT course experienced acute abdominal pain with radiological suspicion of colic perforation; despite timely antibiotic therapy, the patient developed infectious peritonitis and died of septic shock. CMV reactivation was observed in three patients; all of them were diagnosed during the first two courses, were responsive to gancyclovir and/or valgancyclovir, but resulted in chemotherapy delay of up to 3 weeks. G4 nonhematologic toxicities were uncommon. Dose reduction ≥25% was indicated in 12 patients: of araC alone in 3, MTX and araC in 1, araC and thiotepa in 4, and the three drugs in 4. Median relative dose intensity of MTX, araC, and thiotepa was 75%, 67%, and 72%, respectively.
Table 2.
Toxicity
The worst toxicity per organ, per patient was considered for analyses.
aSeries treated with MTX-araC conventional combination reported in [1].
Abbreviations: DVT, deep venous thrombosis; GI, gastrointestinal.
Activity
At the completion of chemotherapy, 4 patients (20%; 95% confidence interval [CI], 3%–37%) achieved a CR, and 3 patients achieved a PR, with an overall response rate of 35% (95% CI, 15%–55%); 12 patients experienced PD and 1 died of toxicity. The 4 patients in CR and the 3 patients in PR after chemotherapy were treated with WBRT, with PD during irradiation in 2 patients. Conversely, only 4 of the 12 patients in PD after chemotherapy were irradiated, with a transient PR in one of them and early PD in the others. No patient interrupted WBRT due to acute toxicity. At the end of the first-line treatment (i.e., chemo- ± radiotherapy), 5 patients achieved a CR (25%; 95% CI, 7%–43%), with a remission duration of 17+, 22+, 25, 30+, and 34+ months, respectively.
Efficacy and Salvage Therapy
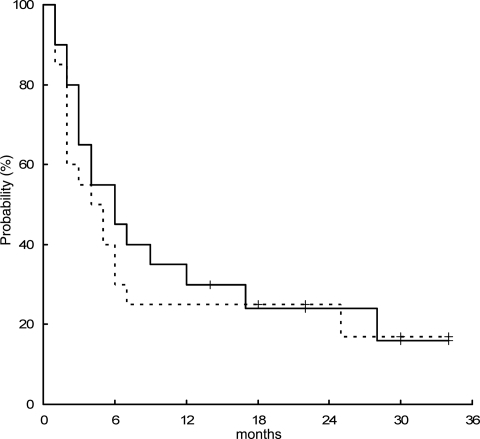
At a median follow-up of 26 months, 14 patients experienced PD during treatment, 1 patient experienced extra-CNS relapse (retroperitoneal lymph nodes) after response, and 1 died of toxicity. At progression or relapse, lymphoma involved the primary site of disease in 13 (87% of failures) patients, meninges in 1 (7%), and extra-CNS organs in 1 (7%) patient. The 2-year FFS was 25% ± 9% (Fig. 1). Salvage therapy consisted of WBRT in 4 patients (PD during MAT), ifosfamide-based chemotherapy in 1 (PD during MAT), and R-CHOP chemoimmunotherapy in 1 (extra-CNS relapse), with transient clinical benefit in only 1 patient; the other 9 failed patients (7 PD during MAT; 2 PD during WBRT) did not receive salvage therapy because of rapid neurological impairment. Sixteen patients died: 1 died of toxicity during MAT, 1 died of septic shock during salvage therapy with R-CHOP, a 74-year-old woman died of progressive neurological impairment while relapse-free, and 13 patients died of lymphoma. Four patients are alive at 14+, 22+, 30+, and 34+ months, with a 2-year OS of 24% ± 9% (Fig. 1).
Figure 1.
Failure-free survival (dotted line) and overall survival curves of the whole series treated with MTX, araC, and thiotepa regimen.
Discussion
This pilot experience on MAT regimen highlights the critical role played by araC dose in the upfront treatment of patients with PCNSL. MAT regimen was an effort to improve outcome with a reduced toxicity with respect to the conventional MTX-araC combination investigated in the randomized IELSG #20 trial [1]. There are two major differences between the MAT regimen and the MTX-araC combination: the addition of thiotepa and the reduction of araC dosage from four doses of 2 g/m2 to four doses of 1 g/m2. In the present series, MAT regimen followed by WBRT was associated with a clinical response rate of 25%, a 2-year FFS of 25%, and a 2-year OS of 24%. These figures are very similar to those reported with HD-MTX alone in the IELSG #20 trial [1], suggesting that a substantial dose reduction could cancel the significant benefit gained by the addition of araC to HD-MTX in patients ≤75 years old with PCNSL.
The major limitations of the present study regards the intrinsic caveats of any noncomparative study. However, this study was conducted by centers with adequate expertise to treat patients with PCNSL that have actively participated to the IELSG #20 trial [1]. Patients were consecutive and selected with the same criteria of the randomized trial, and no eligible patient was excluded. In fact, patient characteristics of both series were very similar, and only a minor difference in the proportion of high-risk patients in the MAT study was observed. Drugs administration schedules, toxicity criteria, response assessment and definition, and follow-up modalities were the same ones used in the randomized trial. Thus, any macroscopic difference between outcomes of both studies should be bone fide attributed to changes in araC dose or thiotepa administration.
Toxic profile of MAT regimen was not significantly different than those reported with MTX-araC; only a slightly higher hematologic toxicity was observed, but this did not have clinical relevance. The addition of thiotepa was not associated with higher rates of septic complications or nonhematologic toxicities. Importantly, only one patient interrupted chemotherapy due to toxicity (the case of toxic death); all the other interruptions were due to lymphoma progression. The proportion of patients treated with dose reductions (60%) or with chemotherapy delay (45%; median cumulative delay, 6 weeks; range, 3–11) was similar to those reported with MTX-araC [1]. Taken together, these results suggest that the addition of thiotepa was not associated with a higher toxicity, and this aspect cannot per se explain the lower efficacy observed with MAT combination.
The reduced success of the MAT regimen should be attributed to the other variable, that is, the reduction of araC delivered dose. The comparison of median relative dose intensity of MTX (77% versus 75%) and araC (68% versus 67%) in MTX + araC standard combination and MAT regimen, respectively, seems to exclude interfering factors related to chemotherapy delay or dose adjustments. Conversely, it is remarkable that patients treated with MAT regimen received araC with a median dose intensity of 898 mg/m2 per week in comparison with the 1.808 mg/m2 per week delivered with standard MTX-araC combination. This could be the actual cause of disappointing results with MAT.
AraC pharmacokinetics could better explain the negative impact of dose reduction in patients with PCNSL. This drug exhibits a dose-related CSF bioavailability [8], with CSF levels of 10%–15% of steady-state plasmatic concentrations [9], and a significant direct correlation between araC dose and CSF end-dose ara-U levels [10]. In a pharmacokinetic study performed on 19 patients with acute leukemia [10], direct correlations between the dose of araC administered and peak plasma araC levels and CSF concentrations have been shown, with a mean end-dose CSF araC level following 3 g/m2 of araC of fourfold the mean end-dose CSF araC level following 0.75 g/m2. In a pharmacokinetics study on 18 patients with acute leukemia or aggressive lymphomas [8], the administration of 1 g/m2 of araC has been associated with a mean drug concentration in the CSF of 347 and 123 ng/ml at 3 and 6 hours, respectively, whereas these concentrations were 1.070 and 507 ng/ml when delivered dose was 3 g/m2, with a mean half-life 3 times longer with the higher dose (2.3 versus 6.3 hours). Importantly, plasmatic concentration of araC has been under the therapeutic drug level at 4 hours in patients treated with 1 g/m2, whereas a therapeutic concentration was detectable even after 6 hours when the delivered dose was 3 g/m2 [8]. Although a comparison between 1 and 2 g/m2 has not been performed in that study [8], there are several pieces of evidence in literature that an araC dose of at least 2 g/m2 is associated with cytotoxic concentrations in the CSF of leukemia patients [11]. Despite variations in administration schedule of araC among reported studies and the existence of a wide interpatient unpredictability in araC levels, delivered dose of araC strongly conditions drug concentration, therapeutic level, and half-lives both in CSF and plasma, which could have a sensible effect on efficacy in the treatment of PCNSL.
In conclusion, MAT experience shows that, when administered in combination with HD-MTX, araC dose reduction to 1 g/m2 is associated with disappointing results in patients with PCNSL. Therefore, four doses of araC at 2 g/m2, as used in the standard MTX-araC combination of the IELSG #20 trial [1], are recommended in the first-line treatment for PCNSL. Accordingly, this araC administration schedule has been included in the three treatment arms of our new PCNSL randomized trial named IELSG #32 (http://clinicaltrials.gov: NCT01011920).
Author Contributions
Conception/Design: Andrés J. M. Ferreri, Michele Reni
Provision of study materials or patients: Andrés J. M. Ferreri, Marco Foppoli, Gaetano Corazzelli, Emanuele Zucca, Caterina Stelitano, Francesco Zaja, Sergio Fava, Rossella Paolini, Alberto Franzin, Letterio Politi, Michele Reni
Collection and/or assembly of data: Andrés J. M. Ferreri, Giada Licata, Gaetano Corazzelli, Emanuele Zucca, Caterina Stelitano, Francesco Zaja, Sergio Fava, Rossella Paolini, Letterio Politi
Data analysis and interpretation: Andrés J. M. Ferreri, Maurilio Ponzoni, Michele Reni
Manuscript writing: Andrés J. M. Ferreri, Maurilio Ponzoni, Michele Reni
Final approval of manuscript: Andrés J. M. Ferreri, Giada Licata, Marco Foppoli, Gaetano Corazzelli, Emanuele Zucca, Caterina Stelitano, Francesco Zaja, Sergio Fava, Rossella Paolini, Alberto Franzin, Letterio Politi, Maurilio Ponzoni, Michele Reni
References
- 1.Ferreri AJ, Reni M, Foppoli M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374:1512–1520. doi: 10.1016/S0140-6736(09)61416-1. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien PC, Seymour JF. Progress in primary CNS lymphoma. Lancet. 2009;374:1477–1478. doi: 10.1016/S0140-6736(09)61488-4. [DOI] [PubMed] [Google Scholar]
- 3.Ansell SM, Rajkumar SV. Hematology: trials and tribulations in primary CNS lymphoma. Nat Rev Clin Oncol. 2010;7:125–126. doi: 10.1038/nrclinonc.2010.9. [DOI] [PubMed] [Google Scholar]
- 4.Hryniuk WM, Goodyear M. The calculation of received dose intensity. J Clin Oncol. 1990;8:1935–1937. doi: 10.1200/JCO.1990.8.12.1935. [DOI] [PubMed] [Google Scholar]
- 5.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 6.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 8.Slevin ML, Piall EM, Aherne GW, et al. Effect of dose and schedule on pharmacokinetics of high-dose cytosine arabinoside in plasma and cerebrospinal fluid. J Clin Oncol. 1983;1:546–551. doi: 10.1200/JCO.1983.1.9.546. [DOI] [PubMed] [Google Scholar]
- 9.Breithaupt H, Pralle H, Eckhardt T, et al. Clinical results and pharmacokinetics of high-dose cytosine arabinoside (HD ARAC) Cancer. 1982;50:1248–1257. doi: 10.1002/1097-0142(19821001)50:7<1248::aid-cncr2820500705>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Damon LE, Plunkett W, Linker CA. Plasma and cerebrospinal fluid pharmacokinetics of 1-beta-D-arabinofuranosylcytosine and 1-beta-D-arabinofuranosyluracil following the repeated intravenous administration of high- and intermediate-dose 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1991;51:4141–4145. [PubMed] [Google Scholar]
- 11.Takashima Y, Matsuyama K. Pharmacokinetic studies of intermediate-to high-dose 1-beta-D-arabinofuranosylcytosine in children with acute leukemia and lymphoma. J Clin Pharmacol. 1987;27:330–333. doi: 10.1002/j.1552-4604.1987.tb03025.x. [DOI] [PubMed] [Google Scholar]