A literature review of in silico planning comparative studies that include proton-based and photon-based irradiation techniques in the treatment of head and neck cancer is presented.
Keywords: Proton therapy, Head and neck cancer, Radiotherapy, In silico treatment planning, Radiation-induced side effects
Abstract
Purpose.
Clinical studies concerning head and neck cancer patients treated with protons reporting on radiation-induced side effects are scarce. Therefore, we reviewed the literature regarding the potential benefits of protons compared with the currently used photons in terms of lower doses to normal tissue and the potential for fewer subsequent radiation-induced side effects, with the main focus on in silico planning comparative (ISPC) studies.
Materials and Methods.
A literature search was performed by two independent researchers on ISPC studies that included proton-based and photon-based irradiation techniques.
Results.
Initially, 877 papers were retrieved and 14 relevant and eligible ISPC studies were identified and included in this review. Four studies included paranasal sinus cancer cases, three included nasopharyngeal cancer cases, and seven included oropharyngeal, hypopharyngeal, and/or laryngeal cancer cases. Seven studies compared the most sophisticated photon and proton techniques: intensity-modulated photon therapy versus intensity-modulated proton therapy (IMPT). Four studies compared different proton techniques. All studies showed that protons had a lower normal tissue dose, while keeping similar or better target coverage. Two studies found that these lower doses theoretically translated into a significantly lower incidence of salivary dysfunction.
Conclusion.
The results of ISPC studies indicate that protons have the potential for a significantly lower normal tissue dose, while keeping similar or better target coverage. Scanned IMPT probably offers the most advantage and will allow for a substantially lower probability of radiation-induced side effects. The results of these ISPC studies should be confirmed in properly designed clinical trials.
Introduction
The main objective of modern radiotherapy is to optimize radiation dose delivery in such a way that the tumor is sterilized while sparing nontarget normal tissues as much as possible. In particular, in head and neck cancer this general objective cannot be easily achieved in the majority of patients because target volumes are generally large and complex and surrounded by many organs at risk (OARs). Hence, the incidences of severe acute and late radiation-induced side effects are relatively high, in particular when radiation is combined with systemic treatment, such as concomitant chemoradiation [1].
The introduction of advanced photon radiation techniques, such as 3-dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiotherapy (IMRT), has already led to significant progress in dose conformity to the tumor and the sparing of normal tissues [2]. However, the physical properties of photon beams do not leave much room for further improvement.
From a physical point of view, charged particles such as protons have an evident advantage over photons. Whereas photons have a maximum dose near the surface followed by a continuous reduction in dose with depth, protons deposit almost all their radiation energy in the so-called Bragg peak. By varying individual proton beam energies, one can produce a spread out Bragg Peak (SOBP) that covers the tumor accurately and delivers a substantially lower dose to normal tissues beyond the tumor. These characteristics allow for better target dose conformity than with the currently used photon techniques.
The superior beam properties of protons over photons can be translated into clinical benefits using two different strategies.
First, protons can be used to escalate the tumor dose, providing possibilities to improve local tumor control without higher doses to healthy surrounding tissue and subsequently without a higher risk for radiation-induced side effects. This strategy may be particularly useful when tumor dose escalation is expected to improve local tumor control, such as in lung and prostate cancer. It should be noted that there is virtually no difference in tumor response per unit dose between protons and photons, which means that the potential benefit of protons in terms of local tumor control can only be the result of physical dose escalation. Moreover, clinically, a relative biological effectiveness for protons (RBEprotons), that is, the ratio of photon dose required to cause an equivalent biological level of effect as a given proton dose, of 1.1 is generally accepted. Therefore, prescribed proton dose values are expressed in Gray equivalents (GyE), that is, the physical proton dose in Gy multiplied by the RBEprotons.
Second, protons can be used to deliver a lower normal tissue dose while keeping the target dose similar. In this case, tumor control is expected to be similar to the results obtained with photons, whereas radiation-induced side effects will most likely be less because the probability and severity of radiation-induced side effects strongly depend on the dose and volume irradiated.
At present, all published reviews on the added value of protons over photons [3–6] mainly focused on the role of protons in terms of treatment efficacy (i.e., local tumor control and overall survival) rather than on the potential benefits of protons in terms of a lower incidence of radiation-induced side effects. The first step in analyzing whether a new radiation technique will have the potential for fewer radiation-induced side effects is comparing dose distributions that can be obtained with the new technique referenced to the current standard technique, also referred to as in silico planning comparative (ISPC) studies. Therefore, the purpose of this study was to review the literature regarding the potential benefits of protons over photons in terms of lower doses to normal tissues and the potential for a subsequently lower incidence of radiation-induced side effects, with the main focus on ISPC studies.
Materials and Methods
Selection of Studies
A literature search was performed for studies that reported on the potential benefit of proton radiotherapy by comparing dose distributions between protons and photons in the same patient cohort (ISPC studies).
The following key words were used: synonyms for head and neck neoplasm or synonyms for head and neck cancer and synonyms for proton radiotherapy. These key words were combined using “AND.” The literature search was carried out in MEDLINE, Embase, and the Cochrane Library databases in November 2009 and was updated through March 2010. In addition, reference lists of papers were screened in order to retrieve additional relevant papers.
To be selected for this review, studies had to fulfill the following eligibility criteria: (a) include patients with cancer of the head and neck area; (b) include the paranasal sinuses, nasal cavity, oral cavity, oropharynx, hypopharynx, nasopharynx, or larynx tumor sites, and (c) include an adult patient population eligible for (chemo)radiotherapy, either primary, adjuvant, or in the reirradiation setting. Review studies and studies published before 1985, studies available only in abstract form, and studies written in languages other than English were excluded from this review.
Two independent observers assessed the relevant studies from the identified papers. Uncertainties with regard to inclusion of a specific paper or its contents were resolved by consensus between the two other reviewers.
ISPC Studies
In the last decades, radiation techniques in head and neck cancer have evolved dramatically from rather simple two-dimensional techniques based on direct simulation to more advanced techniques such as 3D-CRT, IMRT, and helical tomotherapy (HT), enabling better conformity of the high-dose area to the target volume. In addition, protons can also be delivered using different techniques, from three-dimensional conformal proton therapy (3D-CPT) to the more sophisticated intensity-modulated proton therapy (IMPT). In general, protons can be delivered by two main different techniques—passive scattering and the more sophisticated active scanning technique [7]. With scattered protons, each single beam delivers a uniform dose to the target. Because for each field the length of the SOBP is fixed and conformed to the distal edge of the target, this technique does not provide good conformity to the proximal target side. However, with scanned protons, a single beam can deliver any desired nonuniform dose distribution to the target and multiple nonuniform scanned proton beams can be combined to deliver a more uniform dose to the target (referred to as IMPT). Moreover, the length of the SOBP is not fixed, providing more degrees of freedom with regard to OAR sparing and target dose coverage. As a consequence, interpretation of ISPC studies may be influenced because different kinds of photon techniques and proton techniques have been compared. Therefore, for each ISPC study, the different radiation techniques were specified as well.
Normal Tissue Complication Probability Models
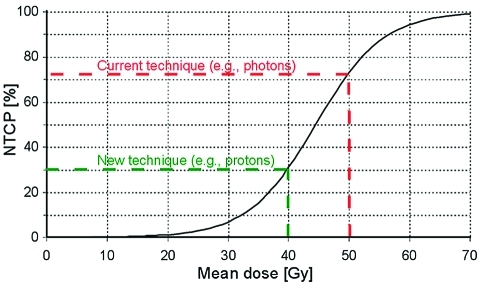
Lower delivered doses to normal tissues do not always translate into clinical benefits, that is, a lower incidence of radiation-induced side effects. The ultimate estimate of a lower incidence of side effects depends on the shape of the normal tissue complication probability (NTCP) curve (Fig. 1). NTCP models describe the relationship between radiation dose distribution parameters and the risk for a given side effect and may vary among different kinds of side effects. In the final step, to determine to what extent an optimized physical dose distribution will translate into an expected beneficial effect, in terms of a lower probability of radiation-induced side effects, data from ISPC studies should be combined with NTCP models (Fig. 1). When reported, these results were also included in this review.
Figure 1.
Example of a possible normal tissue complication probability (NTCP) model with the risk of a given complication (NTCP in %) as a function of radiation dose (in this case the mean dose). NTCP models can be used to estimate the risk for a certain complication as a function of dose and thus also to translate differences in dose into differences in the risk for side effects. In this example, the lower dose that can be obtained with the new technique (−10 Gy) translates into a −42% lower risk. Note that in the case of a dose reduction from 30 to 20 Gy, the benefit in terms of the risk reduction will be much less.
Results
Literature Search
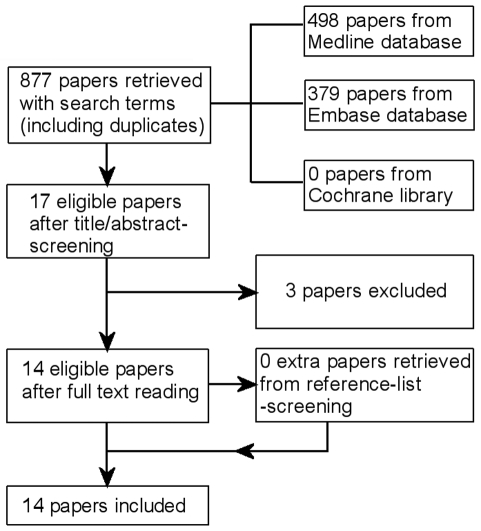
The initial literature search identified 877 papers that included 17 ISPC studies. After screening these papers, 14 ISPC studies that fulfilled the eligibility criteria were ultimately included in this review (Fig. 2). The three excluded studies were two ISPC studies only reporting on proton therapy and one ISPC study that was not completed.
Figure 2.
Papers retrieved from the literature search.
Because radiotherapy treatment of different tumor locations involves irradiation of different relevant OARs, we stratified the ISPC studies into three groups: (a) nasopharyngeal cancer, closely surrounded by critical neural tissues like the visual structures, the brain and brainstem, the pituitary gland, the auditory apparatus, the parotid glands, the larynx, and the oral cavity; (b) paranasal sinus cancer, closely surrounded by similar OARs as mentioned in (a) except for the more caudal structures like the larynx; and (c) the rest, with oropharyngeal, hypopharyngeal, and laryngeal cancer, particularly, surrounded by all major salivary glands, structures in the oral cavity, the spinal cord, the larynx, and thyroid gland.
Paranasal Sinus Cancer
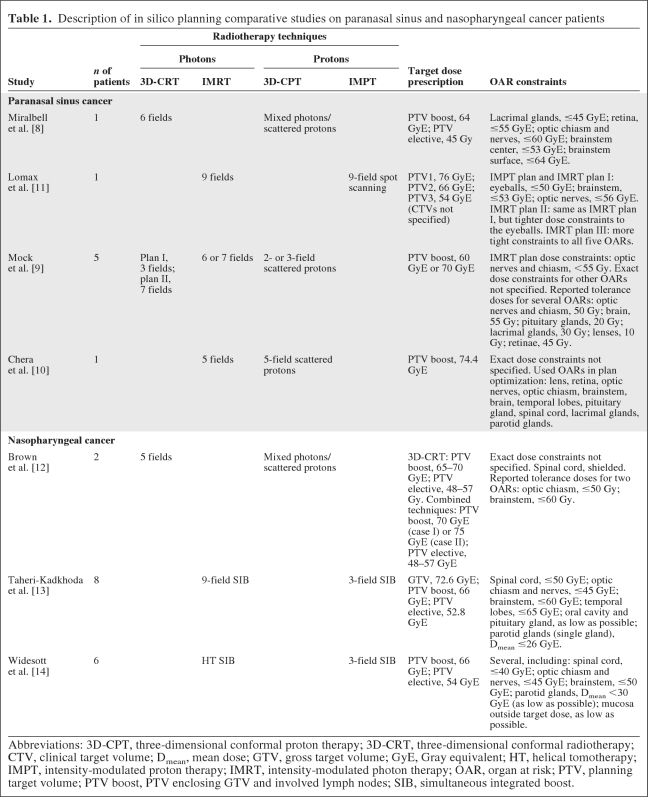
Four studies were identified reporting on ISPC studies among patients with paranasal sinus cancer (Table 1), including a total of eight patients. In most studies, 3D-CRT photons [8, 9] and/or IMRT [9, 10] were compared with scattered protons [8–10]. In one study, only mixed photon–scattered proton plans were compared with 3D-CRT [8]. There was only one study that compared IMRT with spot-scanning protons (IMPT) [11].
Table 1.
Description of in silico planning comparative studies on paranasal sinus and nasopharyngeal cancer patients
Abbreviations: 3D-CPT, three-dimensional conformal proton therapy; 3D-CRT, three-dimensional conformal radiotherapy; CTV, clinical target volume; Dmean, mean dose; GTV, gross target volume; GyE, Gray equivalent; HT, helical tomotherapy; IMPT, intensity-modulated proton therapy; IMRT, intensity-modulated photon therapy; OAR, organ at risk; PTV, planning target volume; PTV boost, PTV enclosing GTV and involved lymph nodes; SIB, simultaneous integrated boost.
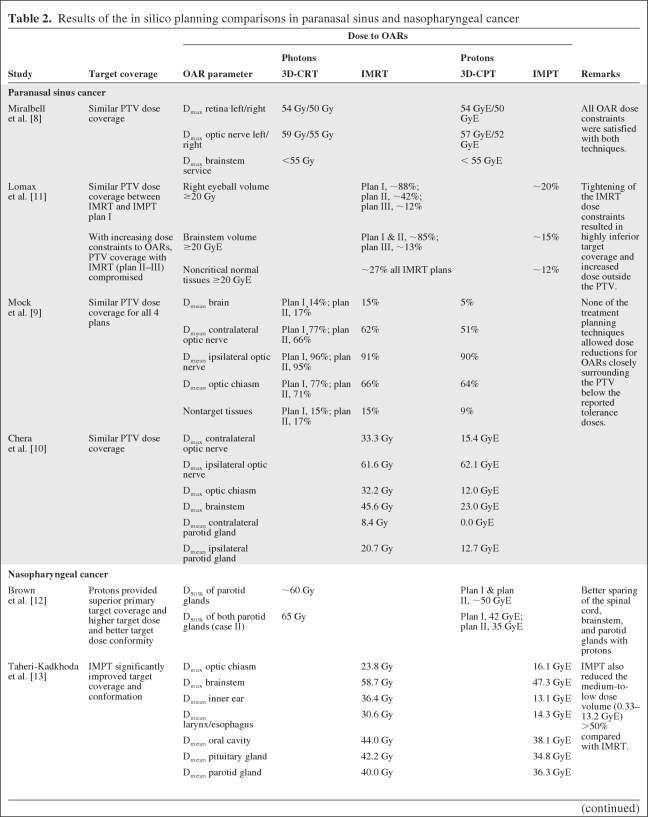
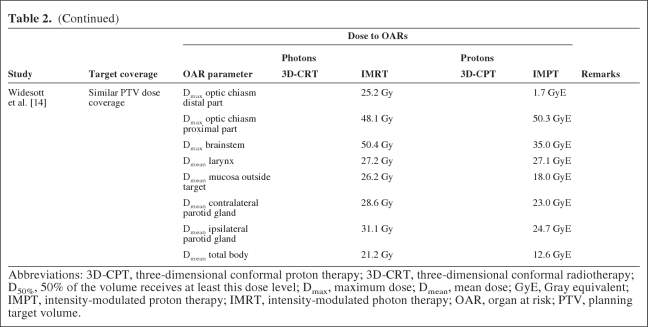
In three out of four studies, the dose coverage to the planning target volume (PTV) obtained with protons was similar to that with photons [8–10] (Table 2). Lomax et al. [11] carried out different comparisons between IMRT and spot-scanning IMPT, showing that increasing dose constraints to OARs resulted in highly inferior dose coverage of the PTV with IMRT, whereas the PTV dose coverage remained within acceptable limits when IMPT was used. Thus, in contrast to IMRT, IMPT enabled radiation dose escalation to the target without exceeding the tolerance dose of critical structures such as the optic chiasm and optic nerves. The dose to most OARs was markedly lower with protons than with photons, even for OARs for which no dose constraints were defined (Table 2). Miralbell et al. [8] showed that by increasing the weight for protons in the mixed photon–proton plans, a further reduction in the dose to OARs could be achieved.
Table 2.
Results of the in silico planning comparisons in paranasal sinus and nasopharyngeal cancer
Table 2.
(Continued)
Abbreviations: 3D-CPT, three-dimensional conformal proton therapy; 3D-CRT, three-dimensional conformal radiotherapy; D50%, 50% of the volume receives at least this dose level; Dmax, maximum dose; Dmean, mean dose; GyE, Gray equivalent; IMPT, intensity-modulated proton therapy; IMRT, intensity-modulated photon therapy; OAR, organ at risk; PTV, planning target volume.
Nasopharyngeal Cancer
Three studies were identified reporting on ISPC studies among patients with nasopharyngeal carcinoma (Table 1), including a total of 16 patients [12–14]. In one study, 3D-CRT was compared with mixed photon–scattered proton plans, whereas in the other two studies, three-field IMPT was compared with IMRT and HT. In one study [12], dose constraints were defined only for critical neural structures, whereas in the other two studies dose constraints for several other OARs, including the parotid glands, were taken into account. In two studies [12, 13], target dose coverage and dose conformity were significantly better using protons, whereas the target dose could be escalated without exceeding the tolerance dose of critical structures [12]. In all studies, the dose to all OARs could be markedly lower (Table 2). Of note is that the medium-to-low dose volume or the mean dose to the total body was lower with IMPT than with IMRT [13, 14], although the exact definition of this endpoint remained unclear.
Oropharyngeal, Hypopharyngeal, and Laryngeal Cancer
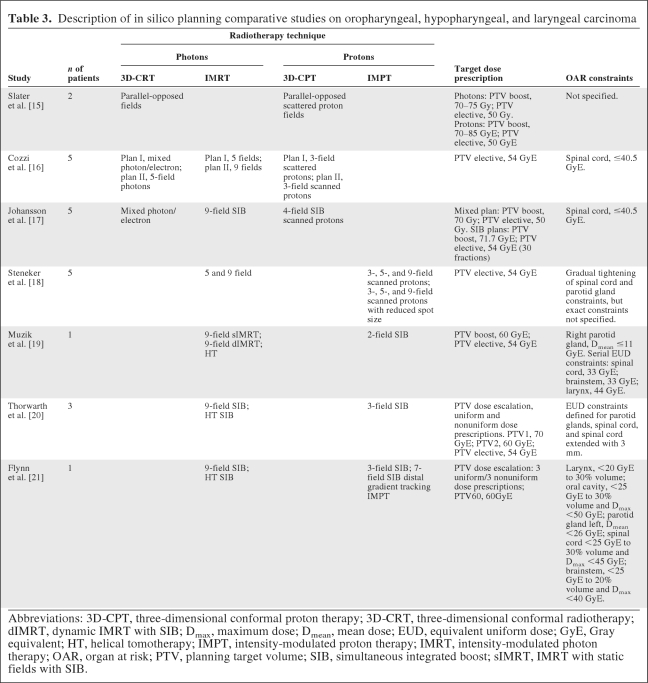
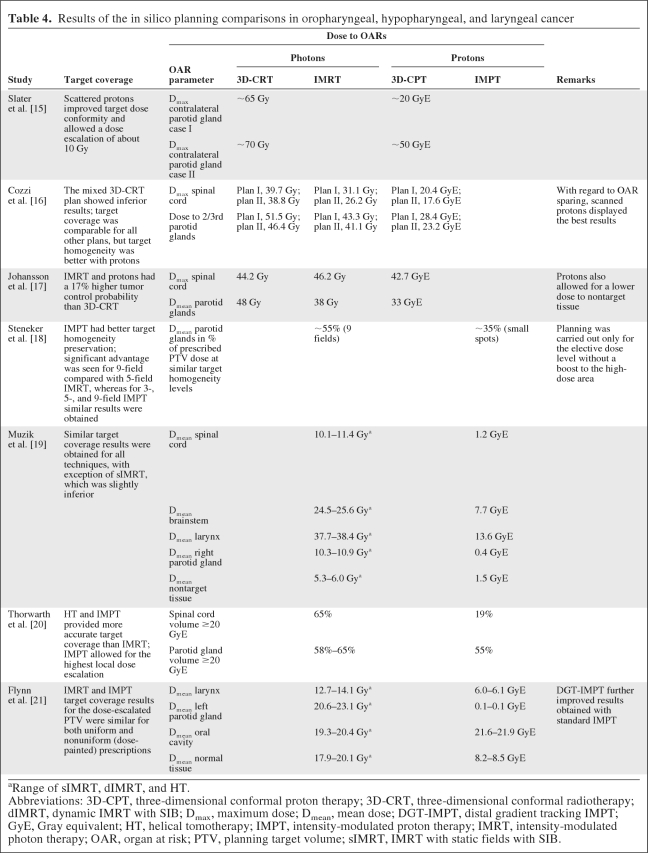
We identified seven studies reporting on ISPC studies among patients with oropharyngeal, hypopharyngeal, and laryngeal carcinoma, including a total of 22 patients [15–21] (Table 3). In one study, only 3D-CRT using parallel-opposed fields was compared with 3D-CPT [15]. However, in the majority of studies, IMRT or HT was compared with scanned and/or scattered 3D-CPT [16, 17] or with IMPT [18–21]. In general, dose conformity and dose coverage to the PTV were better when protons were used (Table 4). In one study [15], the use of scattered protons allowed dose escalation with approximately 10 Gy, without exceeding the tolerance dose to critical structures, which could not be achieved with photon techniques.
Table 3.
Description of in silico planning comparative studies on oropharyngeal, hypopharyngeal, and laryngeal carcinoma
Abbreviations: 3D-CPT, three-dimensional conformal proton therapy; 3D-CRT, three-dimensional conformal radiotherapy; dIMRT, dynamic IMRT with SIB; Dmax, maximum dose; Dmean, mean dose; EUD, equivalent uniform dose; GyE, Gray equivalent; HT, helical tomotherapy; IMPT, intensity-modulated proton therapy; IMRT, intensity-modulated photon therapy; OAR, organ at risk; PTV, planning target volume; SIB, simultaneous integrated boost; sIMRT, IMRT with static fields with SIB.
Table 4.
Results of the in silico planning comparisons in oropharyngeal, hypopharyngeal, and laryngeal cancer
aRange of sIMRT, dIMRT, and HT.
Abbreviations: 3D-CPT, three-dimensional conformal proton therapy; 3D-CRT, three-dimensional conformal radiotherapy; dIMRT, dynamic IMRT with SIB; Dmax, maximum dose; Dmean, mean dose; DGT-IMPT, distal gradient tracking IMPT; GyE, Gray equivalent; HT, helical tomotherapy; IMPT, intensity-modulated proton therapy; IMRT, intensity-modulated photon therapy; OAR, organ at risk; PTV, planning target volume; sIMRT, IMRT with static fields with SIB.
In general, the dose to OARs could be substantially lower, in particular to the parotid glands [15–19, 21], the larynx [19, 21], and the spinal cord [16, 19, 20]. In one study, no difference was noted among three-, five- and nine-field scanning IMPT [18]. There was only one study in which scattered protons were compared with scanned protons showing better OAR sparing with scanning protons [16]. Flynn et al. [21] used distal gradient tracking (DGT) IMPT, a method designed to deliver nonuniform target dose prescriptions by placing proton Bragg peaks only at locations of dose gradients in the prescribed dose distribution. Dose prescriptions for a hypoxic region in a head and neck squamous cell carcinoma patient were designed to either uniformly boost the region or redistribute the dose based on positron emission tomography images with a hypoxia tracer. IMRT and IMPT delivered comparable dose distributions within the boost region for both the uniform and redistributed boosts. However, the doses to the larynx and parotid glands as well as the integral dose to the nontarget tissue were substantially lower when IMPT was used instead of IMRT.
Do Lower Doses to OARs Result in Lower NTCP Values?
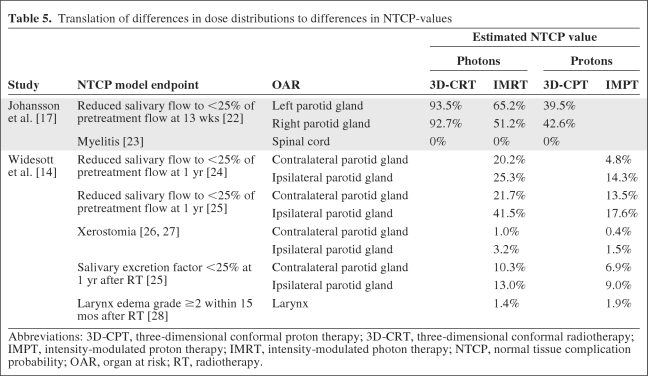
In two studies, the authors tried to translate the differences in dose distributions into clinical benefits in terms of differences in NTCP values using existing NTCP models [14, 17]. The NTCP models used were derived from other studies [22–28]. This model-based approach indicated that, for both parotid glands, scanned protons had significantly lower NTCP values for salivary flow [14, 17] and xerostomia [14] (Table 5). The NTCP model for the larynx did not result in a significant difference between IMPT and HT [14].
Table 5.
Translation of differences in dose distributions to differences in NTCP-values
Abbreviations: 3D-CPT, three-dimensional conformal proton therapy; 3D-CRT, three-dimensional conformal radiotherapy; IMPT, intensity-modulated proton therapy; IMRT, intensity-modulated photon therapy; NTCP, normal tissue complication probability; OAR, organ at risk; RT, radiotherapy.
Discussion
The findings of this review on ISPC studies showed that irradiation with protons in head and neck cancer patients generally results in a lower dose to normal tissues while target dose distributions are better, offering more opportunities for dose escalation, or at least remain similar in terms of homogeneity and conformity. This physical advantage may theoretically allow for improving the therapeutic ratio by reducing the risk for radiation-induced side effects while keeping the target dose the same or by target dose escalation without increasing the risk for radiation-induced side effects.
An ISPC study is one of the first and necessary steps in the development of emerging radiation techniques that allow for a comparison of physical dose distributions between the current standard and new radiation techniques and thus for assessing the potential of improving the therapeutic ratio. If performed properly, the study outcome may serve as a basis for the hypothesis and design of future clinical studies that should confirm the benefit of the new technique. However, proper interpretation of the outcome of ISPC studies requires a critical view on some methodological issues.
An important prerequisite for an ISPC study is that the new technique be compared with the best currently available technique. In addition, a valuable estimation of the new technique's benefit can be properly determined only if the new technique is tested using its full potential properties. In the treatment of head and neck cancer, we consider IMRT as the current standard, whereas the most optimal approach using protons remains to be determined. In this respect, there were only a few studies in which different proton techniques were compared [12, 16, 18, 21]. Brown et al. [12] compared two different mixed 3D-CRT–3D-CPT techniques and showed that OAR sparing was superior with the mixed technique that used the highest proton weight. However, this technique used protons only partly during the radiation course and therefore did not fully use their potential benefits with regard to the sparing of OARs. More recent studies did use protons for the full course. Cozzi et al. [16] showed that scanned 3D-CPT had better OAR sparing than scattered 3D-CPT. As previously discussed, IMPT (nonuniform dose fields) allows for more freedom with regard to OAR sparing than scanned 3D-CPT (uniform dose fields). Given the relatively large and complex-shaped PTVs close to OARs in head and neck cancer, IMPT is more likely to obtain a more optimal dose distribution than scanned 3D-CPT. Steneker et al. [18] found that, using IMPT, OAR sparing was most optimal with three-field IMPT using the smallest spot size. Increasing the number of fields neither improved target dose homogeneity nor further reduced the parotid gland dose. Flynn et al. [21] investigated further refinement of IMPT by comparing DGT-IMPT with IMPT for dose painting and showed that DGT-IMPT provided the most optimal results. Overall, these findings suggest that IMPT provides the most optimal results with regard to OAR sparing while keeping good target coverage results.
In total, seven of the 14 ISPC studies compared the most sophisticated photon technique (IMRT) with the most sophisticated proton technique (IMPT) [11, 13, 14, 18–21]. Therefore, these studies provide the most reliable information with regard to the potential benefit of protons over photons to spare critical structures. The results of these studies all showed markedly lower doses to different OARs with similar or even better dose distributions to the target volume. Of note is that different tumor sites were included in the seven studies and that a certain variety was observed in the dose distribution results. However, although all results point in the same direction, it cannot be ruled out that some bias occurred resulting from the fact that most studies were written by authors from centers with proton therapy.
There were some limitations with regard to the design and required or reported dose distribution results of some ISPC studies. For example, Steneker et al. [18] and Cozzi et al. [16] only used a total dose of 54 GyE for the elective nodal areas and the primary site without a boost to the high-risk areas. On the other hand, Chera et al. [10] and Mock et al. [9] only took into account high-risk areas. Because this is not what is currently considered standard, translation of these results to clinical practice is severely hampered.
Second, some authors did not clearly specify their planning objectives with regard to target coverage acceptance, e.g., the recommendations of the International Commission on Radiation Units and Measurements Report 50 [29], or defined target coverage criteria other than those currently considered standard [8, 11, 12, 15, 18–21].
Third, most authors only presented average results for all cases [9, 13, 14, 16, 18]. It should be noted that the benefit of a new technique in terms of dose distributions in targets and OARs will depend on a number of factors, such as the volume and shape of the target, the position of the target reference to OARs, and the amount of overlap between the PTV and a given OAR. Moreover, the translation of a lower dose to a lower estimated NTCP value strongly depends on the shape of the NTCP curve and the initial dose parameters as obtained by the currently available technique, as illustrated in Figure 1. Therefore, ISPC studies should also report results from individual patients in order to get more insight into the percentage of patients who will eventually benefit from protons in terms of a lower risk for radiation-induced side effects.
In almost all ISPC studies attempts were made to spare various OARs, including the spinal cord, brainstem, optic structures, and parotid glands (Tables 1–4). More recently, it has been recognized that, in addition to xerostomia, dysphagia is also an important side effect that adversely affects quality of life after radiotherapy of the head and neck area [30, 31]. Therefore, sparing of the structures related to both complications, including the salivary glands and pharyngeal constrictor muscles [31], is of importance. Of note is that most ISPC studies did report on the dose to the parotid glands; however, none of the studies reported on the dose to the pharyngeal constrictor muscles. Furthermore, OAR dose constraints used in the optimization process differed among the different studies. Moreover, the exact OAR dose constraints were not always clearly specified in all studies [9, 10, 18]. Of note is that the more recently published studies often included more OARs in their planning optimization.
To compare possibilities of different radiotherapy techniques with respect to target coverage and OAR sparing, different strategies can be handled depending on the study's aim. This makes it hard to allow fair comparisons across different treatment planning studies. To compare the possibilities of different radiotherapy techniques with respect to OAR sparing with similar target coverage in a fair manner, the target dose and OAR dose prescriptions and acceptance criteria should be identical for the different techniques, otherwise the obtained dose differences could also be a consequence of different dose prescriptions. Of note is that, in order to obtain the best plan with different radiation techniques, the dose-volume constraints used during plan optimization may have to be different for different techniques. On the other hand, if the study aim is to examine whether, with a less advanced technique (e.g., IMRT in [11]), similar OAR sparing can be obtained as achieved with a more advanced technique (e.g., IMPT in [11]), the dose prescriptions and acceptance criteria do not have to be the same. These latter studies also focus on the techniques' possible limitations or benefits, though not all treatment plans obtained with this study strategy are suitable for clinical practice. However, both discussed treatment planning comparison strategies are important to investigate differences between radiotherapy techniques.
Furthermore, in general, if no attempts are made to reduce OAR doses as much as possible, the achieved results will not necessarily be the best results that can be achieved with the specific techniques [11, 14]. Consequently, the obtained differences in NTCP cannot be used to adequately determine which technique is best to spare a specific OAR (e.g., the larynx structure spared by Widesott et al. [14]).
The question arises: What would be the most optimal way to deliver protons with regard to fractionation? Cozzi et al. [32] reported on the results of an ISPC study investigating the potential benefit of a full simultaneous integrated boost technique (SIB) in proton therapy compared with conventional fractionation with sequential (SEQ) boost and a mixed SEQ–SIB technique. The final results showed a dosimetric advantage for the SEQ–SIB technique in terms of target coverage while keeping similar OAR dose results. The authors concluded that the clinical introduction of this technique appears promising given the logistic advantages (lower number of fractions) and limited availability of proton therapy facilities.
In addition to fractionation issues, the clinical introduction of proton therapy in head and neck cancer may be hampered by a number of other problems. In general, protons are more sensitive to density heterogeneities (like air gaps, air-tissue-bone transitions) than photons [33–35]. Hence, image guidance and patient positioning are even more important. None of these ISPC studies reported on IMRT and IMPT plan-robustness-analysis comparisons, though such studies would be of interest with regard to the clinical application of these techniques. Furthermore, because there is not much experience with advanced proton techniques and as a result of the fact that proton therapy–based clinical studies reporting on radiation-induced side effects are scarce, the remaining question is whether or not the potential clinical benefit of proton therapy is worth the additional costs. Goitein et al. [36] and Peeters et al. [37] estimated that the relative proton versus photon treatment costs were ∼2.4 or ∼3.2, respectively, but could be reduced to ∼1.7 [36]. Note that the treatment-specific costs and the costs per fraction highly depend on the specific disease site, the treatment technique's complexity, the patient throughput, and clinical experience. Because clinical experience with the advanced proton techniques like IMPT is poor, there is still room to further optimize the application of this technique (also with regard to the treatment schedules used [32]). Note furthermore that the cost analysis studies did not take into account additional costs for treating radiation-induced side effects or for caring for not-cured patients. Additional costs for combined treatments like chemoradiotherapy were also not considered. ISPC studies indicated that protons have the potential for a lower probability of side effects [14, 17] and may lead to better application of dose escalation [20], which subsequently may improve tumor control. Protons also had a lower integral dose, which is of major importance for the treatment of pediatric patients [38, 39]. Therefore, the total costs for proton treatment could be much less than the estimated values. Finally, clinical data should provide the answer to the question of whether or not the potential clinical benefit of proton therapy is worth the additional costs.
Conclusion
ISPC studies indicate that protons have the potential for delivery of a significantly lower dose to several OARs, while keeping similar or even better target coverage. Given the complexity of the target volume in head and neck cancer patients, scanned IMPT probably offers the most advantage and will allow for a substantially lower probability of radiation-induced side effects. The results of these ISPC studies should be confirmed in properly designed clinical trials with a focus on uniform accepted toxicity endpoints.
Acknowledgments
A.J. Lomax (Centre for Proton Therapy, Paul Scherrer Institute, Villigen-PSI, Switzerland) is sincerely acknowledged for accurately reading the review and for his suggestions in improving the review.
Author Contributions
Conception/Design: Tara A. van de Water, Hendrik P. Bijl, Cornelis Schilstra, Johannes A. Langendijk
Provision of study material or patients: Tara A. van de Water, Hendrik P. Bijl
Collection and/or assembly of data: Tara A. van de Water, Hendrik P. Bijl
Data analysis and interpretation: Tara A. van de Water, Hendrik P. Bijl, Cornelis Schilstra, Johannes A. Langendijk
Manuscript writing: Tara A. van de Water, Hendrik P. Bijl, Cornelis Schilstra, Madelon Pijls-Johannesma, Johannes A. Langendijk
Final approval of manuscript: Tara A. van de Water, Hendrik P. Bijl, Cornelis Schilstra, Madelon Pijls-Johannesma, Johannes A. Langendijk
References
- 1.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vergeer MR, Doornaert PA, Rietveld DH, et al. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: Results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. 2009;74:1–8. doi: 10.1016/j.ijrobp.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Schulz-Ertner D. The clinical experience with particle therapy in adults. Cancer J. 2009;15:306–311. doi: 10.1097/PPO.0b013e3181b01922. [DOI] [PubMed] [Google Scholar]
- 4.Brada M, Pijls-Johannesma M, De Ruysscher D. Current clinical evidence for proton therapy. Cancer J. 2009;15:319–324. doi: 10.1097/PPO.0b013e3181b6127c. [DOI] [PubMed] [Google Scholar]
- 5.Laramore GE. Role of particle radiotherapy in the management of head and neck cancer. Curr Opin Oncol. 2009;21:224–231. doi: 10.1097/cco.0b013e328329b716. [DOI] [PubMed] [Google Scholar]
- 6.Chan AW, Liebsch NJ. Proton radiation therapy for head and neck cancer. J Surg Oncol. 2008;97:697–700. doi: 10.1002/jso.21013. [DOI] [PubMed] [Google Scholar]
- 7.Goitein M, Lomax AJ, Pedroni ES. Treating cancer with protons. Phys Today. 2002;55:45–50. [Google Scholar]
- 8.Miralbell R, Crowell C, Suit HD. Potential improvement of three dimension treatment planning and proton therapy in the outcome of maxillary sinus cancer. Int J Radiat Oncol Biol Phys. 1992;22:305–310. doi: 10.1016/0360-3016(92)90047-l. [DOI] [PubMed] [Google Scholar]
- 9.Mock U, Georg D, Bogner J, et al. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:147–154. doi: 10.1016/s0360-3016(03)01452-4. [DOI] [PubMed] [Google Scholar]
- 10.Chera BS, Malyapa R, Louis D, et al. Proton therapy for maxillary sinus carcinoma. Am J Clin Oncol. 2009;32:296–303. doi: 10.1097/COC.0b013e318187132a. [DOI] [PubMed] [Google Scholar]
- 11.Lomax AJ, Goitein M, Adams J. Intensity modulation in radiotherapy: Photons versus protons in the paranasal sinus. Radiother Oncol. 2003;66:11–18. doi: 10.1016/s0167-8140(02)00308-0. [DOI] [PubMed] [Google Scholar]
- 12.Brown AP, Urie MM, Chisin R, et al. Proton therapy for carcinoma of the nasopharynx: A study in comparative treatment planning. Int J Radiat Oncol Biol Phys. 1989;16:1607–1614. doi: 10.1016/0360-3016(89)90970-x. [DOI] [PubMed] [Google Scholar]
- 13.Taheri-Kadkhoda Z, Björk-Eriksson T, Nill S, et al. Intensity-modulated radiotherapy of nasopharyngeal carcinoma: A comparative treatment planning study of photons and protons. Radiat Oncol. 2008;3:4. doi: 10.1186/1748-717X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widesott L, Pierelli A, Fiorino C, et al. Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: Planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys. 2008;72:589–596. doi: 10.1016/j.ijrobp.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 15.Slater JM, Slater JD, Archambeau JO. Carcinoma of the tonsillar region: Potential for use of proton beam therapy. Int J Radiat Oncol Biol Phys. 1992;22:311–319. doi: 10.1016/0360-3016(92)90048-m. [DOI] [PubMed] [Google Scholar]
- 16.Cozzi L, Fogliata A, Lomax A, et al. A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother Oncol. 2001;61:287–297. doi: 10.1016/s0167-8140(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 17.Johansson J, Blomquist E, Montelius A, et al. Potential outcomes of modalities and techniques in radiotherapy for patients with hypopharyngeal carcinoma. Radiother Oncol. 2004;72:129–138. doi: 10.1016/j.radonc.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Steneker M, Lomax A, Schneider U. Intensity modulated photon and proton therapy for the treatment of head and neck tumors. Radiother Oncol. 2006;80:263–267. doi: 10.1016/j.radonc.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Muzik J, Soukup M, Alber M. Comparison of fixed-beam IMRT, helical tomotherapy, and IMPT for selected cases. Med Phys. 2008;35:1580–1592. doi: 10.1118/1.2890085. [DOI] [PubMed] [Google Scholar]
- 20.Thorwarth D, Soukup M, Alber M. Dose painting with IMPT, helical tomotherapy and IMXT: A dosimetric comparison. Radiother Oncol. 2008;86:30–34. doi: 10.1016/j.radonc.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Flynn RT, Bowen SR, Bentzen SM, et al. Intensity-modulated x-ray (IMXT) versus proton (IMPT) therapy for theragnostic hypoxia-based dose painting. Phys Med Biol. 2008;53:4153–4167. doi: 10.1088/0031-9155/53/15/010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schilstra C, Meertens H. Calculation of the uncertainty in complication probability for various dose-response models, applied to the parotid gland. Int J Radiat Oncol Biol Phys. 2001;50:147–158. doi: 10.1016/s0360-3016(00)01553-4. [DOI] [PubMed] [Google Scholar]
- 23.Ågren Cronquist A. Doctoral Thesis. Stockholm, Sweden: Stockholm University; 1995. Quantification of the Response of Heterogeneous Tumours and Organized Normal Tissues to Fractionated Radiotherapy; pp. 1–126. [Google Scholar]
- 24.Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 25.Roesink JM, Moerland MA, Hoekstra A, et al. Scintigraphic assessment of early and late parotid gland function after radiotherapy for head-and-neck cancer: A prospective study of dose-volume response relationships. Int J Radiat Oncol Biol Phys. 2004;58:1451–1460. doi: 10.1016/j.ijrobp.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 27.Burman C, Kutcher GJ, Emami B, et al. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21:123–135. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- 28.Rancati T, Sanguineti G, Fiorino C. NTCP modeling of subacute/late laryngeal edema scored by fiberoptic examination: Evidence of a large volume effect. Radiother Oncol. 2007;84:S158–S159. [Google Scholar]
- 29.International Commission on Radiation Units and Measurements. ICRU Report 50. Bethesda, MD: ICRU; 1993. Prescribing, Recording, and Reporting Photon Beam Therapy; pp. 1–72. [Google Scholar]
- 30.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 31.Dirix P, Nuyts S. Evidence-based organ-sparing radiotherapy in head and neck cancer. Lancet Oncol. 2010;11:85–91. doi: 10.1016/S1470-2045(09)70231-1. [DOI] [PubMed] [Google Scholar]
- 32.Cozzia L, Bolsi A, Nicolinia G, et al. The simultaneous integrated boost with proton beams in head and neck patients. Z Med Physik. 2004;14:180–188. doi: 10.1078/0939-3889-00218. [DOI] [PubMed] [Google Scholar]
- 33.Lomax AJ. Charged particle therapy: The physics of interaction. Cancer J. 2009;15:285–291. doi: 10.1097/PPO.0b013e3181af5cc7. [DOI] [PubMed] [Google Scholar]
- 34.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: The potential effects of calculational uncertainties. Phys Med Biol. 2008;53:1027–1042. doi: 10.1088/0031-9155/53/4/014. [DOI] [PubMed] [Google Scholar]
- 35.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: The potential effects of inter-fraction and inter-field motions. Phys Med Biol. 2008;53:1043–1056. doi: 10.1088/0031-9155/53/4/015. [DOI] [PubMed] [Google Scholar]
- 36.Goitein M, Jermann M. The relative costs of proton and X-ray radiation therapy. Clin Oncol (R Coll Radiol) 2003;15:S37–S50. doi: 10.1053/clon.2002.0174. [DOI] [PubMed] [Google Scholar]
- 37.Peeters A, Grutters JP, Pijls-Johannesma M, et al. How costly is particle therapy? Cost analysis of external beam radiotherapy with carbon-ions, protons and photons. Radiother Oncol. 2010;95:45–53. doi: 10.1016/j.radonc.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Miralbell R, Lomax A, Cella L, et al. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys. 2002;54:824–829. doi: 10.1016/s0360-3016(02)02982-6. [DOI] [PubMed] [Google Scholar]
- 39.Lee CT, Bilton SD, Famiglietti RM, et al. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: How do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys. 2005;63:362–372. doi: 10.1016/j.ijrobp.2005.01.060. [DOI] [PubMed] [Google Scholar]