Controversies in the adjuvant therapy of newly diagnosed high-grade gliomas are analyzed and discussed.
Keywords: Glioblastoma multiforme, Anaplastic astrocytoma, Anaplastic oligodendroglioma, Anaplastic oligoastrocytoma, Low-grade glioma, Adjuvant therapy, Temozolomide, Chemotherapy
Abstract
The 2-year survival rate of patients with glioblastoma accrued to research studies increased from 10% to nearly 40% from 2000 to 2010. These improvements began with the demonstration of a survival benefit when daily temozolomide was administered with 6 weeks of standard radiation and for 6 months thereafter. This treatment regimen is often associated with significant lymphopenia, thrombocytopenia, and progressive blood–brain barrier dysfunction that can result in clinical and radiologic deterioration without true tumor progression (“pseudoprogression”). With new evidence that combining this cytotoxic agent with radiation improves survival in this malignancy, many investigators have modified the regimen to further improve patient outcomes. These largely uncontrolled studies highlight controversies regarding the optimal therapy of this disease. This review focuses on the following selected controversies: (a) What is the appropriate temozolomide dose, schedule, and duration in the postradiation period? (b) How should other U.S. Food and Drug Administration–approved therapies (such as carmustine wafers and bevacizumab) be incorporated into this treatment regimen? (c) Should the results in glioblastoma be extrapolated to patients aged >70 and to patients with lower grade gliomas? and (d) How should novel therapeutic approaches be added to radiation and temozolomide in clinical trials for patients with newly diagnosed glioblastoma?
Introduction
High-grade gliomas represent the largest fraction of the estimated 17,000 newly diagnosed primary brain tumors in the U.S. Until recently, decades of clinical trials designed to discover effective adjuvant chemotherapy regimens were disappointing. A meta-analysis of data from 12 randomized trials (with a total of 3,004 patients) demonstrated that adjuvant nitrosoureas generated slightly longer median survival times with considerable myelosuppression and no difference in the long-term survival rate [1]. It is with this background that the introduction of temozolomide (TMZ), an oral alkylating agent, opened a new era in the treatment and investigation of these tumors [2, 3].
The State of the Art
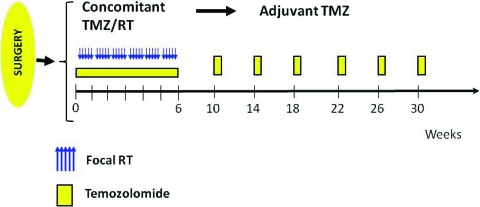
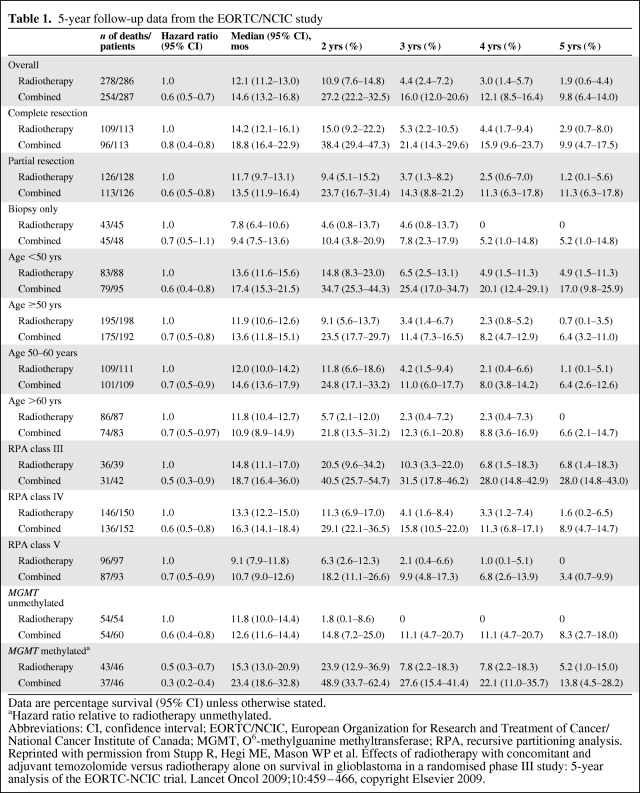
The current treatment of patients with newly diagnosed glioblastoma (GBM) is based on the results of the large European Organization for Research and Treatment of Cancer (EORTC)/National Cancer Institute of Canada (NCIC) phase III multicenter study published in 2005 [2]. Five hundred seventy-three patients from 85 centers who had undergone surgery were randomized to receive either radiotherapy (RT) alone (60 Gy in 30 2-Gy daily fractions over 6 weeks) or the same RT plus daily low-dose TMZ at 75 mg/m2. This was followed by a treatment break of 4 weeks after which TMZ was administered at a dose of 150–200 mg/m2 per day for five consecutive days each month for a total of 6 months (Fig. 1). That study documented a longer median survival time, 14.6 months, versus 12.1 months, when TMZ was added to RT. In addition, there was a higher 2-year survival rate—26.5% in the combination therapy group, versus 10.4% in patients only receiving RT. A recently published analysis of long-term results demonstrated that the survival advantage conferred by TMZ persisted at 3 years (4.4% versus 16%), 4 years (3% versus 12.1%), and 5 years (1.9% versus 9.8%) [3]. A subgroup analysis of these data was performed examining the extent of the initial resection, age, recursive partitioning analysis class, and O6-methylguanine methyltransferase (MGMT) promoter methylation status. Survival in all subgroups was longer in the combination therapy arm than in the RT monotherapy arm (Table 1) [3]. These randomized prospective data are the first to demonstrate a significant and meaningful survival benefit when a chemotherapeutic agent is given in combination with RT in this disease. As a result, chemoradiation with TMZ followed by six cycles of TMZ as in the EORTC/NCIC trial, constitutes the current standard of care for the adjuvant treatment of GBM.
Figure 1.
Current standard treatment schema (based on the European Organization for Research and Treatment of Cancer/National Cancer Institute of Canada design).
Abbreviations: RT, radiotherapy; TMZ, temozolomide.
Table 1.
5-year follow-up data from the EORTC/NCIC study
Data are percentage survival (95% CI) unless otherwise stated.
aHazard ratio relative to radiotherapy unmethylated.
Abbreviations: CI, confidence interval; EORTC/NCIC, European Organization for Research and Treatment of Cancer/National Cancer Institute of Canada; MGMT, O6-methylguanine methyltransferase; RPA, recursive partitioning analysis.
Reprinted with permission from Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–466, copyright Elsevier 2009.
There are toxicities associated with this treatment regimen. Myelosuppression, in particular significant thrombocytopenia, observed in approximately 12%–20% of patients, can substantially limit the amount of TMZ that can be administered [2, 4]. Especially in the initial phase of therapy when TMZ is being given daily in combination with RT, platelet counts frequently continue to fall for weeks after the drug is discontinued. As a result, most clinicians hold further doses of TMZ when a platelet count <100,000/μL is detected on a weekly blood count. Recent data also suggest that the combination of RT, TMZ, and dexamethasone has a profound effect on total lymphocyte and CD4 counts, which requires prolonged Pneumocystis jiroveci prophylaxis [5]. In addition, severe treatment-related immunosuppression may be associated with early tumor recurrence.
This regimen is also associated with treatment-related injury to the blood–brain barrier. This is evident on magnetic resonance imaging (MRI) and computed tomography scans as greater contrast enhancement, edema, and mass effect. In addition, it is frequently associated with clinical deterioration and a need for higher glucocorticoid doses to control peritumoral edema. These findings, commonly referred to as “pseudoprogression,” have been seen in 20%–30% of patients [6]. They are frequently noted on neuroimaging studies performed 1 month following completion of RT and concurrent TMZ and may last for many months thereafter. These early clinical and radiologic outcomes are not usually sufficient to discontinue TMZ because this is the only treatment documented to prolong survival in patients with GBM. Unfortunately, other radiologic studies, such as positron emission tomography or nuclear magnetic resonance spectroscopy, have not been useful in this setting. As a result, in selected cases, tissue may be required to distinguish treatment-related effects from progressive neoplasm if clinically indicated.
Despite the observed improvement in survival, the prognosis for patients with these tumors is still grim, and virtually all patients die as a result of their disease. It is therefore imperative to explore novel treatment options for these patients. Some of the more important questions that have been posed in recent years include: (a) What is the appropriate TMZ dose, schedule, and duration in the post-RT period? (b) How should other U.S. Food and Drug Administration (FDA)-approved therapies (such as carmustine wafers and bevacizumab) be incorporated into this treatment regimen? (c) Should the results in GBM be extrapolated to patients aged >70 years and patients with lower grade gliomas? and (d) How should novel therapeutic approaches be added to RT and TMZ in clinical trials for patients with newly diagnosed GBM?
Dose, Schedule, and Duration of TMZ in the Post-RT Period
TMZ Dose and Schedule
There is little controversy about dosing 75 mg/m2 per day of TMZ during the time of RT. Survival data supporting this dose and schedule exist and the associated myelosuppression will likely not permit further dose intensity in this portion of the treatment regimen. There is, however, uncertainty about the optimal timing of TMZ prior to RT. Current practice is to administer the TMZ 1 hour before RT. Microdialysis data suggest that TMZ achieves a maximum concentration in the brain much later than this, suggesting that results might be improved by providing TMZ hours before RT is administered [7]. Further studies to optimize the best possible timing are needed.
Significant controversy exists regarding TMZ doses and schedules in the period following chemoradiation. Data from single-arm studies suggest that alternative TMZ dose schedules that provide a longer exposure and a higher cumulative dose might be more efficacious. This approach is designed to increase the depletion of MGMT, which could render cells more susceptible to the cytotoxic effect of TMZ [8]. The proposed regimens are a protracted low-dose schedule of TMZ (75 mg/m2 for 21 days of each 28-day cycle), daily administration of TMZ at a lower dose (e.g., 50 mg/m2, “metronomic” schedule), and a so-called “dose-dense” regimen, with high-dose TMZ at 150 mg/m2, 1 week on/1 week off.
The more dose-intense TMZ schedule of 75 mg/m2 daily for 3 weeks followed by 1 week off was tested in an Italian phase II trial on 33 chemotherapy-naïve patients with recurrent or progressive GBM that showed a 6-month progression-free survival rate of 30%. That study also demonstrated a high rate of lymphopenia and associated infections. The MGMT promoter methylation status was not associated with a difference in survival [9]. Profound lymphopenia was also reported in another trial looking at this treatment schedule, with a dose of 100 mg/m2, in the setting of recurrent disease [10]. A phase II trial of 90 patients with recurrent gliomas using the dose-dense schedule was feasible and safe and showed similar activity in patients with and without MGMT gene promoter methylation [11]. One randomized phase II trial compared chemoradiation followed by either a dose-dense (150 mg/m2, 1 week on/1 week off) or metronomic (50 mg/m2 daily) 6-month course of TMZ [12]. The toxicity in both arms appeared to be reasonably similar and the 1-year survival rate appeared favorable in the dose-dense treatment arm.
To date, there has been one randomized trial in chemotherapy-naïve patients with recurrent high-grade gliomas that directly compared a 5-day/month regimen (200 mg/m2 for 5 days every 28 days) with a metronomic schedule with TMZ (100 mg/m2 daily for 21 days). Patients were randomized to receive either chemotherapy with procarbazine, lomustine, and vincristine (PCV) or TMZ; patients who received TMZ were randomized to either the 5-day or the 21-day schedule. Among the patients who received TMZ (223 patients in both arms combined), the data showed the superiority of the 5-day schedule with regard to overall progression-free survival and median survival times (p = .023 and p = .056, respectively), and a quality of life assessment also favored the 5-day over the 21-day schedule of TMZ (p = .005) [13]. Additional information on how more intense treatment schedules compare with the current standard of care (150–200 mg/m2 on days 1–5 of each cycle) will be definitively provided by an ongoing trial by the Radiation Treatment Oncology Group (RTOG0525). This is a randomized comparison between the metronomic regimen of adjuvant TMZ (given on days 1–21 of each 28-day cycle) and conventional dosing (days 1–5 of each adjuvant cycle) in newly diagnosed GBM patients. Recruitment is complete and the analysis is currently pending.
Duration of Chemotherapy
Another controversy exists regarding the duration of adjuvant therapy in newly diagnosed patients. Although six cycles of adjuvant TMZ were used in the EORTC/NCIC trial, there are significant differences in real practice, and some clinicians recommend longer durations of therapy. To date, no trial has compared the different durations of therapy in a randomized fashion. The rationale for maintenance treatment is that residual disease is expected in virtually all patients, and it is hoped that additional cycles could delay recurrence. However, ample experience with chemotherapy in other solid tumors suggests that the maximum benefit in terms of tumor cell kill is achieved with the first two to four cycles of chemotherapy. In no other solid tumor has the administration of cytotoxic chemotherapy continued “until disease progression” been beneficial in improving overall survival. This is reflected in common clinical practice and guidelines for a variety of other solid tumors (http://www.nccn.org). As a result, adjuvant chemotherapy is usually prescribed for a defined period, usually lasting 4–6 months, to avoid additional toxicity when there are no data documenting added benefit to continued therapy.
How Should Other FDA-Approved Therapies (Such as Carmustine Wafers and Bevacizumab) Be Incorporated Into This Treatment Regimen?
Carmustine Wafers
The implantation of carmustine polymer wafers (Gliadel®; Eisai Inc., Woodcliff Lake, NJ) after surgical resection is an FDA-approved treatment option that facilitates local delivery of BCNU [14]. Two hundred forty patients with newly diagnosed anaplastic astrocytomas and GBMs were randomly assigned to placement of up to eight carmustine or placebo wafers followed by standard RT with no concurrent or adjuvant chemotherapy. Patients on the carmustine arm had a statistically significant longer median survival time, 13.9 months (versus 11.6 months), without a demonstrable difference in survival at 2 years [15]. More impact was observed in patients with anaplastic astrocytomas than in patients with GBMs. Toxicities included an increase in cerebrospinal fluid leak, intracranial hypertension, and potentially serious cerebral edema [16]. Comparing the outcomes in patients with GBM in the carmustine wafer and EORTC/NCIC TMZ trial, both studies showed a benefit in terms of the median survival duration, but only the EORTC/NCIC data demonstrated a difference in the survival rate at 2–5 years (Table 1). Based on this, it can be concluded that carmustine wafers should not be used in lieu of TMZ in newly diagnosed GBM patients. The question of whether carmustine wafers can be safely added to standard treatment has been studied. A retrospective study of 33 patients who received carmustine wafers in addition to standard chemoradiation suggested that the treatments can be combined safely. The median survival duration of these patients, who, by definition, all had extensive surgical debulking, was nearly 21 months [17]. A large prospective randomized trial is needed to clearly document the added survival benefit of carmustine wafers to standard therapy in this patient population.
Bevacizumab
Bevacizumab received provisional accelerated approval by the FDA in 2009 in the U.S., but not in Europe, for patients with recurrent GBM based on radiologic and clinical improvement without data on survival prolongation [18]. The effect of this agent on the survival of patients with newly diagnosed GBM is currently the focus of two large phase III trials that are accruing patients. The early use of bevacizumab in these patients deserves careful study in light of the risks for wound healing, bleeding, and blood clots, and recent data suggesting greater invasiveness of GBM when treated with anti–vascular endothelial growth factor therapy [19, 20]. Furthermore, survival, rather than response or progression-free survival, is required as the primary endpoint for these studies because the effect of bevacizumab on blood–brain barrier permeability is dramatic and directly affects the appearance of MRI scans. Additional concerns on the upfront use of this agent center on the recurrence of brain edema once the agent is discontinued and the lack of responsiveness and poor survival of patients progressing on bevacizumab.
Patient Selection
Should the Results in GBM Be Extrapolated to Patients Aged >70 Years?
High-grade gliomas, in particular GBM, are diseases of the middle-aged to elderly, with most patients diagnosed in their seventh decade of life. In the EORTC/NCIC trial, the age limit was <70 years, with median ages of 57 in the RT arm and 56 in the RT plus TMZ arm [2]. Nonetheless, looking at the 5-year survival data from that trial, it was found that, even though the overall survival time in older patients was worse than in younger subgroups, there was still a significant survival benefit in the cohort treated with combination therapy, compared with RT alone [3]. There are limited data on the treatment of high-grade gliomas in the elderly.
Many investigators, concerned about added toxicity in the elderly, have studied reducing the intensity of RT or chemotherapy in this population. A randomized French trial comparing RT (total dose, 50 Gy) plus supportive care with supportive care alone in patients aged ≥70 years showed a modestly longer survival time with RT without significant differences in cognitive function or quality of life [21]. Other groups evaluated whether chemotherapy or RT might be a superior treatment for these patients, assuming that combination therapy might be too toxic for elderly patients with these tumors. Two recent European studies, presented at the 2010 Annual Meeting of the American Society of Clinical Oncology, that compared RT with single-agent TMZ showed conflicting results. The Nordic Brain Tumor Study Group trial looked at outcomes in GBM patients aged ≥60 years treated in three different treatment arms—RT alone at a dose of 60 Gy, RT alone at a dose of 34 Gy, and single-agent TMZ for six cycles [22]. The overall survival duration was not statistically different when looking at all patients; however TMZ alone appeared to be superior to RT in patients aged >70 years. The Neurooncology Working Group of the German Cancer Society looked at GBM patients aged ≥65 years [23]. Patients were randomized to either high-dose TMZ (100 mg/m2 per day, every other week until treatment failure) or RT alone. In comparison with the Nordic trial, however, this study suggested that daily TMZ, given every other week, was superior to RT, although TMZ appeared to be more toxic. None of these three trials, however, included a direct comparison with combined chemoradiation with TMZ followed by six cycles of TMZ. Based on the assumption that combination therapy might be more toxic in elderly patients, the ongoing EORTC26062/NCIC-CE6 trial is comparing concomitant short RT (40 Gy over 15 days) and TMZ with short RT alone, followed by adjuvant TMZ in both treatment arms. Also in this trial, there is no direct comparison with the Stupp regimen, and conclusions will need to be drawn from a historical comparison. Survival appears substantially inferior with single-modality chemotherapy or irradiation to that seen with combination therapy in elderly patients [3, 24, 25].
One important question is how to best identify patients who would be expected to tolerate combined therapy and whether it would be better to define and use criteria for fitness and frailty than to select therapies just based on chronological age. Although the reported rates of acute toxicities do not seem much different between younger and older patients, the elderly may have a higher rate of delayed cognitive decline [25]. There are data suggesting that MGMT promoter methylation status in elderly patients has a similar distribution and prognostic importance to those seen in younger patients [24, 25]. Although testing the MGMT promoter methylation status in younger patients (outside a clinical trial) currently does not influence the decision about which treatment the patient should receive, this might be a helpful adjunct in elderly, more frail patients in whom the risks and benefits of adding TMZ need to be weighed with particular caution.
Based on the currently available data, however, the Stupp regimen remains the standard of care for all patients aged <70 years, and probably all patients, regardless of age, who appear fit enough to undergo this therapy.
Should Data from GBM Patients Be Extrapolated to Patients with Lower Grade Gliomas?
Another controversy is whether newly diagnosed patients with anaplastic astrocytomas, anaplastic oligodendrogliomas, or anaplastic oligoastrocytomas should be treated with the same initial regimen as GBM patients. To date, there has not been any high-level evidence comparing the outcome of combined chemoradiation with that of TMZ in these patients. Two previous phase III trials that looked at the role of PVC chemotherapy in anaplastic oligodendroglial tumors demonstrated a longer progression-free survival time; however, there was no difference in overall survival and treatment was associated with significant hematologic toxicity [26, 27]. These trials did not include a treatment arm with concomitant RT and TMZ, and the interpretation was complicated by the interim discovery of the favorable implication of codeletion of 1p and 19q on treatment response and prognosis. Because the survival rate of patients with anaplastic oligodendrogliomas with codeletion of 1p and 19q is significantly better than the survival rate of patients with GBM, the potential benefit of combination therapy needs to be carefully weighed against the risk from potentially added long-term toxicity. There are currently two ongoing international trials investigating the role of combined therapy with TMZ in anaplastic glioma patients. The N0577/EORTC 26081–22086 trial, including only patients with anaplastic gliomas with 1p–19q codeletion, is comparing three different upfront treatment arms: RT alone versus chemoradiation followed by six to 12 cycles of TMZ (Stupp regimen) versus chemotherapy alone (12 months of TMZ). The EORTC26053–22054/RTOG0834 (CATNON) trial is randomizing patients with anaplastic gliomas without 1p–19q codeletion to either upfront chemoradiation or RT alone with or without adjuvant TMZ. Accrual to these studies began recently. A German trial (NOA-4) is evaluating sequences of different monotherapies in a four-arm model with either RT or chemotherapy first (with randomization of chemotherapy to either PCV or TMZ), followed by the other treatment modality at the time of progression [28]. Comparison with combined therapy with RT and TMZ, however, was not part of this study, which limits the conclusions from this trial.
In the U.S., patients with anaplastic gliomas are often treated with the same initial therapy as GBM patients. This may complicate accrual to these very important clinical trials.
The same considerations also apply to patients with grade II gliomas. Randomized controlled trials are needed, such as the Eastern Cooperative Oncology Group study E3F05, which aims to compare outcomes of treatment with RT with or without TMZ in patients with symptomatic or progressive low-grade gliomas.
How Should Novel Therapeutic Agents Be Added to RT and TMZ in Clinical Trials for Patients with Newly Diagnosed GBM?
Prior to 2005, novel agents studied in patients with newly diagnosed GBM were often added to RT or started after the completion of RT, with overall survival as the primary endpoint. However, with the demonstration of a survival benefit from TMZ administered with and following RT, the introduction of new agents requires a more complex development pathway. Cytotoxic agents could increase the myelosuppression associated with TMZ resulting in reductions in TMZ doses that could compromise survival. As a result, these agents usually need to be studied in dose-escalation trials with TMZ before an efficacy study is considered. As a complicating factor, the standard RT plus TMZ regimen contains two separate TMZ dosing schedules (Fig. 1). The daily dose administered with RT is 75 mg/m2 for 42 days, whereas the adjuvant portion is given at a dose of 150–200 mg/m2 for five consecutive days each month. The novel cytotoxic agent will need to be evaluated in each of these settings before it can be added to both segments of therapy in patients with newly diagnosed GBM. Noncytotoxic agents without overlapping toxicities with RT and TMZ can be considered differently. Adding these directly to the standard RT and TMZ regimen has been safe with selected noncytotoxic agents—talampanel [29], cilengitide [30], poly-ICLC [31], bevacizumab, hydroxychloroquine, erlotinib, etc. Caution is recommended in comparisons of single-arm phase II trials of novel agents added to RT and TMZ and the published results of the EORTC/NCIC trial. Several of these new studies show dramatically better median and 2-year survival data, which could be a result of the effects of the new agents or better results with the standard therapy in 2010 than in 2004, with better salvage therapy, patient selection, and supportive care [29–32]. These survival questions will be answered shortly by looking at the survival time of patients on the control arm of several large randomized phase III trials in GBM.
Conclusions
Significant progress has been made in the treatment of high-grade gliomas over the past 10 years. The current standard of care for patients with GBM remains concomitant chemoradiation with TMZ at 75 mg/m2 followed by 6 months of adjuvant TMZ at 150–200 mg/m2 on days 1–5 of each 28-day cycle. Carmustine wafers can be used safely in combination with standard RT and TMZ in appropriate patients. Until more data are available, bevacizumab is best used only as a part of a research study in newly diagnosed patients with GBM. Patients aged >70 years should probably be treated in a fashion similar to all other patients and decisions about the treatment regimen should be made based on the patient's individual morbidity and frailty rather than chronological age alone. The role of adding TMZ to RT in grade III and grade II tumors needs to be determined by randomized phase III trials before being adopted as an international standard. Further progress in the treatment of these malignancies requires carefully designed and conducted clinical trials.
Author Contributions
Conception/Design: Matthias Holdhoff, Stuart A. Grossman
Collection and/or assembly of data: Matthias Holdhoff, Stuart A. Grossman
Data analysis and interpretation: Matthias Holdhoff, Stuart A. Grossman
Manuscript writing: Matthias Holdhoff, Stuart A. Grossman
Final approval of manuscript: Matthias Holdhoff, Stuart A. Grossman
References
- 1.Stewart LA. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Gerber DE, Grossman SA, Zeltzman M, et al. The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas. Neuro Oncol. 2007;9:47–52. doi: 10.1215/15228517-2006-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman SA, Ye X, Lesser GJ, et al. Iatrogenic immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide: A NABTT CNS Consortium study. J Clin Oncol. 2010;28(15 suppl):2013. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 7.Portnow J, Badie B, Chen M, et al. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: Potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15:7092–7098. doi: 10.1158/1078-0432.CCR-09-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolcher AW, Gerson SL, Denis L, et al. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88:1004–1011. doi: 10.1038/sj.bjc.6600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandes AA, Tosoni A, Cavallo G, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: Phase II study from Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br J Cancer. 2006;95:1155–1160. doi: 10.1038/sj.bjc.6603376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neyns B, Chaskis C, Joosens E, et al. A multicenter cohort study of dose-dense temozolomide (21 of 28 days) for the treatment of recurrent anaplastic astrocytoma or oligoastrocytoma. Cancer Invest. 2008;26:269–277. doi: 10.1080/07357900701708393. [DOI] [PubMed] [Google Scholar]
- 11.Wick A, Felsberg J, Steinbach JP, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25:3357–3361. doi: 10.1200/JCO.2007.10.7722. [DOI] [PubMed] [Google Scholar]
- 12.Clarke JL, Iwamoto FM, Sul J, et al. Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol. 2009;27:3861–3867. doi: 10.1200/JCO.2008.20.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brada M, Stenning S, Gabe R, et al. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin Oncol. 2010;28:4601–4608. doi: 10.1200/JCO.2009.27.1932. [DOI] [PubMed] [Google Scholar]
- 14.Lawson HC, Sampath P, Bohan E, et al. Interstitial chemotherapy for malignant gliomas: The Johns Hopkins experience. J Neurooncol. 2007;83:61–70. doi: 10.1007/s11060-006-9303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber EL, Goebel EA. Cerebral edema associated with Gliadel wafers: Two case studies. Neuro Oncol. 2005;7:84–89. doi: 10.1215/S1152851704000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110:583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 19.Verhoeff JJ, van Tellingen O, Claes A, et al. Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer. 2009;9:444. doi: 10.1186/1471-2407-9-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:4589–4599. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- 21.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 22.Malmstrom A, Gronberg BH, Stupp R, et al. Glioblastoma (GBM) in elderly patients: A randomized phase III trial comparing survival in patients treated with 6-week radiotherapy (RT) versus hypofractionated RT over 2 weeks versus temozolomide single-agent chemotherapy (TMZ) J Clin Oncol. 2010;28(18 suppl):2002. [Google Scholar]
- 23.Wick W, Engel C, Combs SE, et al. NOA-08 randomized phase III trial of 1-week-on/1-week-off temozolomide versus involved-field radiotherapy in elderly (older than age 65) patients with newly diagnosed anaplastic astrocytoma or glioblastoma (Methusalem) J Clin Oncol. 2010;28(18 suppl):2001. [Google Scholar]
- 24.Gerstner ER, Yip S, Wang DL, et al. Mgmt methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology. 2009;73:1509–1510. doi: 10.1212/WNL.0b013e3181bf9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandes AA, Franceschi E, Tosoni A, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: Correlation with MGMT promoter methylation status. Cancer. 2009;115:3512–3518. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
- 26.van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: A randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715–2722. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 27.Intergroup Radiation Therapy Oncology Group Trial 9402. Cairncross G, Berkey B, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 28.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 29.Grossman SA, Ye X, Chamberlain M, et al. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: A multicenter phase II trial. J Clin Oncol. 2009;27:4155–4161. doi: 10.1200/JCO.2008.21.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nabors LB, Mikkelsen T, Batchelor T, et al. NABTT 0306: A randomized phase II trial of EMD 121974 in conjunction with concomitant and adjuvant temozolomide with radiation therapy in patients with newly diagnosed glioblastoma multiforme (GBM) J Clin Oncol. 2009;27(15 suppl):2001. [Google Scholar]
- 31.Rosenfeld MR, Chamberlain MC, Grossman SA, et al. A multi-institution phase II study of poly-ICLC and radiotherapy with concurrent and adjuvant temozolomide in adults with newly diagnosed glioblastoma. Neuro Oncol. 2010;12:1071–1077. doi: 10.1093/neuonc/noq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16:2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]