The frequency, nature, trends, predictors, and outcomes of chemotherapy-related hospitalizations among a nonselected population of cancer patients treated at a community cancer center are described. The feasibility of implementing continuous quality improvement methodologies in routine oncology practice is explored.
Keywords: Chemotherapy, Adverse events, Cancer, Quality improvement
Learning Objectives
After completing this course, the reader will be able to:
Define the potential risks of chemotherapy and evaluate the relationship between multiple comorbid conditions and the likelihood of experiencing these risks.
Describe a process for tracking severe chemotherapy toxicity in a patient population, enumerate the barriers to implementation, and describe ways to overcome the barriers.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Purpose.
To describe the frequency, nature, trends, predictors, and outcomes of chemotherapy-related hospitalizations (CRHs) among a nonselected population of cancer patients treated at a community cancer center, and to explore the feasibility of implementing continuous quality improvement methodologies in routine oncology practice.
Methods.
We conducted a prospective cohort study of consecutive adult cancer patients who received chemotherapy at a community cancer center January 2003 to December 2006. Demographic, comorbidity, diagnosis, treatment, and laboratory data were collected via medical record abstraction. Hospitalizations were classified as chemotherapy related or unrelated by a multidisciplinary panel. Patients who experienced CRHs were compared with those who did not. Using a randomly sampled subset of cases and controls, we built a logistic regression model to identify independent predictors of CRH.
Results.
Of 2,068 chemotherapy recipients, 179 (8.7%) experienced 262 CRHs. Most hospitalizations were not chemotherapy related (73.7%). The mean monthly rate of CRH was 1.5%, the median length of stay was 5 days, the most common type of CRH was gastrointestinal (46.1%) followed by infectious (31.4%), and 0.9% of chemotherapy recipients had a fatal CRH. Significant predictors of CRH included having a comorbidity score of 3–4 versus 0 and having a higher creatinine level.
Conclusions.
Although the vast majority of chemotherapy recipients did not experience a CRH, these events were, unfortunately, not without serious consequences. Care should be taken when offering chemotherapy to patients with multiple comorbid conditions. Systematic efforts to monitor toxicity can lead directly to improvements in quality of care.
Introduction
Chemotherapy can offer substantial benefit to patients with many types of cancer, but also has the potential to cause significant toxicity. Unlike other medical therapies, the therapeutic index for chemotherapy, the ratio of the effective dose to the toxic dose, is often much lower and the risk for toxicity much higher. Consequently, decision making for chemotherapy requires careful consideration of and accurate information on both the potential benefits and the potential risks. Reports of clinical trials provide detailed information regarding the benefits of chemotherapy; these benefits appear to be similar regardless of whether patients are treated within or outside a clinical trial setting [1, 2]. However, these same reports provide relatively limited and potentially incomplete data regarding the risks of chemotherapy [3–5]. Moreover, although the vast majority of cancer patients are treated in community centers or subspecialty clinics, little is known about the risks of chemotherapy in these settings.
There are several reasons to suspect that the risks of chemotherapy administered in routine practice may be different from those observed during a clinical trial. Patients enrolled in clinical trials are clearly different—they tend to be younger, have fewer comorbid health conditions, and are less likely to represent socioeconomically disadvantaged populations [6–16]. In addition to selecting patients who tend to be healthier and have more extensive social supports, the more frequent follow-up often required by clinical trials raises the possibility that adverse effects are caught earlier, making serious toxicity less common. A retrospective analysis of claims data supported this hypothesis [17]. Unfortunately, prospectively collected data on chemotherapy-related toxicities experienced outside clinical trials are hard to come by.
Drug-related serious adverse effects have been defined as any untoward medical occurrences that are related to drug use and that result in death or significant disability/incapacity, require hospital admission or prolong an existing hospital stay, or are life threatening [18, 19]. We decided to focus on chemotherapy-related hospitalizations (CRHs) as a surrogate for chemotherapy-related serious adverse effects, because they are relatively easy to identify and are meaningful to patients, providers, and payers. Our goals were to describe the frequency, nature, trends, and outcomes of CRHs among a nonselected population of cancer patients treated at a community cancer center, to identify predictors of these events, and to explore the feasibility of implementing continuous quality improvement methodologies in routine oncology practice.
Methods
Setting
We conducted a prospective cohort study of consecutive adult cancer patients who received chemotherapy at a community cancer center in northeastern Massachusetts. The North Shore Cancer Center (NSCC) was a freestanding ambulatory cancer facility in Peabody, MA—located 20 miles north of Boston (now centered in Danvers, MA and called the Mass General/North Shore Cancer Center). Residents of the surrounding county have a median age of 39.2 years and a median household income of $61,505 (U.S. 2007 inflation-adjusted dollars); 83.2% are white, 3.2% are black, 2.9% are Asian, and 9% identify with some other race [20]. The NSCC provides ambulatory cancer services, including chemotherapy and radiation therapy, to a region that encompasses >200,000 adults. During the data collection period, January 1, 2003 to December 31, 2006, approximately 1,200 new cancer cases were evaluated and 3,000 chemotherapy administrations were delivered by the cancer center each year. The NSCC is affiliated with two local hospitals—Salem Hospital, Salem, MA, and Union Hospital, Lynn, MA—that are responsible for the vast majority of hospitalizations experienced by patients treated at the cancer center.
Case Identification and Data Collection
As part of an internal quality improvement project, the NSCC initiated a prospective effort to identify and collect data on all chemotherapy-related hospital admissions experienced by its patients. A detailed description of the procedures for this program were described previously [21]. In brief, the clinic relied on multiple, overlapping case-identification methods to identify admissions. Because all oncology admissions to the regional hospitals are seen by dedicated hematology/oncology subspecialty hospitalists, daily comparison of the patients on these services and the patients receiving chemotherapy at the NSCC identified nearly all admissions. For the rare cases in which patients were admitted to nonregional hospitals, these events were identified by missed clinic or treatment appointments and confirmed by clinic staff through direct contact with patients or families and the outside hospital.
For each admission, a standardized data collection form was completed by a nurse using the ambulatory and hospital charts as the primary data sources. Information collected included patient age and sex, cancer type, concurrent comorbid medical conditions, date and cycle number for the most recent chemotherapy treatment, intent of chemotherapy (curative, adjuvant, or palliative), date of hospital admission, reason for admission, length of hospital stay, and outcome of the admission (discharged versus in-hospital death). Comorbid medical conditions were coded according to the method developed by Charlson and colleagues, and included the optional age addition (+1 if 50–59, +2 if 60–69, +3 if 70–79, +4 if 80–89, +5 if 90–99) [22, 23]. When patients were admitted to hospitals other than the two mentioned above, records from the admitting hospital and reports from the patients and families were used to help complete the data collection form. A second reviewer confirmed the information entered on the data collection form by comparing it with primary source documents.
Each month, a team of medical oncologists, hematology/oncology subspecialty hospitalists, nurse practitioners, nurses, and pharmacists met to review each admission and determine whether or not it should be considered chemotherapy related. An admission was considered chemotherapy related if it occurred within 30 days following a chemotherapy administration, and it was judged to be definitely, probably, or possibly a result of a chemotherapy medication the patient was receiving. All other admissions were thought to be related to the underlying cancer diagnosis or to a noncancer diagnosis, and were considered unrelated to chemotherapy. If an admission could be attributed to both chemotherapy and cancer, the team chose to be inclusive and considered the admission chemotherapy related. Participants in the monthly team meetings numbered approximately six to 10 and were not blinded to the identities of the patients. Attributions were consensus based.
Derivation and Analysis of the Primary Dataset
We used the cancer center's pharmacy database to identify the total population of NSCC patients receiving chemotherapy in the ambulatory clinic. Because the pharmacy database records every newly started chemotherapy regimen, it provided a reliable method for determining the size and characteristics of the population of chemotherapy recipients at the NSCC. Pharmacy data were merged with CRH data (information on patients who were hospitalized with chemotherapy-related adverse effects) to create the primary analytic dataset. Pairings between the two datasets were based on matches between multiple unique identifiers, including medical record number, patient name, and patient date of birth.
We used this merged dataset to characterize CRHs, the patients who experienced these hospitalizations, and the frequencies of these events among all new chemotherapy regimens. Individual patients could experience multiple CRHs and start multiple chemotherapy regimens; these analyses focused on hospitalizations per new chemotherapy cycle, rather than patients, as the units of analysis. To facilitate interpretability, hospitalizations were categorized based on the primary reason for admission: infectious (e.g., pneumonia, bacteremia, or febrile neutropenia), hematologic (e.g., anemia or thrombocytopenia), gastrointestinal (e.g., emesis or diarrhea), cardiac (e.g., heart failure), metabolic (e.g., hyponatremia), or other. Primary cancer diagnoses were categorized as breast, lung, colorectal, and other—separating out each individual diagnosis that accounted for >10% of the total cohort.
Risk Factor Identification: Nested Case–Control Analysis
We collected additional data for a subset of hospitalized and nonhospitalized patients to build a model to identify independent predictors (i.e., risk factors) for CRH. Through random selection, we identified 100 cases (patients who received chemotherapy and experienced a CRH) and 200 controls. Data on 33 demographic and clinical variables that we hypothesized might be associated with experiencing a CRH were gathered via retrospective chart review. For time-sensitive variables, data were collected on the day a chemotherapy regimen was initiated. Using bivariate analysis, we determined which covariates were associated with CRH. Those that demonstrated a significant association with CRH on bivariate analysis and those considered potentially significant based on previous clinical experience (13 total covariates) were entered into a logistic regression model. Comorbidity score and performance status score were entered as categorical variables to facilitate interpretability; aspartate transaminase was entered as a log-transformed variable because the primary data were not normally distributed. Age was not part of the baseline model because the comorbidity score included age as a component (age and comorbidity score demonstrated a correlation of 0.6). However, as part of the sensitivity analysis, age was added to the model as a continuous predictor and separately as a categorical variable using groupings that mirrored the age component of the comorbidity score (<50, 50–59, 60–69, 70–79, and 80+).
All primary data were entered into a Microsoft Excel file (Microsoft Corporation, Redmond, WA) and subsequently converted to SAS files. Data merging, matching, and statistical analyses were conducted using SAS, version 9.1 (SAS Institute Inc., Cary, NC). All p-values were two sided. p < .05 was considered statistically significant. The NSCC institutional review board reviewed and approved the protocols for this study.
Results
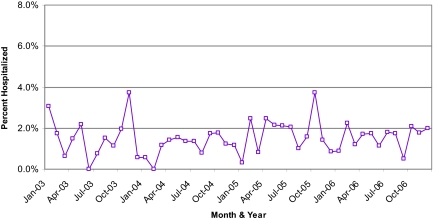
Among 2,068 cancer patients who started at least one new chemotherapy regimen at the NSCC between January 2003 and December 2006, we identified 826 total hospitalizations. Of these, 262 (26.3%) were classified as chemotherapy related; the remaining were considered disease related or unrelated to cancer or its associated therapy. There were 361 chemotherapy recipients (range, 268–434), 17 total hospitalizations (range, 2–30), and five CRHs (range, 0–14) on average per month. Approximately 1.5% of chemotherapy recipients experienced a CRH each month (range, 0%–3.7%). The proportion hospitalized each month remained relatively stable throughout the 4-year study (Fig. 1). The 262 CRHs occurred in 179 patients. Most chemotherapy recipients (91.3%) experienced no CRHs.
Figure 1.
Chemotherapy-related hospitalization rate over time. The monthly chemotherapy-related hospitalization rate, defined as the number of chemotherapy-related hospitalizations divided by the number of patients on active chemotherapy, remained relatively stable throughout the 4-year study.
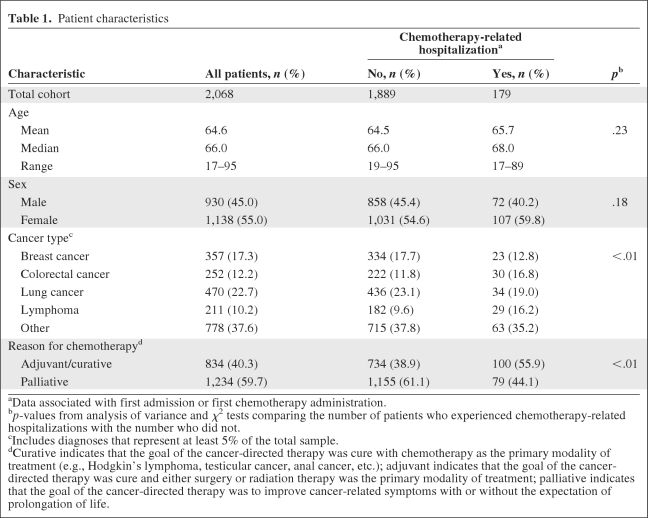
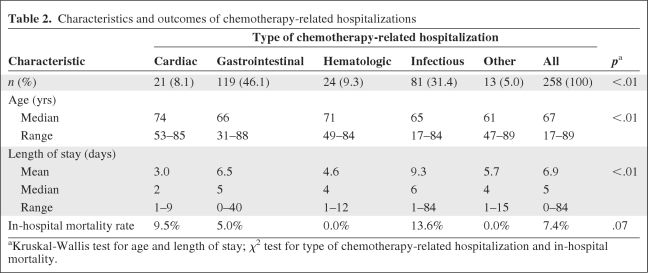
We were able to gather detailed information on 258 (98.5%) CRHs. Comparing patients who did with those who did not experience a CRH, the mean age (65.7 years versus 64.5 years; p = .23) and percentage female (59.8% versus 54.6%; p = .18) were not significantly different (Table 1). Those who experienced a CRH were more likely to have received chemotherapy for adjuvant/curative than for palliative purposes (55.9% versus 38.9%; p < .01). The proportion of chemotherapy recipients who experienced a CRH varied by cancer type—it was 14.2% for lymphoma, 11.9% for colorectal cancer, 7.2% for lung cancer, 6.4% for breast cancer, and 8.1% for other cancers. The CRH rate was higher when chemotherapy was given for adjuvant/curative than for palliative purposes among lymphoma (37% versus 10%) and colorectal cancer (15% versus 8%) patients, but not among breast cancer patients (3% versus 7%). The characteristics and outcomes of CRH appear in Table 2. Gastrointestinal problems, such as nausea, vomiting, or diarrhea, were responsible for the largest fraction of CRHs, followed by infectious problems, such as pneumonia or febrile neutropenia. The median length of stay for all CRHs was 5 days. Hospitalizations related to cardiac problems tended to be the shortest, whereas those related to infectious problems tended to be the longest.
Table 1.
Patient characteristics
aData associated with first admission or first chemotherapy administration.
bp-values from analysis of variance and χ2 tests comparing the number of patients who experienced chemotherapy-related hospitalizations with the number who did not.
cIncludes diagnoses that represent at least 5% of the total sample.
dCurative indicates that the goal of the cancer-directed therapy was cure with chemotherapy as the primary modality of treatment (e.g., Hodgkin's lymphoma, testicular cancer, anal cancer, etc.); adjuvant indicates that the goal of the cancer-directed therapy was cure and either surgery or radiation therapy was the primary modality of treatment; palliative indicates that the goal of the cancer-directed therapy was to improve cancer-related symptoms with or without the expectation of prolongation of life.
Table 2.
Characteristics and outcomes of chemotherapy-related hospitalizations
aKruskal-Wallis test for age and length of stay; χ2 test for type of chemotherapy-related hospitalization and in-hospital mortality.
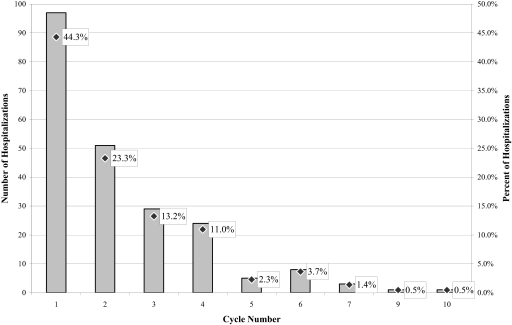
Mortality during chemotherapy was uncommon—only 0.9% of all chemotherapy recipients experienced a CRH that ended in death. Among those patients who experienced at least one CRH, the in-hospital mortality rate was 7.4%. Patients admitted for infectious problems experienced the highest in-hospital mortality rate at 13.6%. Fatalities were more frequent among recipients of palliative than adjuvant/curative chemotherapy (10.1% versus 4.2%; p = .07), though on multivariate analysis this was only a borderline-significant predictor (odds ratio, 2.8; 95% confidence interval, 0.9–8.4; p = .07). The average time from chemotherapy to admission was shorter for fatal than for nonfatal admissions (3.6 days versus 7.7 days; p < .01). The distribution of CRHs by chemotherapy cycle number in which each hospitalization occurred appears in Figure 2. Approximately two thirds of all CRHs occurred during the first two cycles of chemotherapy; only 8% occurred after the fourth cycle of chemotherapy. It is not surprising that most CRHs occurred early on during the course of therapy, because delayed/cumulative adverse effects from chemotherapy are less common and patients who experience serious adverse effects from chemotherapy usually do not continue to receive multiple cycles of the offending chemotherapy medication. We were not able to compare the risk for experiencing a CRH during each cycle because the total number of chemotherapy recipients at risk during each cycle was not known.
Figure 2.
Hospitalizations by cycle of chemotherapy. Shown are the total number of hospitalizations by the chemotherapy cycle number during which the hospitalizations occurred (left axis) and the percent of hospitalizations by chemotherapy cycle number (right axis). Although the exact number of patients eligible to receive each cycle of chemotherapy was not known, this number generally decreased as the cycle number increased.
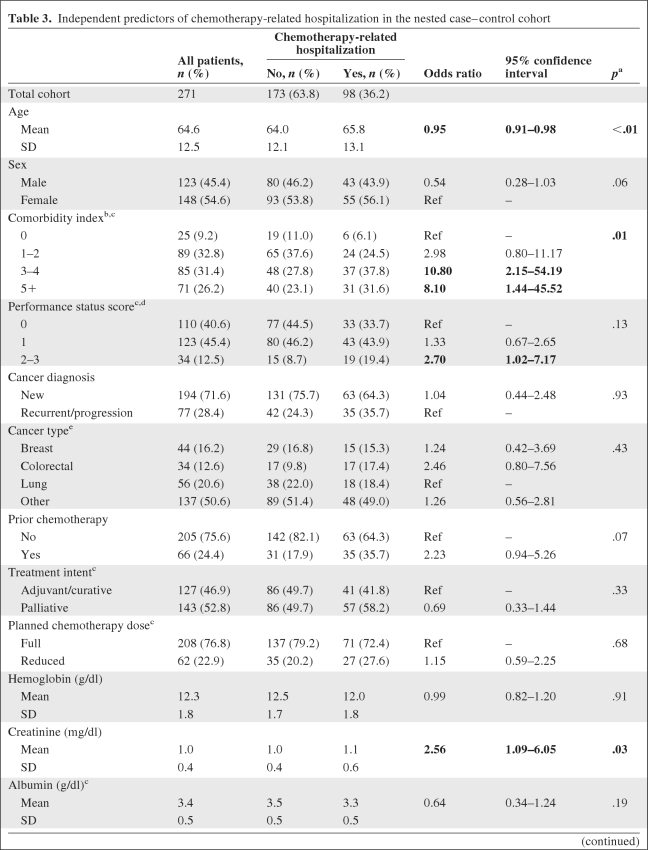
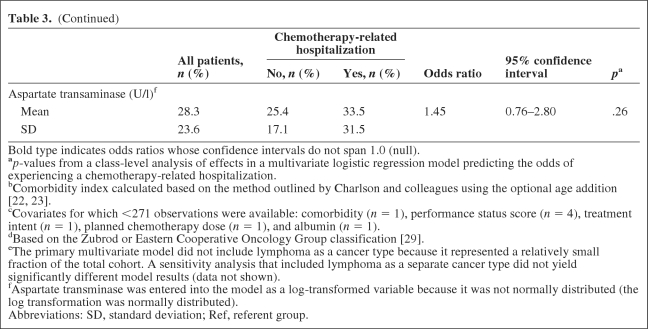
Of the 100 cases and 200 controls randomly selected for the nested case–control cohort analysis, chart abstraction was completed for 98 cases (98%) and 173 controls (86%). Patients for whom complete medical record data were unavailable were excluded from further analysis. Significant predictors of CRH on bivariate analysis included the mean comorbidity score (3.9 for cases versus 3.0 for controls; p < .01), a performance status score of 2–3 versus 0 (19.4% versus 8.7%; p = .02), a recurrent/progressive versus new cancer diagnosis (35.7% versus 24.3%; p = .04), and receipt of prior chemotherapy (35.7% versus 17.9%; p < .01). In addition, the mean creatinine and aspartate transaminase values were significantly higher and the mean albumin was significantly lower among those who experienced a CRH. On multivariate analysis excluding age as a covariate, only two variables were significant predictors of CRH—comorbidity and creatinine. Having a comorbidity score ≥3 was the strongest predictor of CRH. Having a higher creatinine level at the initiation of chemotherapy was also associated with a greater odds for experiencing a CRH (Table 3). Forward or stepwise selection did not result in significantly different model output than that generated from backward selection. When age was entered into the model as a continuous predictor, younger patients demonstrated a slightly greater risk for CRH, potentially because they were more likely to receive intensive chemotherapy administered for curative intent. Age as a categorical variable did not significantly change the model output (results not shown).
Table 3.
Independent predictors of chemotherapy-related hospitalization in the nested case–control cohort
Table 3.
(Continued)
Bold type indicates odds ratios whose confidence intervals do not span 1.0 (null).
ap-values from a class-level analysis of effects in a multivariate logistic regression model predicting the odds of experiencing a chemotherapy-related hospitalization.
bComorbidity index calculated based on the method outlined by Charlson and colleagues using the optional age addition [22, 23].
cCovariates for which <271 observations were available: comorbidity (n = 1), performance status score (n = 4), treatment intent (n = 1), planned chemotherapy dose (n = 1), and albumin (n = 1).
dBased on the Zubrod or Eastern Cooperative Oncology Group classification [29].
eThe primary multivariate model did not include lymphoma as a cancer type because it represented a relatively small fraction of the total cohort. A sensitivity analysis that included lymphoma as a separate cancer type did not yield significantly different model results (data not shown).
fAspartate transminase was entered into the model as a log-transformed variable because it was not normally distributed (the log transformation was normally distributed).
Abbreviations: SD, standard deviation; Ref, referent group.
Discussion
In a cohort of >2,000 community cancer center patients, CRH occurred in 8.7% of chemotherapy recipients and accounted for approximately one quarter of all hospitalizations. Relatively few chemotherapy recipients experienced a CRH each month (≤3.7%). Despite changes in chemotherapy prescribing patterns, the proportion of chemotherapy recipients who experienced a CRH did not change significantly over time. Gastrointestinal and infectious problems accounted for a majority of all CRHs. Two thirds of CRHs occurred during the first two cycles of chemotherapy; only 8% occurred after the fourth cycle. Chemotherapy for palliative purposes was more common, but the risk for experiencing a CRH was greater among those receiving chemotherapy for adjuvant/curative purposes (12.0% versus 6.4%). Fatal CRHs were uncommon and could have been disease and/or treatment related. In a nested case–control analysis, comorbidity score was the strongest predictor of CRH.
This study is one of only a small number to characterize the risks associated with chemotherapy in a community-based setting. In a previous analysis, Hassett and colleagues [17] used insurance claims from a population-based sample of women aged ≤63 years old with breast cancer to identify hospitalizations for chemotherapy-related adverse effects in the year after diagnosis. This previous analysis was limited to breast cancer patients, did not provide as much detail regarding the risk factors for and outcomes of CRHs, and was not able to independently validate hospitalizations as truly chemotherapy related, but it did report a similar rate of CRH, that is, 9%. Whereas infectious CRHs were more common in the previous study gastrointestinal CRHs were more common in this analysis, probably because the other study focused only on breast cancer patients, who are less likely to receive chemotherapy medications that cause diarrhea. Not unexpectedly, the type of CRH correlated with the length of hospitalization, and the goal of therapy (curative versus palliative) correlated with in-hospital mortality. Performance status score was not an independent predictor of CRH in this study, but the number of chemotherapy recipients with a poor performance status was small, suggesting there may have been overestimation of performance status; continued caution when offering chemotherapy to patients with a performance status score >2 is warranted.
Beyond simply estimating the frequency of and risk factors for CRH in routine practice, this study demonstrates that chemotherapy-related toxicity can be assessed as part of routine clinical practice. Moreover, we found that monitoring toxicity on a routine basis using a structured format fostered continuous quality improvement. We identified several interventions that we believe may help reduce the frequency of selected types of CRHs in our practice setting. For example, adopting routine glucose monitoring for patients receiving high-dose corticosteroids may help reduce hyperglycemia-related CRHs. And making modest modifications to the chemotherapy regimens used for colorectal cancer may prevent some admissions for diarrhea. The ability to integrate quality measurement and improvement efforts into the daily activities of ambulatory care is likely to become increasingly important in the future. Professional boards are requiring this for recertification, and the federal government considers quality monitoring to be an integral part of meaningful use. The recently published American Society of Clinical Oncology/Oncology Nursing Society Chemotherapy Administration Safety Standards require that practices have processes in place to document chemotherapy-related toxicity and use these data to plan subsequent treatment [24].
This study is not without its limitations. All the patients were treated at one community cancer center, so it is difficult to know the extent to which these results can be generalized to other centers. The cohort included a relatively small fraction of patients from traditionally underrepresented sociodemographic groups. Also, it did not include patients with acute leukemia, bone marrow transplant recipients, or patients receiving oral chemotherapy medications, and we could not control specifically for the type of chemotherapy administered. Consensus and expert opinion were used to characterize hospitalizations as chemotherapy related or not, but a significant proportion of hospitalizations may have been multifactorial. Because the panel tended to be overinclusive when characterizing which hospitalizations were chemotherapy related, the rates of CRH reported herein may represent overestimates. More research is needed to help understand the extent to which comorbidity causes hospitalizations for noncancer diagnoses among chemotherapy recipients or potentiates the development of CRHs. The sample size for the nested case–control cohort was relatively small, so the multivariate model offered relatively limited power to identify significant predictors of CRH. With a larger sample, it may have been possible to identify additional predictors and more accurately characterize the magnitude of effect for the predictors that were identified. For example, while sex was not a significant predictor in this model, there was a trend toward fewer CRHs among men (p = .06) that warrants further analysis.
Although the vast majority of chemotherapy recipients did not experience a CRH, these events were, unfortunately, not infrequent and not without serious consequences. Our findings suggest that care should be taken when offering chemotherapy to patients with multiple comorbid conditions. Although this study advances our understanding of the toxicities experienced by patients treated in routine clinical practice, additional studies are needed to further explore the frequency of and risk factors for chemotherapy-related serious adverse effects in the community setting. This information can only help facilitate informed decision making. In light of the increasing focus being placed on quality by professional organizations, the federal government, and recertification bodies, demonstrating that it is feasible to introduce routine quality monitoring into clinical practice is meaningful. Systematic efforts to monitor toxicity can lead directly to improvements in quality of care. Professional organizations have begun to develop resources that facilitate practices' efforts to measure and improve quality of care—the Quality Oncology Practice Initiative [25–28] is one widely available, generally accepted, and maturing example. Significant advances in quality improvement will be possible only if we are able to enhance existing quality measurement/improvement tools (e.g., by adding new measures and facilitating quality improvement efforts) and address barriers to participating meaningfully in these efforts.
Acknowledgments
We acknowledge and thank Maryann Papi and Rosemary Stankiewicz for their generous assistance with data collection and study coordination.
The Norman H. Reed Charitable Trust provided financial assistance for the study. The American Society of Clinical Oncology Career Development Award provided salary support to Dr. Hassett. The sponsors had no direct influence on the design of the study, analysis of the data, interpretation of the results, or writing of the manuscript.
This study was presented as a poster at the 2009 American Society of Clinical Oncology Annual Meeting, Orlando, Florida.
Author Contributions
Conception/design: Michael J. Hassett, Joel H. Schwartz, Joseph O. Jacobson
Provision of study materials or patients: Joel H. Schwartz, Betty Maloney, Joseph O. Jacobson
Collection/assembly of data: Suzana Brozovic, Joel H. Schwartz, Betty Maloney, Joseph O. Jacobson
Data analysis and interpretation: Michael J. Hassett, Sowmya R. Rao, James E. Stahl, Joel H. Schwartz, Joseph O. Jacobson
Manuscript writing: Michael J. Hassett, Sowmya R. Rao, Suzana Brozovic, Joel H. Schwartz, Joseph O. Jacobson
Final approval of manuscript: Michael J. Hassett, Sowmya R. Rao, Suzana Brozovic, James E. Stahl, Joel H. Schwartz, Betty Maloney, Joseph O. Jacobson
References
- 1.Peppercorn JM, Weeks JC, Cook EF, et al. Comparison of outcomes in cancer patients treated within and outside clinical trials: Conceptual framework and structured review. Lancet. 2004;363:263–270. doi: 10.1016/S0140-6736(03)15383-4. [DOI] [PubMed] [Google Scholar]
- 2.Vist GE, Hagen KB, Devereaux PJ, et al. Systematic review to determine whether participation in a trial influences outcome. BMJ. 2005;330:1175. doi: 10.1136/bmj.330.7501.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fromme EK, Eilers KM, Mori M, et al. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22:3485–3490. doi: 10.1200/JCO.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: An evaluation of 7 medical areas. JAMA. 2001;285:437–443. doi: 10.1001/jama.285.4.437. [DOI] [PubMed] [Google Scholar]
- 5.Ladewski LA, Belknap SM, Nebeker JR, et al. Dissemination of information on potentially fatal adverse drug reactions for cancer drugs from 2000 to 2002: First results from the research on adverse drug events and reports project. [Erratum in J Clin Oncol 2004;22:1169] J Clin Oncol. 2003;21:3859–3866. doi: 10.1200/JCO.2003.04.537. [DOI] [PubMed] [Google Scholar]
- 6.Aapro MS, Kohne CH, Cohen HJ, et al. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. The Oncologist. 2005;10:198–204. doi: 10.1634/theoncologist.10-3-198. [DOI] [PubMed] [Google Scholar]
- 7.Adams-Campbell LL, Ahaghotu C, Gaskins M, et al. Enrollment of African Americans onto clinical treatment trials: Study design barriers. J Clin Oncol. 2004;22:730–734. doi: 10.1200/JCO.2004.03.160. [DOI] [PubMed] [Google Scholar]
- 8.Antman K, Amato D, Wood W, et al. Selection bias in clinical trials. J Clin Oncol. 1985;3:1142–1147. doi: 10.1200/JCO.1985.3.8.1142. [DOI] [PubMed] [Google Scholar]
- 9.Britton A, McKee M, Black N, et al. Threats to applicability of randomised trials: Exclusions and selective participation. J Health Serv Res Policy. 1999;4:112–121. doi: 10.1177/135581969900400210. [DOI] [PubMed] [Google Scholar]
- 10.Bugeja G, Kumar A, Banerjee AK. Exclusion of elderly people from clinical research: A descriptive study of published reports. BMJ. 1997;315:1059. doi: 10.1136/bmj.315.7115.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross CP, Filardo G, Mayne ST, et al. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103:483–491. doi: 10.1002/cncr.20792. [DOI] [PubMed] [Google Scholar]
- 12.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 13.Simon MS, Du W, Flaherty L, et al. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol. 2004;22:2046–2052. doi: 10.1200/JCO.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 15.Elting LS, Cooksley C, Bekele BN, et al. Generalizability of cancer clinical trial results: Prognostic differences between participants and nonparticipants. Cancer. 2006;106:2452–2458. doi: 10.1002/cncr.21907. [DOI] [PubMed] [Google Scholar]
- 16.Tournoux C, Katsahian S, Chevret S, et al. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006;106:258–270. doi: 10.1002/cncr.21613. [DOI] [PubMed] [Google Scholar]
- 17.Hassett MJ, O'Malley AJ, Pakes JR, et al. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98:1108–1117. doi: 10.1093/jnci/djj305. [DOI] [PubMed] [Google Scholar]
- 18.Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis, and management. Lancet. 2000;356:1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 19.Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: A clinician's guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140:795–801. doi: 10.7326/0003-4819-140-10-200405180-00009. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Census Bureau. American FactFinder. [accessed August 27, 2009]. Available at http://factfinder.census.gov/home/saff/main.html?_lang=en.
- 21.Krzyzanowska MK, Treacy J, Maloney B, et al. Development of a patient registry to evaluate hospital admissions related to chemotherapy toxicity in a community cancer center. J Oncol Pract. 2005;1:15–19. doi: 10.1200/jop.2005.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36:453–471. doi: 10.1016/s0959-8049(99)00319-6. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson JO, Polovich M, McNiff KK, et al. American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. Oncol Nurs Forum. 2009;36:651–658. doi: 10.1188/09.ONF.651-658. [DOI] [PubMed] [Google Scholar]
- 25.Blayney DW, McNiff K, Hanauer D, et al. Implementation of the Quality Oncology Practice Initiative at a university comprehensive cancer center. J Clin Oncol. 2009;27:3802–3807. doi: 10.1200/JCO.2008.21.6770. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the Quality Oncology Practice Initiative. J Clin Oncol. 2008;26:1893–1898. doi: 10.1200/JCO.2007.14.2992. [DOI] [PubMed] [Google Scholar]
- 27.McNiff KK, Neuss MN, Jacobson JO, et al. Measuring supportive care in medical oncology practice: Lessons learned from the Quality Oncology Practice Initiative. J Clin Oncol. 2008;26:3832–3837. doi: 10.1200/JCO.2008.16.8674. [DOI] [PubMed] [Google Scholar]
- 28.Neuss MN, Desch CE, McNiff KK, et al. A process for measuring the quality of cancer care: The Quality Oncology Practice Initiative. J Clin Oncol. 2005;23:6233–6239. doi: 10.1200/JCO.2005.05.948. [DOI] [PubMed] [Google Scholar]
- 29.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]