We performed a multicenter retrospective comparative study to assess the impact of age on potential differences in clinical characteristics, treatment patterns, and outcome in HCC patients. Characteristics that distinguish elderly from younger HCC patients include lower M/F ratio, worse performance status, lower rate of HCV infection, and less advanced underlying cirrhosis. Elderly patients were less likely to have a liver transplant and more likely to receive supportive care only. However, overall and HCC-specific survival were similar between the two groups.
Keywords: Hepatocellular carcinoma, Epidemiology, Elderly, Treatment pattern, Survival
Abstract
Background.
There is a paucity of information on the clinical presentation and outcome of elderly hepatocellular carcinoma (HCC) patients. We performed a multicenter retrospective comparative study to assess the impact of age on potential differences in clinical characteristics, treatment patterns, and outcome in HCC patients.
Methods.
We retrospectively analyzed HCC patients treated at two U.S. tertiary institutions from 1998 to 2008. Demographics, tumor parameters, etiology and severity of cirrhosis, treatment, and survival from diagnosis were collected and analyzed. After exclusion of transplanted patients, survival analyses were performed using the Kaplan-Meier method with log-rank tests and Cox proportional hazards models.
Results.
Three hundred thirty-five HCC patients were divided into two groups: “elderly” (95 patients, age ≥70 years) and “younger” (240 patients, aged <70 years). The male/female (M/F) ratio was 5.8:1 and 1.7:1 in the younger and elderly groups, respectively (p < .0001). Hepatitis C virus (HCV) infection rate was 48.3% in younger and 21.1% in elderly patients (p < .0001); Child class B and C cirrhosis accounted for 35.8% in younger and 25.3% in elderly patients (p = .063). Compared with younger patients, the elderly received transplant less frequently (19.6% versus 5.3%, p = .0002) and were more likely to receive supportive care only (22.9% versus 36.8%, p = .01). No significant differences between the two age groups were seen in tumor parameters or other treatments received. Overall (p = .47) and HCC-specific survival rates (p = .38) were similar in both age groups.
Conclusions.
Characteristics that distinguish elderly from younger HCC patients include lower M/F ratio, worse performance status, lower rate of HCV infection, and less advanced underlying cirrhosis. Elderly patients were less likely to have a liver transplant and more likely to receive supportive care only. However, overall and HCC-specific survival were similar between the two groups.
Introduction
The incidence and mortality of hepatocellular carcinoma (HCC) have been dramatically increasing in the United States [1]. Age is a known risk factor for HCC [2–4]. In the United States, the average age of HCC diagnosis is 65 years [5]. The incidence rate of HCC among the elderly is progressively increasing [6]. This trend may be partially attributable to screening, improved management of chronic liver disease, the rising incidence of nonalcoholic steatohepatitis-related cirrhosis, and an epidemic of hepatitis C virus (HCV) infection in the late 1970s [7]. As the population ages, it is becoming increasingly important to recognize age-specific cancer epidemiologic trends and differences in cancer outcomes to aid in prevention strategies, diagnostic approaches, and treatment decisions.
Historically, the elderly represent an important population particularly vulnerable to disparities in cancer treatment [8, 9]. There is a paucity of information on the possible differences in the clinical presentation, treatment patterns, and outcomes of HCC based on age. The limited available data are mainly from Asia, where HCC has a distinct underlying etiology and different treatment pattern [10–17]. Additional studies from Europe reflect disease trends inherent to a less demographically diverse population and particular patterns of clinical practice, thus making it difficult to extrapolate to U.S. patients [18, 19].
The increasing HCC incidence, aging population, development of new HCC treatment modalities, and limited age-related data available prompted the current study. By using a multi-institutional database, we designed the present study to examine the clinical characteristics, treatment patterns, and outcomes in elderly patients with HCC.
Patients and Methods
Patient Selection
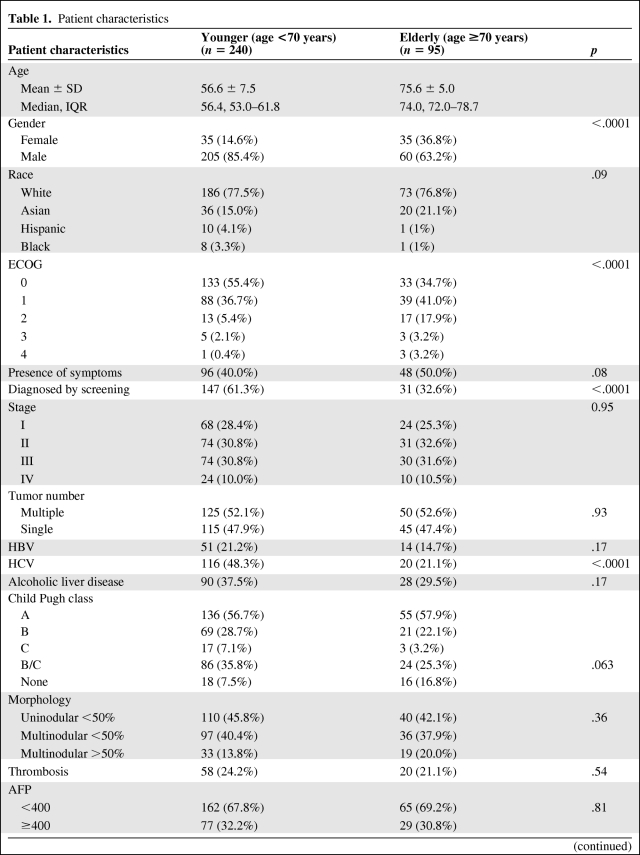
Retrospective analysis of HCC patients treated at two U.S. tertiary institutions from January 1998 to December 2008 was performed. A diagnosis of HCC was confirmed histopathologically or according to the noninvasive European Association for the Study of the Liver (EASL) diagnostic criteria [20]. The age of 70 years was chosen as the cutoff point for the analysis because the 70 years of age landmark has been described as the lower boundary of senescence and the incidence of age-related organ dysfunction and the development of comorbid conditions start to increase after the age of 70 [21]. Additionally, 70 years of age has been the most frequently used cutoff age for comparisons between younger and older patients in previous analyses of HCC patients, thus allowing for some comparability with these other studies. The demographics, etiology of liver disease, tumor parameters, comorbidities, performance status, body mass index, detailed treatment information, length of survival from diagnosis, and cause of death were captured. Race/ethnicity was categorized as White, Black, Asians, and Hispanic. The etiology of the liver disease was categorized as infection with hepatitis B (HBV), HCV, alcoholic liver disease, nonalcoholic fatty liver disease, hemochromatosis, autoimmune hepatitis, and primary biliary cirrhosis. The etiology of cirrhosis was considered to be cryptogenic if no cause for chronic liver disease was identified after exhaustive testing. No quantitative analysis of alcohol consumption was performed; assessment of habitual alcohol use was based on patient self-reporting of habitual drinking.
Underlying cirrhosis was confirmed histologically or based on clinical and/or radiological criteria including nodular liver contour, presence of ascites, varices, enlargement of the caudate lobe, splenomegaly, and collateral portal-venous anastomoses. We also assessed whether the diagnosis of HCC was made during evaluation for possible HCC based on presenting signs or symptoms or as a result of surveillance.
Multimodality treatment was defined as therapy that combines more than one method (e.g., combination of chemotherapy and embolization) of treatment. Death was categorized as attributable to HCC based on review of death certificate, discharge summary, or hospice documentation. Institutional Review Board approval was granted from each institution for this retrospective study.
Statistical Analysis
The patient characteristics were summarized using mean ± SD, median and interquartile range for continuous variables, and percentages for categorical variables. Chi-square tests and Wilcoxon rank sum tests were used to compare the patient characteristics between the two age groups. Three types of survival analysis were performed to compare the clinical outcome between the elderly and younger patients: (a) Overall survival (OS) defined as the interval between the date of HCC diagnosis and the date of death due to any cause; (b) HCC-specific survival defined as the time from HCC diagnosis to death from HCC (deaths from non-HCC causes were treated as censoring); (c) HCC-specific survival, but non-HCC death was treated as a competing risk. The Kaplan-Meier method was used to estimate survival distribution for OS and HCC-specific survival. Cumulative incidence rate for HCC death was estimated for the two age groups when non-HCC death was treated as a competing risk.
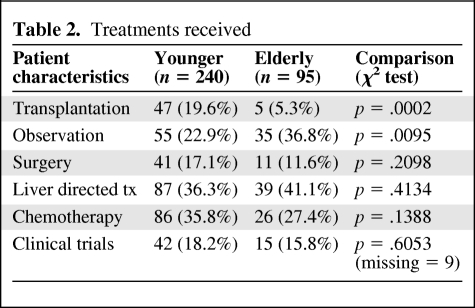
A Cox proportional hazard regression model was developed to evaluate the survival difference between the elderly and nonelderly groups while other prognostic factors were controlled. All factors listed in Table 1, but not age, were included in the original model, and a stepwise method was used for variable selection. Eastern Cooperative Oncology Group (ECOG) performance status (PS), cancer stage, presence of symptoms, Child-Turcotte-Pugh (CTP) class, portal vein thrombosis (PVT), BMI, and HCV were identified as the significant prognostic factors for OS and HCC-specific survival and included in the final model. Analyses were performed separately for patients with and without orthotopic liver transplantation (OLT) because transplanted HCC patients have substantial long-term survival and the majority of transplanted patients were in the younger group.
Table 1.
Patient characteristics
Table 1.
(Continued)
Abbreviations: AFP, alpha-fetoprotein; CAD, coronary artery disease; CLIP, Cancer of the Liver Italian Program; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; HTN, hypertension; IQR, interquartile range.
All tests were two-sided with significance level p < .05. All statistical analyses were conducted using SAS 9.2 statistical software (SAS Institute, Cary, NC).
Results
Patients Characteristics
A total of 335 patients with HCC were included. Ninety-five patients ≥70 years old (median age of 74 years) at diagnosis of HCC were defined as the elderly group. Two hundred forty patients aged <70 years (median age of 56) were regarded as the younger group. The patient characteristics are summarized in Table 1. The male (M)-to-female (F) ratio was 1.7:1 in the elderly and 5.8:1 in the younger group, showing that there was a higher proportion of women in the elderly group (p < .0001). The proportion of patients with HCV infection was significantly lower in the elderly group (21.1% versus 48.3%, p < .0001). There were no significant differences in HBV infection rates between younger and elderly patients (21.2% and 14.7%, respectively). Alcohol-induced liver disease was found in 37.5% of younger patients and 29.5% of the elderly group; the difference was not statistically significant (p = .1657). There was a trend toward more advanced CTP class in the younger group: CTP class B and C cirrhosis 35.8% versus 25.3% (p = .063). younger patients were more frequently diagnosed as a result of HCC screening or surveillance (61.3% versus 32.6%, p < .0001). More patients in the elderly group had ECOG PS > 1 than in the younger group (24.2% versus 7.9%, p < .0001). There were no significant differences in the following: race distribution, stage, number of lesions, symptomatic at presentation, PVT, alpha-fetoprotein (AFP) level, Cancer of the Liver Italian Program score, diabetes mellitus, or BMI.
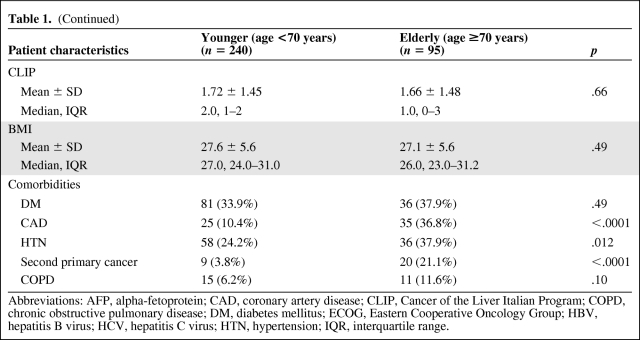
The types of treatments received are summarized in Table 2. Forty-seven (19.6%) of the younger patients underwent liver transplant compared to 5.3% of the elderly patients (p = .0002). Conversely, a higher proportion of elderly patients received only symptomatic treatment (36.8% versus 22.9%, p = .01). There was no difference in the number of liver resections or liver directed treatments, including radiofrequency ablation, transarterial chemoembolization, radiation, and chemotherapy. The rate of utilization of multimodality treatment was 28.3% in the younger group and 23.2% in the elderly group; the difference was not statistically significant.
Table 2.
Treatments received
Comorbidities
Eighty nine of 95 patients (93%) in the elderly group had comorbid conditions compared with 102 of 240 patients (42.5%) in the younger group (p < .0001). Cardiovascular and cerebrovascular disease, chronic lung conditions, second primary malignancies, chronic renal disease, or cognitive disorders constituted the most common comorbid conditions. The following statistically significant differences between the two groups were observed: 36.8% elderly patients had coronary artery disease compared to 10.4% in the younger group (p < .0001); similar trends were observed for hypertension (37.9% versus 24.2%, p = .0117) and second primary malignancy (21.1% versus 3.8%, p < .0001).
Overall Survival
Median follow-up was 15 months for the younger group and 11 months for the elderly group. For subjects who were alive at the time of this analysis, the median follow-up time was 27 months for the elderly and 31 months for the younger.
For all patients, the 1-, 2-, and 3-year survival rates were 52.9 ± 5.3%, 38.3 ± 5.2%, and 27.1 ± 5.2%, respectively, for the elderly group and 63.1 ± 3.2%, 46.8 ± 3.4%, and 35.9 ± 3.4%, respectively, for the younger group. Figure 1A shows the overall survival curves for the elderly and younger group. This difference was borderline significant (log-rank test, p = .06). One hundred forty-eight of the 190 patients in the younger group and 66 of the 90 patients in the elderly group died from all causes. Figure 1B shows the overall survival curves of the elderly group and the younger group after exclusion of patients who underwent OLT. The median survival time was 12.5 months (95% CI, 7.9–18.7 months) for the elderly group and 13.9 months (95% CI, 10.2–16.4 months) for the younger group. After exclusion of transplanted patients, the 1-, 2-, and 3-year survival rates were 51.4 ± 5.4%, 35.7 ± 5.3%, and 23.0 ± 5.2%, respectively, for the elderly group and 54.4 ± 3.7%, 35.9 ± 3.7%, and 23.5 ± 3.5%, respectively, for the younger group. This difference was not statistically significant (log-rank test, p = .47).
Figure 1.
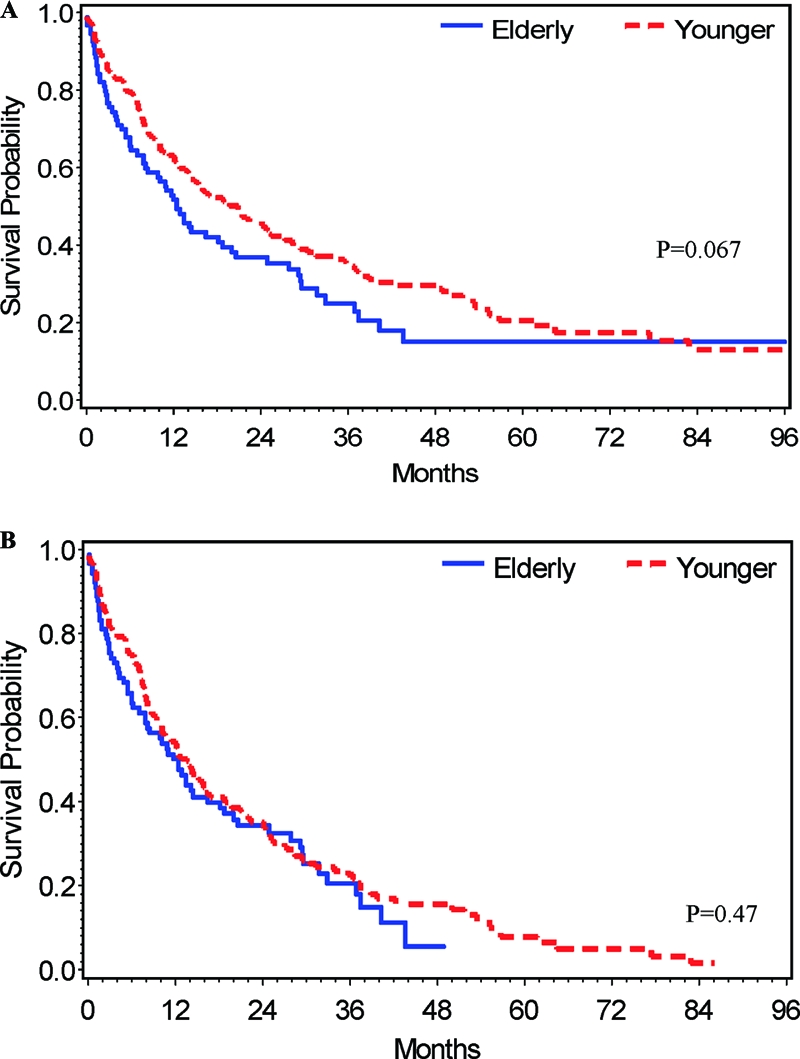
Kaplan-Meier overall survival curves. (A): Elderly (n = 95) and younger (n =240) patients. (B): Overall survival curves of elderly (n = 90) and younger (n = 193) patients after exclusion of patients who underwent orthotic liver transplantation. p values were calculated with the use of the log-rank test.
HCC-Specific Survival
One hundred eighty-six HCC-related deaths were documented: 46 in the elderly group and 140 in younger group. The median HCC specific survival time was 27.8 months (95% CI, 13.4–36.9 months) for the elderly group and 24.5 months (95% CI, 18.7–31.3 months) for the younger group.
For all patients, the 1-, 2-, and 3-year HCC-specific survival rates were 66.5 ± 5.3%, 52.3 ± 5.9%, and 39.1 ± 6.5%, respectively, for the elderly group and 66.7 ± 3.2%, 51.7 ± 3.5%, and 40.3 ± 3.6%, respectively, for the younger group. Figure 2A shows the HCC-specific survival curves for the elderly and younger group. This difference was not statistically significant (log-rank test, p = .89).
Figure 2.
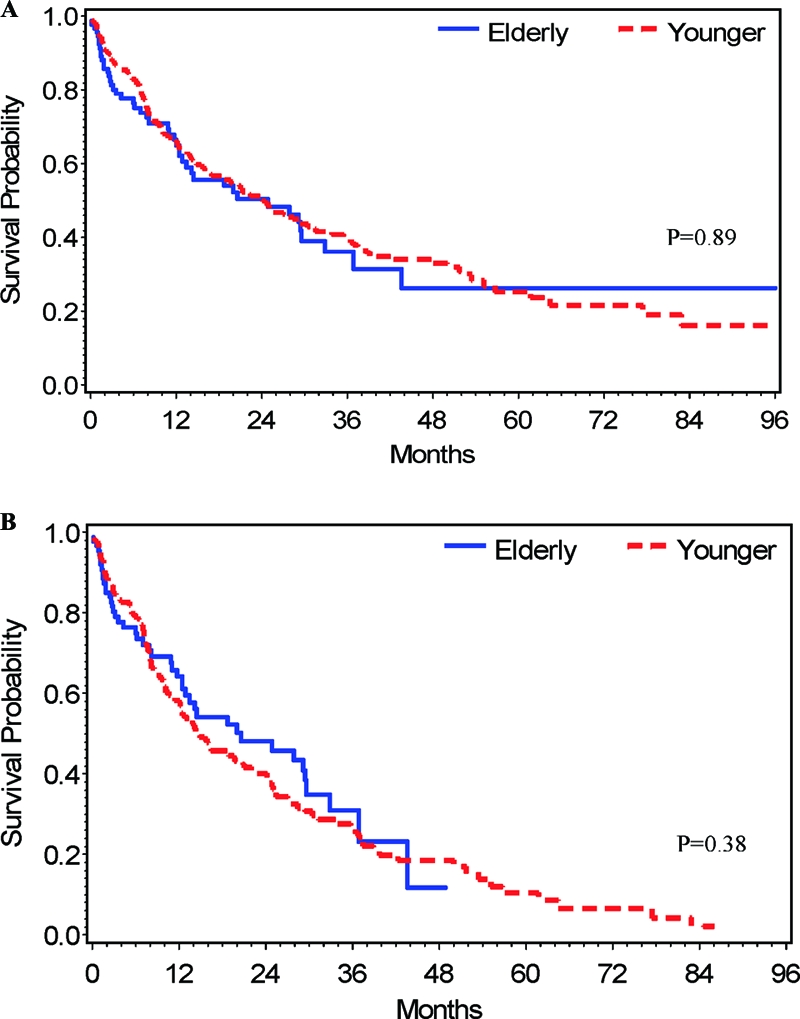
Kaplan-Meier HCC-specific survival curves. (A): All elderly (n = 95) and younger (n = 240) patients. (B): HCC-specific survival for elderly (n = 90) and younger (n =193) patients after exclusion of patients who underwent orthotic liver transplantation. p-values were calculated with the use of the log-rank test.
For the 280 patients who did not receive OLT, 45 (50%) of the 90 elderly patients died from HCC, versus 131 patients (69%) of 190 patients in the younger group. The median disease-specific survival time was 24.9 months (95% CI, 12.9–32.9 months) for the elderly group and 14.9 months (95% CI, 1–21.0 months) for the younger group. The estimated 1-, 2-, and 3-year HCC-specific survival rates were 65.9 ± 5.4%, 50.3 ± 6.2% and 34.9 ± 6.9%, respectively, for the elderly group and 58.4 ± 3.8%, 40.9 ± 3.9%, and 27.6 ± 3.9%, respectively, for the younger group. Figure 2B shows the HCC-specific survival curves of the elderly group and the younger group after exclusion of patients who underwent OLT. This difference was not statistically significant (log-rank test, p = .38).
HCC-Specific Survival Adjusted for Competing Risk
Forty-one non-HCC-related deaths were documented: 21 in the elderly group and 20 in younger group. Although non-HCC death was considered as a competing risk, there was no statistically significant difference in the cumulative incidence of the HCC-related death between the two patients groups, hazard ratio of 1.02 (95% CI, 0.73–1.43, p = .89). There were a total of 38 non-HCC deaths after transplanted patients were excluded: 21 in the elderly group and 17 in the younger group. The non-HCC-related deaths included infections, myocardial infarction, stroke, renal failure, suicide, and trauma.
Although non-HCC death was considered as a competing risk, there was no statistically significant difference in the cumulative incidence of the HCC-related death among the two patients groups, hazard ratio of 0.86 (95% CI, 0.61–1.21, p = .39). However, comparing with younger patients, elderly patients had a significantly worse survival for non-HCC related deaths with a hazard ratio of 3.27 (95% CI, 1.76–6.05, p = .0002).
Multivariate Analysis for Survival
Multivariate analysis did not show a significant difference in clinical outcomes between the two age groups. When the effects of the important prognostic factors were adjusted, the estimated hazard ratio was 1.33 (95% CI, 0.97–1.82, p = .07) for OS; 0.98 (95% CI, 0.68–1.41, p = .92) for HCC-specific survival; and 0.98 (95% CI, 0.66–1.46, p = .92) for HCC-specific survival adjusted for competing risk.
The results of multivariate analysis for patients without liver transplant are summarized in Table 3. No significant difference between the two age groups was found. The estimated hazard ratio was 1.23 (95% CI, 0.89–1.71, p = .21) for OS; 0.90 (95% CI, 0.62–1.30, p = .57) for HCC-specific survival; and 0.90 (95% CI, 0.59–1.36, p = .61) for HCC-specific survival adjusted for competing risk.
Table 3.
Estimated hazard ratios from Cox proportional hazards analysis for patients without liver transplant
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; PVT, portal vein thrombosis.
aHigh if BMI >27 (median); low otherwise.
Discussion
In this retrospective multicenter study, we compared the clinical characteristics, treatment modalities, and outcome data for elderly (age ≥70 years) compared to younger patients (age <70 years) with HCC. Characteristics that distinguished the elderly group included lower M/F ratio, fewer patients with HCV infections, and less advanced underlying liver disease than the younger group. Several previous studies have shown differences in clinico-pathological characteristics of elderly patients with HCC including a lower rate of viral hepatitis positivity, lower M/F ratio, lower AFP values, smaller tumor diameter, and a higher proportion of stage I-II patients in elderly group. However, most of these studies were conducted in Asia where HCC has a distinct epidemiologic pattern [22–24]. Additional data available from small-scale retrospective European studies reflect a relatively homogenous patient population and particular patterns of clinical practice, thus making it difficult to extrapolate to U.S. patients. Both of these studies found shorter survival in elderly patients with HCC that could not be accounted for just by differences in tumor extent or liver failure with the conclusion reached that this difference might be due to a lower rate of therapeutic intervention in elderly patients [18, 19].
A recent retrospective report from a U.S. institution illustrated differences in clinicopathological characteristics between young and elderly HCC patients. This cohort of patients differed from ours in that they were selected for transarterial embolization and therefore had relatively preserved hepatic function and less severe medical comorbidities. Similar to our findings, they found a lower M/F ratio and a lower rate of HCV/HBV co-infection in the elderly group. As opposed to our finding where no difference in stage distribution between the two age groups was observed, they found that the younger group had higher clinical tumor-node-metastasis stage [25]. The differences found in stage between the elderly and younger patients in the two studies may in part be due to the fact that elderly patients selected for transarterial embolization might have lower stage on average than younger patients.
It is interesting that a higher proportion of women in elderly HCC patients were observed in a recent epidemiologic study that analyzed age-adjusted trends in HCC incidence using U.S. Surveillance, Epidemiology, and End Results (SEER) registry data [1]. Although the reason for this difference has not been defined, it is possible that behavioral risk factors in younger males leading to earlier infection with HCV or HBV, and alcohol abuse might lead to a disproportionate increase in HCC incidence in younger males [26]. However, further studies are needed to confirm this finding, which could have implications for public health measures to develop strategies to decrease the higher rate of HCC in younger males. Moreover, SEER registry data demonstrated increased HCC incidence rates among Hispanic and black middle-aged men over the last 3 decades [1]. In our study, a higher proportion of Hispanic and black patients in the younger group were seen, although the numbers were small and not statistically different.
HCC screening and surveillance in a well-defined risk population is considered the standard of care in the United States. Nevertheless, in our study only 32.6% of patients in the elderly group were diagnosed via active screening and surveillance, as compared to 62% of the younger patient group. Despite this, there was no difference in stage distribution between the two groups. This lack of difference may be partially explained by accidental discovery of HCC in an elderly patient undergoing state-of-the-art medical care for comorbidities. It also reflects the relative inefficiency of current surveillance and screening approaches in detecting early HCC.
In contrast to other solid tumors, the presence of underlying liver cirrhosis in patients with HCC adds an important dimension that cannot be overemphasized when the prognosis and treatment of HCC are assessed. Interestingly, elderly patients in our study had less advanced liver disease, with CTP class B/C in 25.3% of patients compared to 35.8% in younger counterparts, although it did not reach statistical significance (p = .063). A higher proportion of younger patients (92.5%) had underlying chronic liver disease, based on histological and/or clinical and radiological studies as opposed to 83.2% of elderly patients. This may be partially explained by the fact that a proportion of patients with poor liver function might succumb to liver failure and not survive into elderly age. It is also possible that fewer HCV infections in the elderly group may partially contribute to these findings, although genetic, environmental, and other less clearly defined factors of hepatocarcinogenesis may play a role.
The prognosis of HCC patients is largely determined by tumor burden, hepatic function, and performance status [27, 28]. In our study, no difference was found in tumor burden, hepatic function, and symptomatic presentation in the two groups, although performance status was worse in the elderly group. Given that HCC disease characteristics (number of lesions, AFP levels, and stage) were similar between the elderly and younger patients, this poor performance status is likely a reflection of the higher number of comorbidities in the elderly, rather than directly related to HCC.
Several trends in selection of treatment modalities were noted between the two groups in our study. Because the majority of patients with HCC have underlying cirrhosis, it is the degree of hepatic dysfunction that dictates the choice of treatment [29, 30]. Not surprisingly, liver transplantation was uncommon in the elderly group, as only 10% of liver transplantation across the United States is performed in patients over 65 years old [29]. Supportive care alone was a more common course of action in the elderly, despite equal stage distribution and more preserved liver function on average in the elderly group. This discrepancy likely reflects decisions based on a poor performance status and competing medical conditions. Age-based patterns of care, as defined by practitioner and the patient, are likely implicated in this complex decision process. Our study complements the findings from the largest retrospective Western HCC database presented at ASCO 2009. Although this work illuminates differences in the pattern of care in different age groups, it does not provide details of the degree of liver dysfunction, comorbidities, and performance status, which are important for HCC treatment decisions [30].
In recent years, the chemotherapeutic armamentarium for HCC has expanded with introduction of the targeted therapy. Clinical trials are a major source of information for clinical decision making. However, historically, elderly patients have been underrepresented in clinical trials [31]. In contrast to lung cancer, lymphoma, breast cancer, and myeloma, there are few prospective studies designed for elderly patients with liver cancer. Elderly subgroup analyses have not been reported from the largest clinical trials involving patients with HCC. As a consequence, limited data are available about toxicity and tolerability of systemic therapy in this population. In our study, 27% of elderly patients received chemotherapy at some point of their treatment, which was not statistically different from the younger patient group. Moreover, 15% of elderly patients in our study were enrolled in clinical trials, a number similar to that of their younger counterparts. Advanced liver disease commonly presents a major limitation, when considering enrolling a HCC patient into a clinical trial. Given our results suggesting that elderly patients, on average, have less advanced liver disease, they should be offered the chance to participate in appropriate clinical trials. On the basis of our observations, elderly patients as a group are as willing to participate in clinical research as younger patients, when presented with an opportunity to enroll into a clinical trial.
Despite a higher prevalence of comorbidities and a significantly higher mean age, overall and HCC-specific survival of elderly patients was comparable to that of their younger counterparts. Low survival rate in both groups indicating morbid nature of HCC could potentially explain this result as HCC mortality surpasses the impact of both comorbidity and age-adjusted life expectancy. These findings are in line with results previously reported focusing on liver-directed therapy and surgical intervention, which include a healthier subset of the HCC population [12, 25, 32, 33]. Given the high mortality for HCC in both the younger and the elderly populations, clinical trials that have proportionate representation of the elderly would permit extrapolation of the results to the elderly and allow for direct comparisons of outcomes for younger and older patients. The complexity of HCC diagnosis and management argues for development of a prospective multi-institutional HCC registration database. Prospective data collection is required to optimize evidence-based treatment choices appropriate for elderly patients with HCC and ensure meaningful gains in survival and quality of life in the elderly population.
Limitation
An advantage of this analysis is that there was access to demographic and clinical variables not available in many population-based databases, providing the ability to analyze associations that have not been well characterized in the past. However, the retrospective nature of this study is its biggest limitation. All patients in this study were managed at tertiary care hospitals with liver transplant programs, interventional radiology experts, and clinical trials available. Thus, our findings may not be applicable to the patients managed in settings without similar resources available.
Acknowledgments
We thank the registrars of the Massachusetts Cancer Registry for collecting additional data on mortality.
Author Contributions
Conception/Design: Olga N. Kozyreva, Dorcas Chi, Jeffrey W. Clark, Hejing Wang, Andrew X. Zhu
Provision of study material or patients: Olga N. Kozyreva, Kathy P. Theall, David P. Ryan, Andrew X. Zhu
Collection and/or assembly of data: Olga N. Kozyreva, Dorcas Chi
Data analysis and interpretation: Olga N. Kozyreva, Dorcas Chi, Jeffrey W. Clark, Hejing Wang, Andrew X. Zhu
Manuscript writing: Olga N. Kozyreva, Jeffrey W. Clark, David P. Ryan, Andrew X. Zhu
Final approval of manuscript: Olga N. Kozyreva, Dorcas Chi, Jeffrey W. Clark, Hejing Wang, Kathy P. Theall, David P. Ryan, Andrew X. Zhu
References
- 1.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lok AS, Seeff LB, Morgan TR, et al. HALT-C Trial Group Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarbah SA, Gramlich T, Younoszai A, et al. Risk factors for hepatocellular carcinoma in patients with cirrhosis. Dig Dis Sci. 2004;49:850–853. doi: 10.1023/b:ddas.0000030098.75759.32. [DOI] [PubMed] [Google Scholar]
- 4.N'Kontchou G, Paries J, Htar MT, et al. Risk factors for hepatocellular carcinoma in patients with alcoholic or viral C cirrhosis. Clin Gastroenterol Hepatol. 2006;4:1062–1068. doi: 10.1016/j.cgh.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975–2006. [Accessed December 1, 2009]. Available at http://seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- 6.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 8.Bouchardy C, Rapiti E, Blagojevic S, et al. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25:1858–1869. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- 9.Gross CP, Smith BD, Wolf E, et al. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazama H, Omagari K, Matsuo I, et al. Clinical features and treatment of hepatocellular carcinoma in eight patients older than eighty years of age. Hepatogastroenterology. 2001;48:1692–1696. [PubMed] [Google Scholar]
- 11.Dohmen K, Shirahama M, Shigematsu H, et al. Optimal treatment strategy for elderly patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:859–865. doi: 10.1111/j.1440-1746.2003.03306.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaibori M, Matsui K, Ishizaki M, et al. Hepatic resection for hepatocellular carcinoma in the elderly. J Surg Oncol. 2009;99:154–160. doi: 10.1002/jso.21221. [DOI] [PubMed] [Google Scholar]
- 13.Nomura F, Ohnishi K, Honda M, et al. Clinical features of hepatocellular carcinoma in the elderly: a study of 91 patients older than 70 years. Br J Cancer. 1994;70:690–693. doi: 10.1038/bjc.1994.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poon RT, Fan ST, Lo CM, et al. Hepatocellular carcinoma in the elderly: results of surgical and nonsurgical management. Am J Gastroenterol. 1999;94:2460–2466. doi: 10.1111/j.1572-0241.1999.01376.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoshida Y, Ikeda K, Kobayashi M, et al. Chronic liver disease in the extremely elderly of 80 years or more: clinical characteristics, prognosis and patient survival analysis. J Hepatol. 1999;31:860–866. doi: 10.1016/s0168-8278(99)80287-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Rui JA, Wang SB, et al. Clinicopathological features, post-surgical survival and prognostic indicators of elderly patients with hepatocellular carcinoma. Eur J Surg Oncol. 2006;32:767–772. doi: 10.1016/j.ejso.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 17.Fujishima T, Ishikawa T, Shiratori Y, et al. Age-related comparison of the profiles of patients with hepatocellular carcinoma. Hepatogastroenterology. 2006;53:913–918. [PubMed] [Google Scholar]
- 18.Fernández-Ruiz M, Guerra-Vales JM, Llenas-García J, et al. Hepatocellular carcinoma in the elderly: clinical characteristics, survival analysis, and prognostic indicators in a cohort of Spanish patients older than 75 years. Rev Esp Enferm Dig. 2008;100:625–631. doi: 10.4321/s1130-01082008001000005. [DOI] [PubMed] [Google Scholar]
- 19.Pignata S, Gallo C, Daniele B, et al. Characteristics at presentation and outcome of hepatocellular carcinoma (HCC) in the elderly. A study of the Cancer of the Liver Italian Program (CLIP) Crit Rev Oncol Hematol. 2006;59:243–249. doi: 10.1016/j.critrevonc.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 21.Balducci L. Geriatric oncology: challenges for the new century. Eur J Cancer. 2000;36:1741–1754. doi: 10.1016/s0959-8049(00)00169-6. [DOI] [PubMed] [Google Scholar]
- 22.Jing-Lin X, Sharma D, Bing-Hui Y, et al. Analysis of the clinicopathological features of hepatocellular carcinoma in elderly patients. J Nepal Med Assoc. 2008;47:132–135. [PubMed] [Google Scholar]
- 23.Huang J, Li BK, Chen GH, et al. Long-term outcomes and prognostic factors of elderly patients with hepatocellular carcinoma undergoing hepatectomy. J Gastrointest Surg. 2009;13:1627–1635. doi: 10.1007/s11605-009-0933-4. [DOI] [PubMed] [Google Scholar]
- 24.Tsukioka G, Kakizaki S, Sohara N, et al. Hepatocellular carcinoma in extremely elderly patients: an analysis of clinical characteristics, prognosis and patient survival. World J Gastroenterol. 2006;12:48–53. doi: 10.3748/wjg.v12.i1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton RH, Covey A, Petre EN, et al. A comparison of outcomes from treating hepatocellular carcinoma by hepatic artery embolization in patients younger or older than 70 years. Cancer. 2009;115:5000–5006. doi: 10.1002/cncr.24556. [DOI] [PubMed] [Google Scholar]
- 26.Paulozzi L, Annest J. Unintentional poisoning deaths—United States, 1999–2004. CDC. MMWR. 2007;56:93–96. [PubMed] [Google Scholar]
- 27.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 28.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–715. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 29.The Organ Procurement and Transplantation Network. [Accessed December 1, 2009]. Available at http://optn.transplant.hrsa.gov/latestData/rpt.
- 30.Sanyal AJ, Moyneur E, Barghout V. Retrospective claims database analysis of elderly compared with nonelderly patients (pts) with newly diagnosed hepatocellular carcinoma (HCC) J Clin Oncol. 2009;27 abstract 9552. [Google Scholar]
- 31.Townsley CA, Chan KK, Pond GR, et al. Understanding the attitudes of the elderly towards enrolment into cancer clinical trials. BMC Cancer. 2006;6:34–34. doi: 10.1186/1471-2407-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanazaki K, Kajikawa S, Shimozawa N, et al. Hepatic resection for hepatocellular carcinoma in the elderly. J Am Coll Surg. 2001;192:38–46. doi: 10.1016/s1072-7515(00)00778-x. [DOI] [PubMed] [Google Scholar]
- 33.Mirici-Cappa F, Gramenzi A, Santi V, et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: a 20-year multicentre experience. Gut. 2010;59:387–396. doi: 10.1136/gut.2009.194217. [DOI] [PubMed] [Google Scholar]