The presenting characteristics and outcomes of patients with advanced/metastatic non-small cell lung cancer treated in MD Anderson Cancer Center phase I clinical studies are reported.
Keywords: Phase I, Non-small cell lung cancer, Survival
Abstract
Background.
The outcomes of patients with advanced non-small cell lung cancer (NSCLC) treated in phase I clinical trials have not been systematically analyzed.
Methods.
We reviewed the records of consecutive patients with advanced/metastatic NSCLC who were treated in the Phase I Clinical Trials Program at MD Anderson from August 2004 to May 2009.
Results.
Eighty-five patients (51 men, 34 women) treated on various phase I protocols were identified. The median age was 62 years (range, 30–85). The median number of previous systemic therapies was two (range, 0–5). A partial response was observed in eight patients (9.5%) and stable disease lasting >4 months was observed in 16 patients (19%). The median overall survival time was 10.6 months and median progression-free survival (PFS) time was 2.8 months, which was 0.6 months shorter than the median PFS of 3.4 months following prior second-line therapy. Factors predicting longer survival in the univariate analysis were an Eastern Cooperative Oncology Group performance status (PS) score of 0–1, no prior smoking, two or fewer organ systems involved, a hemoglobin level ≥12 g/dL, liver metastases, a history of thromboembolism, and a platelets count > 440 × 109/L. In the multivariate analysis, a PS score of 0–1 and history negative for smoking predicted longer survival. Sixty-two (73%) patients had grade ≤2 toxicity, and there were no treatment-related deaths.
Conclusion.
Phase I clinical trials were well tolerated by selected patients with advanced NSCLC treated at M.D. Anderson. Nonsmokers and patients with a good PS survived longer. PFS in our population was shorter in smokers/ex-smokers and patients with a PS score of 2. It is reasonable to refer pretreated patients with a good PS to phase I clinical trials.
Introduction
Lung cancer is the most frequent cause of cancer-related death in the U.S. [1]. Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancers [2]. Most patients are diagnosed with advanced/metastatic NSCLC, a setting in which palliative chemotherapy produces only modestly longer survival times and better quality of life [3, 4].
Patients progressing after first-line chemotherapy (platinum-containing doublets) can be treated with second-line docetaxel, pemetrexed, or erlotinib. Unfortunately, the response rate (RR) usually does not exceed 10%, with a progression-free survival (PFS) duration of approximately 2.5 months and overall survival (OS) time of 7–8 months [5–7]. After failing conventional treatment, patients with a good performance status (PS) are considered candidates for enrollment in phase I clinical trials [8].
Treatment outcomes of patients with advanced/metastatic NSCLC treated in phase I clinical trials have not been systematically analyzed. The Phase I Clinic at The University of Texas MD Anderson Cancer Center (MD Anderson) focuses on treating patients with targeted agents in early clinical trials, alone or in combination regimens. Here we report the presenting characteristics and outcomes of patients with advanced/metastatic NSCLC treated in our phase I clinical program.
Methods
Patients
An electronic chart review of consecutive patients with NSCLC treated in the Phase I Clinical Trials Program at MD Anderson from August 2004 to May 2009 was performed. Phase I clinical trials varied over time depending on protocol availability at the time of presentation.
Trial-eligible patients were ≥18 years old and had histologically proven (by an MD Anderson pathologist) NSCLC for which approved therapies were no longer effective. Disease was measured by the Response Evaluation Criteria in Solid Tumors (RECIST) [9]. PS was measured by the Eastern Cooperative Oncology Group (ECOG) PS score. Premenopausal women were required to have a negative pregnancy test, and patients of childbearing potential were required to use contraception. A washout period of 3–4 weeks preceding the initiation of each phase I therapy was required. Further eligibility criteria varied according to each specific study.
After initiation of an investigational therapy, patients were evaluated at 3- to 4-week intervals depending on the particular phase I protocol requirements. At each visit, a history review and physical examination were performed along with comprehensive metabolic and hematologic panels.
All data collection, trial participation, and consent procedures were performed in accordance with MD Anderson Institutional Review Board guidelines.
Endpoints and Statistical Methods
Responses were assessed from computed tomography scans and/or magnetic resonance imaging scans at baseline before treatment initiation and then every two cycles (one cycle is 3–4 weeks, depending on the protocol). All radiographs were read in the Department of Radiology at MD Anderson and reviewed in the Department of Investigational Cancer Therapeutics using the RECIST [9]. A waterfall plot analysis was used to illustrate antitumor efficacy, as previously described [10]. Toxicities were assessed using the National Cancer Institute Common Terminology Criteria (NCI CTC) for Adverse Events, version 3.0 [11].
The OS time was measured from the initiation of treatment with the first phase I trial drug administered until death. The PFS time was measured from the date of the first phase I trial drug administration until disease progression or death, whichever came first. Patients without an event (death for OS and progression or death for PFS) were censored at the time of their last follow-up. For patients enrolled on more than one phase I protocol, data for OS and PFS times from the time of enrollment in the first trial were used. OS and PFS probabilities were estimated using the method of Kaplan and Meier [12] and were compared among subgroups of patients using the log-rank test [13]. The PFS time in the phase I trial was compared with PFS times on previous therapies (first and second line). Univariate and multivariate Cox proportional hazards models [14] were fit to assess the association between prognostic factors and OS, in which the prognostic factors included: gender, age, ethnicity, histology, history of smoking, ECOG PS score, number of prior therapies, number of metastatic sites, hemoglobin, lactate dehydrogenase (LDH), albumin, liver metastases, history of thromboembolism, and platelet count >440 × 109/L. A p-value < .05 was considered statistically significant. All statistical analyses were carried out using SAS 9.1 (SAS Institute, Cary, NC), S-Plus version 7.0 (Insightful Corp., Seattle, WA), and SPSS version 17 (SPSS, Chicago, IL) software.
Results
Patient Characteristics
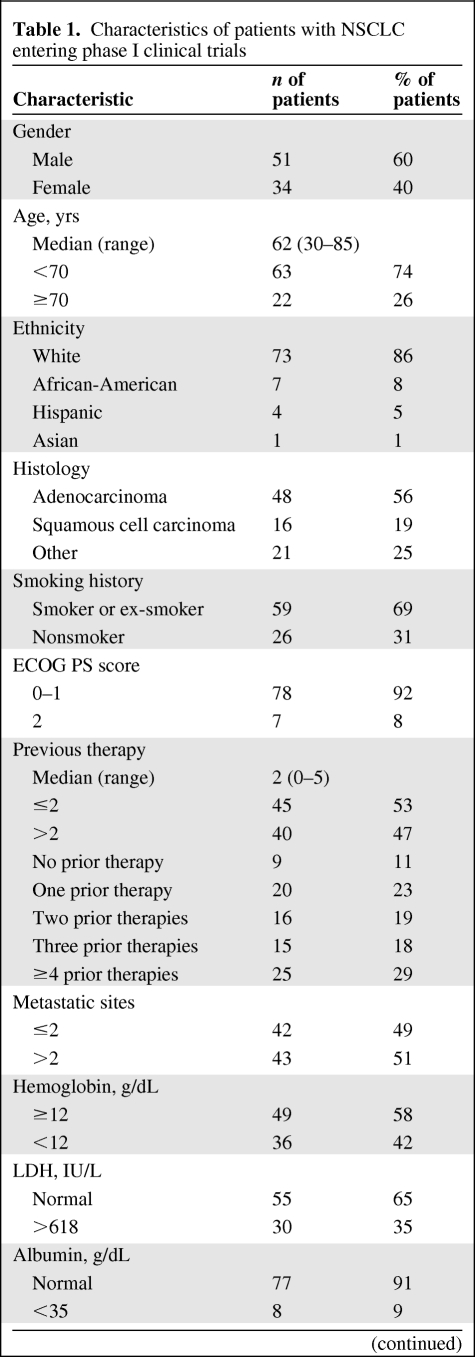
Overall, 85 patients with NSCLC treated in phase I clinical trials were identified (Table 1). There were 51 men and 34 women. The median age was 62 years (range, 30–85 years). Histologic subtypes were adenocarcinoma (n = 48), squamous cell carcinoma (n = 16), and other (n = 21). The most frequent site of distant metastasis was the contralateral lung (40 of 85, 47%), bones (24 of 85, 28%), and liver (18 of 85, 21%). Fifteen (18%) patients had brain metastases. Most patients had a PS score of 0–1 (Table 1). The median time from the diagnosis of advanced disease (stage III/IV) to phase I clinical trial initiation was 17.5 months (95% confidence interval [CI], 12.6–22.4 months).
Table 1.
Characteristics of patients with NSCLC entering phase I clinical trials
Table 1.
(Continued)
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; NSCLC, non-small cell lung cancer.
Of the 85 patients, 20 (23%) received only one line of prior therapy, 16 (19%) received two lines of prior therapy, 15 (18%) received three lines of prior therapy, and 25 (29%) received four or more lines of treatment (Table 1). Nine (11%) patients did not receive any prior therapy for advanced cancer for the following reasons: prior neoadjuvant/adjuvant therapy (n = 5) and referral to a phase I trial that included a platinum-containing doublet at standard doses in combination with experimental therapy (n = 4).
Treatment
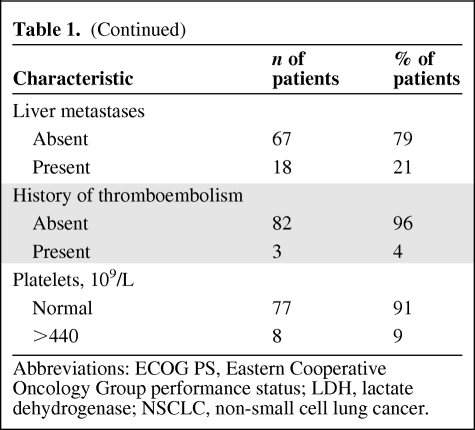
Of 85 patients treated, 50 (59%) received single-agent targeted phase I therapy [15–23], 14 (16%) received a combination of targeted agents, 13 (15%) received chemotherapy combined with targeted agents [24, 25], and eight (10%) received chemotherapy alone (Table 2) [26]. None of the patients was treated in a genotype-specific trial such as with anti–epidermal growth factor receptor (EGFR) therapy in patients with tumors harboring EGFR mutations or anti–anaplastic lymphoma kinase (ALK) therapy in tumors with EML4–ALK fusion. Patients who were entered in phase I clinical trials with single-agent targeted therapy were more heavily pretreated (60% received more than two lines of prior therapy) than patients entered in phase I trials with combinations of targeted therapies, combinations of targeted therapy and chemotherapy, and chemotherapy alone (26% received more than two prior therapies; p = .0004, Fisher's exact test).
Table 2.
Phase I treatment used in 85 NSCLC patients
Abbreviations: EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; VEGF, vascular endothelial growth factor.
Thirteen of 85 (15%) patients received further therapy in another phase I trial after disease progression on phase I treatment.
Response
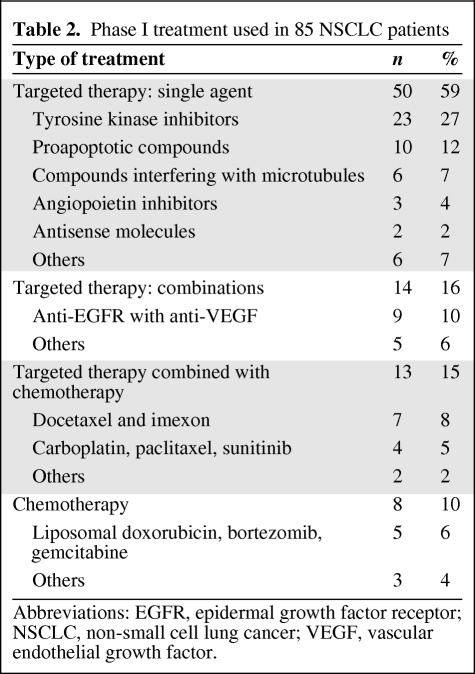
Baseline tumor measurements prior to phase I treatment initiation were available for all 85 patients. A decrease in tumor measurements (range, −1% to −60%) was observed in 26 (31%) patients (Fig. 1). A partial response (PR) was documented in eight (9.5%) patients and stable disease (SD) was documented in 45 (53%) patients, including 16 (19%) patients with SD lasting >4 months. Progressive disease (PD) was documented in 25 (29.5%) of 85 patients. Seven (8%) patients did not have restaging scans because of early treatment discontinuation (withdrawal of consent, n = 5; toxicity, n = 1; death, n = 1).
Figure 1.
Waterfall plot of patients with non-small cell lung cancer treated in phase I clinical trials. Twenty-six (31%) patients had some tumor shrinkage, including eight partial responses (9.5%). Patients treated with a combination of targeted therapy and chemotherapy (green bars) were most likely to respond (5 of 13, 38.5%).
Abbreviations: PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
PR rates in patients treated with single-agent targeted therapy, combination of targeted therapies, combinations of targeted therapy with chemotherapy, and chemotherapy alone were 2% (one of 50), 7% (one of 14), 38.5% (five of 13), and 12.5% (one of 8), respectively (p = .002, Fisher's exact test) (supplemental online Table 1).
OS
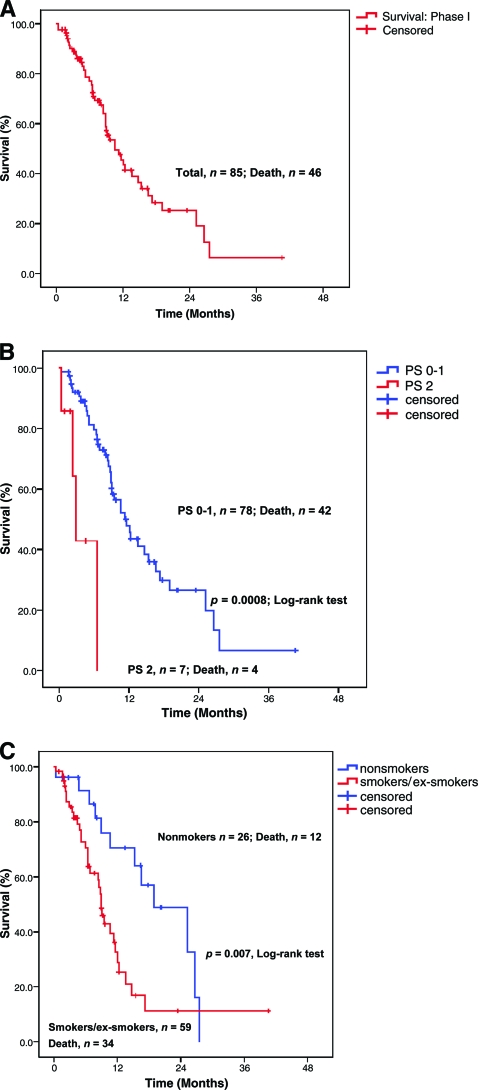
The median OS time in 85 patients treated in phase I studies was 10.6 months (95% CI, 8.9–14.7 months) (Fig. 2A). The 90-day mortality rate was 10.5% (nine of 85 patients). At the time of analysis, 39 patients (46%) were alive or lost to follow-up. The 1-year survival rate was 26%. Patients having a PR on a phase I therapy had a median OS time of 13.6 months (95% CI, 8.9 to not estimable), which was not significantly different from the median OS time of 10.6 months (95% CI, 8.5–14.7 months) for patients without a response (SD, PD, not assessed) (p = .17). Similarly, 24 patients with a PR or SD lasting >4 months had a median OS duration of 12.3 months (95% CI, 9.1–25.2 months), which was not significantly different from the median OS time of 9 months (95% CI, 6.9–14.7 months) in patients who had SD lasting ≤4 months, had PD, or were not assessed (p = .29). Thirteen (15%) patients who received further postprotocol phase I therapy had a median OS time of 25.2 months (95% CI, 10.6–26.6 months). Patients not receiving further phase I treatment had a median OS duration of 9.5 months (95% CI, 8.5–13.6 months; p = .10).
Figure 2.
Kaplan–Meier estimates of OS. (A): The median OS time in phase I trials was 10.6 months (95% CI, 8.9–14.7 months). (B): Patients with an ECOG PS score of 0–1 had a longer OS time than patients with an ECOG PS score of 2—11.6 months (95% CI, 8.7–14.4 months) versus 2.9 months (95% CI, 1.9–3.9 months) (p = .0008). (C): Nonsmokers had a longer OS time than smokers and ex-smokers—19.9 months (95% CI, 10.2–27.8 months) versus 9 months (95% CI, 7.9–10.1 months) (p = .007).
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; OS, overall survival; PS, performance status.
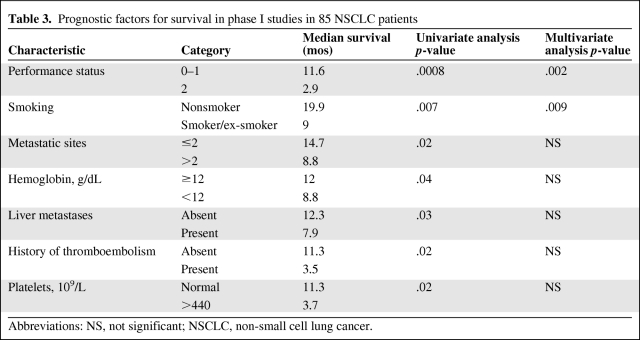
On univariate analysis, factors associated with a longer OS time were an ECOG PS score of 0–1 (p = .0008) (Fig. 2B), no prior smoking history (p = .007) (Fig. 2C), a lesser tumor burden (two or fewer metastatic sites; p = .02), a hemoglobin level ≥12 g/dL (p = .04), liver metastases (p = .03), a history of thromboembolism (p = .02), and a platelet count >440 × 109/L (p = .02) (Table 3). On multivariate analysis, factors associated with a longer OS time were an ECOG PS score of 0–1 (p = .002) and a history negative for smoking (p = .009) (Table 3).
Table 3.
Prognostic factors for survival in phase I studies in 85 NSCLC patients
Abbreviations: NS, not significant; NSCLC, non-small cell lung cancer.
PFS
The median PFS interval for phase I treatment was 2.8 months (95% CI, 2.4–3.4 months). Patients with a PR had a significantly longer median PFS time of 7.8 months (95% CI, 6.0–11.5 months) than patients without a response, who had a median PFS time of 2.6 months (95% CI, 2.3–3.1 months) (p = .0005). Patients who received up to two previous therapies had a longer median PFS time, 3.7 months, than patients pretreated with more than two prior therapies, who had a median PFS time of 2.4 months (p = .004).
Comparison of PFS in Phase I Trials with PFS on Previous Lines of Therapy
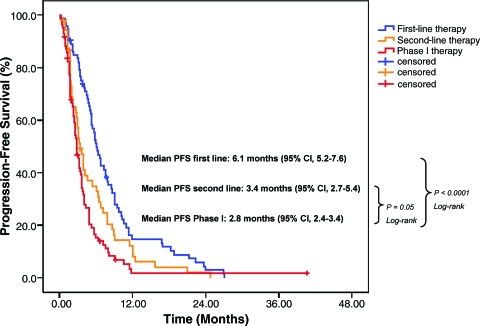
Of 85 patients treated in phase I trials, 76 (89%) received previous first-line therapy and 56 (66%) received previous second-line therapy. Three patients were excluded from the first- and second-line therapy PFS analysis because of insufficient data. The median PFS interval of 2.8 months for phase I treatment (95% CI, 2.4–3.4 months) was significantly shorter than the median PFS time of 6.1 months (95% CI, 5.2–7.6 months; p < .0001) on previous first-line systemic therapy. It was also shorter (0.6 months/18 days) than the median PFS time of 3.4 months on previous second-line therapy at borderline statistical significance (95% CI, 2.7–5.4 months; p = .05) (Fig. 3) (supplemental online Table 2).
Figure 3.
Kaplan–Meier estimates of PFS. PFS on first-line systemic therapy (blue), second-line systemic therapy (orange), and phase I therapy (red). The median PFS time on first-line treatment was significantly longer than the median PFS time on phase I treatment (p < .0001). The median PFS time on second-line treatment was longer than the median PFS time on phase I treatment and at borderline statistical significance (p = .05).
Abbreviations: CI, confidence interval; PFS, progression-free survival.
Toxicity
Drug-related NCI CTC grade 3 or 4 toxicity occurred in 23 (27%) of 85 patients. There were no treatment-related deaths. The most common grade 3 or 4 toxicity was hematologic toxicity, followed by diarrhea and hand–foot syndrome. There was a significant association between the type of treatment and the risk for grade 3 or 4 toxicity (p < .0001, Fisher's exact test). Patients treated with single-agent targeted therapy, compared with other treatments (combinations of targeted therapy, combinations of chemotherapy with targeted therapy, and chemotherapy alone), were least likely to develop grade 3 or 4 treatment-related toxicity (five of 50, 10%), and patients treated with chemotherapy alone, compared with other treatments (single-agent targeted therapy, combinations of targeted therapy, and targeted therapy combined with chemotherapy) were most likely to suffer from grade 3 or 4 treatment-related toxicity (six of eight, 75%).
Discussion
This is the first study to summarize the clinical outcomes of patients with advanced/metastatic NSCLC enrolled in phase I clinical trials in which the majority of therapeutic regimens contained targeted agents. The overall RR was 9.5% and SD lasting >4 months was observed in an additional 19% of patients (total, 28.5%), which was similar to previously published data from non–disease-specific phase I clinical trials (RR, 4.1%–10.6%) [27–30]. In addition, data obtained from our phase I clinic on 683 participants having various tumor types showed that patients treated with low dose levels did not fare worse [31]. Previously reported RRs in heavily pretreated NSCLC patients treated in disease-specific phase I clinical trials were in the range of 3%–15% [32–34]. Most of our patients with NSCLC (77 of 85, 90.5%) were enrolled in studies that administered targeted agents. Patients receiving targeted therapy combined with chemotherapy were most likely to achieve a PR (38.5%). Patients treated with single-agent targeted therapy were least likely to achieve a PR (2%); however, they were also the most heavily pretreated. The ability of combined targeted therapy and cytotoxic agents to induce response has been previously documented [35]. For instance, the anti–vascular endothelial growth factor monoclonal antibody bevacizumab, which yielded no objective response as a single agent in a dose-escalation phase I study, showed activity in combination [35, 36]. In our study, the median OS time was about 3 months longer in patients who achieved a PR, but this did not reach statistical significance, perhaps because of the relatively small number of patients attaining a PR or possibly because having a response did not impact survival.
The median OS time in our study was 10.6 months. The median OS time observed in our analysis compares favorably with previously reported medians of approximately 5 months from other phase I clinical trials in heavily pretreated NSCLC patients [33, 37]. The median PFS interval in our study was 2.8 months, similar to previously reported data [37]. However, some studies of less heavily pretreated or treatment-naïve patients with NSCLC reported better outcomes, with median PFS times of 4–5 months [32, 38]. In advanced/metastatic NSCLC, the median OS time on first-line therapy rarely exceeds 11 months and the median PFS duration is usually around 5 months [39–42]. In the second-line setting, the median OS time is typically around 7–8 months [5–7]. The median PFS time usually does not exceed 3 months, which is similar to what we observed in our patient population. The OS time on phase I therapy was similar to the OS times reported from large phase III randomized trials with frontline therapies, which could be explained by selection bias. It is likely that patients with NSCLC treated in phase I clinical trials have a better prognosis than that observed in an unselected patient population. In our phase I study–treated population, the median PFS time on previous lines of therapy was similar (6.1 months on first-line and 3.4 months on second-line therapy) to the above-mentioned previously published data.
Most phase I protocols require a life expectancy ≥90 days for enrollment. Several retrospective studies evaluated prognostic factors for survival to determine the risks for early mortality in the phase I setting [43–48]. A group from Royal Marsden Hospital in London developed a prognostic score for predicting survival, which included albumin, LDH, and number of metastatic sites, and validated it in a prospective study with 78 patients [49]. Previously, we reported a retrospectively developed prognostic score that included the presence of liver metastases, a history of thromboembolism, and an elevated platelet count (>440 × 109/L) [44]. In our retrospective study, among other prognostic factors, we evaluated both prognostic scores in patients with NSCLC. The number of metastatic sites, presence of liver metastases, a history of thromboembolism, and an elevated platelet count (>440 × 109/L), but not LDH and albumin, had prognostic significance in a univariate analysis. None of these factors remained statistically significant in the multivariate analysis. Among other prognostic factors in the univariate analysis, patients with a PS score of 0–1, patients who never smoked, and patients with a hemoglobin level ≥12 g/dL had significantly longer survival times. On multivariate analysis, a PS score of 0–1 and a history negative for smoking remained statistically significant factors for longer survival.
Patients evaluated in this study were treated with various regimens, which precluded detailed toxicity analyses. In general, drug-related grade 3 or 4 toxicity occurred in 27% of patients. The most common grade 3 or 4 drug-related toxicity was myelosuppression, followed by diarrhea and hand–foot syndrome. The incidence was lower in patients treated with targeted therapy than in patients who received chemotherapy. These observations were similar to those of previously published reports [46, 47]. Treatment-related mortality in phase I trials is generally low [29, 30, 50]. In our study, there were no treatment-related deaths.
Our study has several limitations, including its retrospective design. Patients were treated in different trials, frequently with diverse outcomes. The median time from an advanced disease diagnosis to phase I clinical trial initiation was 17.5 months. That time frame suggests that our patients had an overall better prognosis than usually seen in advanced NSCLC patients.
In recent years, with the advent of targeted therapies, studies have demonstrated that patients who participate in cancer trials have a very low risk for treatment-related death, although RRs also remain low [29–31, 50]. Observations from select studies suggest that matching patients with targeted drugs based on molecular profile can, however, result in high RRs, even in the phase I setting, [51–54], and for some categories of patients, these treatments may prove better than the available approved care [45, 48, 52]. In our patients with NSCLC, the median PFS interval was modest (2.8 months), and was shorter than that for their prior second-line therapy by 18 days, a time period of borderline statistical significance. These observations suggest that clinical trials are a reasonable alternative for patients with NSCLC who have completed two lines of conventional therapies.
Supplementary Material
Acknowledgments
The authors thank Joann Aaron (MD Anderson Cancer Center) for scientific review and editing. We thank Mr. Sijin Wen for discussion about statistical analyses. This study was supported in part by grant number RR024148 from the National Center for Research Resources, a component of the NIH Roadmap for Medical Research (http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp).
Author Contributions
Conception/Design: Filip Janku, Apostolia M. Tsimberidou,Ignacio Garrido-Laguna, Henrique A. Parsons, Razelle Kurzrock
Administrative support: Razelle Kurzrock
Provision of study material or patients: Filip Janku, Apostolia M. Tsimberidou, David S. Hong, Aung Naing, Ralph G. Zinner, Razelle Kurzrock
Collection and/or assembly of data: Filip Janku, Jing Gong, Ignacio Garrido- Laguna, Ralph G. Zinner
Data analysis and interpretation: Filip Janku, Apostolia M. Tsimberidou, Xuemei Wang, David S. Hong, Jing Gong, Ignacio Garrido-Laguna, Henrique A. Parsons, Razelle Kurzrock
Manuscript writing: Filip Janku, Apostolia M. Tsimberidou, Xuemei Wang, David S. Hong, Aung Naing, Razelle Kurzrock
Final approval of manuscript: Filip Janku, Apostolia M. Tsimberidou, Xuemei Wang, David S. Hong, Aung Naing, Jing Gong, Ignacio Garrido-Laguna, Henrique A. Parsons, Ralph G. Zinner, Razelle Kurzrock
References
- 1.National Cancer Institute. SEER Cancer Statistics Review 1975–2006. Estimated New Cancer Cases and Deaths for 2009. [accessed November 29, 2010]. Available at http://www.seer.cancer.gov/csr/1975_2006/results_single/sect_01_table.01.pdf.
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: A systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26:4617–4625. doi: 10.1200/JCO.2008.17.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis PA, Smith IE, Hardy JR, et al. Symptom relief with MVP (mitomycin C, vinblastine and cisplatin) chemotherapy in advanced non-small-cell lung cancer. Br J Cancer. 1995;71:366–370. doi: 10.1038/bjc.1995.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 6.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 8.Higgins MJ, Ettinger DS. Chemotherapy for lung cancer: The state of the art in 2009. Expert Rev Anticancer Ther. 2009;9:1365–1378. doi: 10.1586/era.09.115. [DOI] [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) [accessed February 4, 2010]. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 12.Kaplan EM, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 14.Cox D. Regression models and life tables (with discussion) J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 15.Bahleda RF, Herbst E, Hanna RS, et al. Phase I multicenter trial of BMS-690514: Safety, pharmacokinetic profile, biological effects, and early clinical evaluation in patients with advanced solid tumors and non-small cell lung cancer. J Clin Oncol. 2008;26 Abstract 2564. [Google Scholar]
- 16.LoRusso PH, Heath D, Kurzrock E, et al. First-in-human study of AMG 655, a pro-apoptotic TRAIL receptor-2 agonist, in adult patients with advanced solid tumors. J Clin Oncol. 2007;25 Abstract 3534. [Google Scholar]
- 17.Rosen LS, Kurzrock R, Mulay M, et al. Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:2369–2376. doi: 10.1200/JCO.2006.07.8170. [DOI] [PubMed] [Google Scholar]
- 18.Johnson FM, Agrawal S, Burris H, et al. Phase 1 pharmacokinetic and drug-interaction study of dasatinib in patients with advanced solid tumors. Cancer. 2010;116:1582–1591. doi: 10.1002/cncr.24927. [DOI] [PubMed] [Google Scholar]
- 19.Camidge DH, Gordon RS, Eckhardt M, et al. A phase I safety and pharmacokinetic study of apomab, a human DR5 agonist antibody, in patients with advanced cancer. J Clin Oncol. 2007;25 Abstract 3582. [Google Scholar]
- 20.Tsimberidou AM, Camacho LH, Verstovsek S, et al. A phase I clinical trial of darinaparsin in patients with refractory solid tumors. Clin Cancer Res. 2009;15:4769–4776. doi: 10.1158/1078-0432.CCR-08-2984. [DOI] [PubMed] [Google Scholar]
- 21.Herbst RS, Hong D, Chap L, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27:3557–3565. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- 22.Kurzrock RA, Hong W, Ng D, et al. Two phase 1 studies of MPC-6827, a novel vascular disrupting agent (VDA), in patients with advanced solid tumors and CNS metastases. J Clin Oncol. 2007;25 Abstract 3604. [Google Scholar]
- 23.Stephenson JL, Martin N, Ho JC, et al. Phase I multicenter study to assess the safety, tolerability, and pharmacokinetics of AZD4877 administered twice weekly in adult patients with advanced solid malignancies. J Clin Oncol. 2008;26 Abstract 2516. [Google Scholar]
- 24.Moulder S, Dhillon N, Ng C, et al. A phase I trial of imexon, a pro-oxidant, in combination with docetaxel for the treatment of patients with advanced breast, non-small cell lung and prostate cancer. Invest New Drugs. 2010;28:634–640. doi: 10.1007/s10637-009-9273-1. [DOI] [PubMed] [Google Scholar]
- 25.Heath EB, Cohen GR, LoRusso RB, et al. Sunitinib in combination with paclitaxel and carboplatin in patients with advanced solid tumors: Updated phase I study results. J Clin Oncol. 2009;27 Abstract e14509. [Google Scholar]
- 26.Falchook GD, Moulder N, Duvic S, et al. Age-stratified phase I trial of a combination of bortezomib, gemcitabine, and liposomal doxorubicin in patients with advanced malignancies. J Clin Oncol. 2007;25 doi: 10.1007/s00280-011-1808-4. Abstract 14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itoh K, Sasaki Y, Miyata Y, et al. Therapeutic response and potential pitfalls in phase I clinical trials of anticancer agents conducted in Japan. Cancer Chemother Pharmacol. 1994;34:451–454. doi: 10.1007/BF00685653. [DOI] [PubMed] [Google Scholar]
- 28.Decoster G, Stein G, Holdener EE. Responses and toxic deaths in phase I clinical trials. Ann Oncol. 1990;1:175–181. doi: 10.1093/oxfordjournals.annonc.a057716. [DOI] [PubMed] [Google Scholar]
- 29.Horstmann E, McCabe MS, Grochow L, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352:895–904. doi: 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 30.Kurzrock R, Benjamin RS. Risks and benefits of phase 1 oncology trials, revisited. N Engl J Med. 2005;352:930–932. doi: 10.1056/NEJMe058007. [DOI] [PubMed] [Google Scholar]
- 31.Jain RK, Lee JJ, Hong D, et al. Phase I oncology studies: Evidence that in the era of targeted therapies patients on lower doses do not fare worse. Clin Cancer Res. 2010;16:1289–1297. doi: 10.1158/1078-0432.CCR-09-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adjei AA, Molina JR, Mandrekar SJ, et al. Phase I trial of sorafenib in combination with gefitinib in patients with refractory or recurrent non-small cell lung cancer. Clin Cancer Res. 2007;13:2684–2691. doi: 10.1158/1078-0432.CCR-06-2889. [DOI] [PubMed] [Google Scholar]
- 33.Baas P, Szczesna A, Albert I, et al. Phase I/II study of a 3 weekly oral taxane (DJ-927) in patients with recurrent, advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:745–750. doi: 10.1097/JTO.0b013e31817c73ff. [DOI] [PubMed] [Google Scholar]
- 34.Borghaei H, Alpaugh K, Hedlund G, et al. Phase I dose escalation, pharmacokinetic and pharmacodynamic study of naptumomab estafenatox alone in patients with advanced cancer and with docetaxel in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:4116–4123. doi: 10.1200/JCO.2008.20.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margolin K, Gordon MS, Holmgren E, et al. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: Pharmacologic and long-term safety data. J Clin Oncol. 2001;19:851–856. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 36.Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 37.Haura EB, Tanvetyanon T, Chiappori A, et al. Phase I/II study of the Src inhibitor dasatinib in combination with erlotinib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1387–1394. doi: 10.1200/JCO.2009.25.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thienelt CD, Bunn PA, Jr, Hanna N, et al. Multicenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non-small-cell lung cancer. J Clin Oncol. 2005;23:8786–8793. doi: 10.1200/JCO.2005.03.1997. [DOI] [PubMed] [Google Scholar]
- 39.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 40.Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–4291. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 41.Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: The TAX 326 study group. J Clin Oncol. 2003;21:3016–3024. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 42.Kelly K, Crowley J, Bunn PA, Jr, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol. 2001;19:3210–3218. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 43.Arkenau HT, Olmos D, Ang JE, et al. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: The Royal Marsden Hospital experience. Br J Cancer. 2008;98:1029–1033. doi: 10.1038/sj.bjc.6604218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheler J, Tsimberidou AM, Hong D, et al. Survival of patients in a phase 1 clinic: The M. D. Anderson Cancer Center experience. Cancer. 2009;115:1091–1099. doi: 10.1002/cncr.24018. [DOI] [PubMed] [Google Scholar]
- 45.Tsimberidou AM, Vaklavas C, Wen S, et al. Phase I clinical trials in 56 patients with thyroid cancer: The M. D. Anderson Cancer Center experience. J Clin Endocrinol Metab. 2009;94:4423–4432. doi: 10.1210/jc.2009-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bachelot T, Ray-Coquard I, Catimel G, et al. Multivariable analysis of prognostic factors for toxicity and survival for patients enrolled in phase I clinical trials. Ann Oncol. 2000;11:151–156. doi: 10.1023/a:1008368319526. [DOI] [PubMed] [Google Scholar]
- 47.Han C, Braybrooke JP, Deplanque G, et al. Comparison of prognostic factors in patients in phase I trials of cytotoxic drugs vs new noncytotoxic agents. Br J Cancer. 2003;89:1166–1171. doi: 10.1038/sj.bjc.6601218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garrido-Laguna I, Janku F, Falchook GS, et al. Patients with advanced head and neck cancers have similar progression-free survival on phase I trials and their last food and drug administration-approved treatment. Clin Cancer Res. 2010;16:4031–4037. doi: 10.1158/1078-0432.CCR-10-0672. [DOI] [PubMed] [Google Scholar]
- 49.Arkenau HT, Barriuso J, Olmos D, et al. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol. 2009;27:2692–2696. doi: 10.1200/JCO.2008.19.5081. [DOI] [PubMed] [Google Scholar]
- 50.Roberts TG, Jr, Goulart BH, Squitieri L, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. 2004;292:2130–2140. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- 51.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 52.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janku F, Stewart DJ, Kurzrock R. Targeted therapy in non-small-cell lung cancer—is it becoming a reality? Nat Rev Clin Oncol. 2010;7:401–414. doi: 10.1038/nrclinonc.2010.64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.