A literature review of recently published trials, reviews, and practice guidelines outlining the surgical and adjuvant management of early breast cancer in older women is presented.
Keywords: Breast neoplasms, Aged, Geriatrics, General surgery, Adjuvant chemotherapy, Adjuvant radiation
Abstract
Background.
Women aged ≥65 are generally underrepresented in early breast cancer studies. Therefore, the optimal management of this group of women remains less certain.
Methods.
A literature review of recently published trials, reviews, and practice guidelines outlining the surgical and adjuvant management of early breast cancer in older women was performed.
Results.
Surgery remains as the cornerstone treatment for early breast cancer in the elderly. Adjuvant radiation is generally considered if the projected lifespan is >5 years. Hormone receptor–positive disease is best treated with adjuvant endocrine treatment; aromatase inhibitors and tamoxifen are both options. Evidence for the use of adjuvant chemotherapy and trastuzumab for high-risk disease in the elderly is more limited. Polychemotherapy is still preferred in fit older women. Certain toxicities from systemic treatments can be more pronounced and should be carefully managed. Treatment with systemic agents should be individualized, with consideration of patient preference, performance status, comorbidities, and projected lifespan. Molecular tumor signatures may help better select patients for treatment in the future.
Conclusions.
Age in itself should not be an absolute contraindication to any breast cancer therapy. Comprehensive, multidisciplinary assessment of elderly patients is imperative in evaluating eligibility for beneficial therapies.
Introduction
Every year, >22,000 new diagnoses of breast cancer are made in Canada. It is the most common cancer in Canadian women, and kills >5,000 patients every year [1]. Ongoing advances in breast cancer management have significantly changed patient outcomes, particularly in the developed world, where mortality rates have been decreased by approximately 30% in the last two decades [1, 2]. However, the general applicability of these treatment advances in women aged >65, and especially aged >70, often remains unclear. Almost 40% of breast cancers are diagnosed in women aged >65 [2], and age in itself is a significant risk factor for developing breast cancer [1]. Despite this, older women are often underrepresented in clinical trials [3], and therefore extrapolation of trial results to this population can be challenging. Nonetheless, carefully selected elderly women can still benefit from efficacious breast cancer treatments, and undertreatment of elderly patients does result in poorer outcomes [4]. Despite this, evidence suggests that these women do not always receive standard treatments, and physician practice patterns can vary greatly. In general, the unique challenges posed by older women need to be carefully addressed in order to optimize breast cancer care in this population.
Methods
A literature review using MEDLINE and PubMed databases was performed. Relevant gray literature (epidemiologic statistics and some practice guidelines) were searched using an online search engine. The search strategy was first limited to include English-language articles of clinical trials and practice guidelines highlighting early breast cancer management in the geriatric population (using “geriatric,” “older,” “age greater than 65,” and “age greater than 70” as keywords). In MEDLINE, searches were also limited to the categories “All Aged: 65 and over,” and then “Aged: 80 and over.” Included publication years were 1990 and later, and then 2005 and later for systemic treatments specifically (because of changes in the management of human epidermal growth factor receptor (HER)-2+ early breast cancer during this time). The evaluated breast cancer treatments were surgery, radiation, chemotherapy, endocrine, and targeted treatments in the adjuvant setting. Articles on toxicity from treatment in the elderly were included, as were screening, diagnostic, and follow-up practices. When no specific publications highlighting treatment in elderly women were found, the strategy was broadened to include general practice guidelines and reviews in early breast cancer treatment. These publications were then hand-searched to find any age-based recommendations or findings. When redundant reviews and practice guidelines were found, the most recent publications were used.
Results
Age as a Risk Factor for Breast Cancer
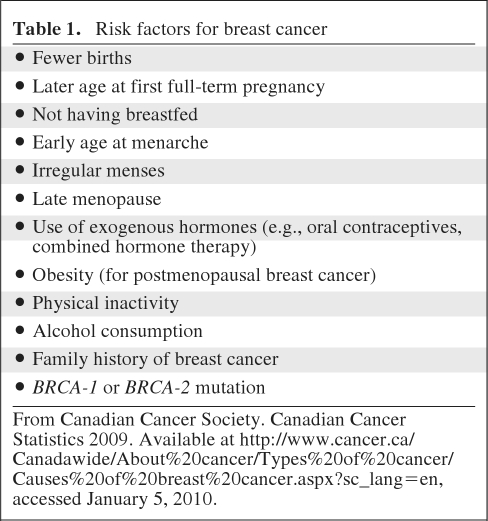
The greatest risk for developing breast cancer is prolonged estrogen exposure, which makes female gender and advancing age significant contributors. A woman's lifetime risk is estimated as 9%; this is a population-based estimate that means one in nine women will develop breast cancer at some point during their lives [1]. For an individual woman, risk is largely based on age, and is often estimated by decade. After menopause, and particularly after age 65, the risk increases several-fold. For instance, for ages 30–39, the risk is 0.43%, for ages 40–49, the risk is 1.44%, for ages 50–59, the risk is 2.63%, and for ages 60–69, the risk is 3.65% [2]. Other risk factors for breast cancer are listed in Table 1.
Table 1.
Risk factors for breast cancer
From Canadian Cancer Society. Canadian Cancer Statistics 2009. Available at http://www.cancer.ca/Canadawide/About%20cancer/Types%20of%20cancer/Causes%20of%20breast%20cancer.aspx?sc_lang=en, accessed January 5, 2010.
Elderly Women and Breast Cancer Screening and Diagnosis
There is conflicting evidence as to whether breast cancer screening is beneficial in women age >70 [5]. Nonetheless, screening mammography likely continues to have benefit in the older population, as long as life expectancy is reasonable; those with a life expectancy <5 years are unlikely to benefit. The choice to continue mammography beyond the age of 70 should be individualized [6]. The biology of breast cancer may be more favorable in older women, with more hormone receptor–positive and more HER-2− disease; however, presentation is often at a later stage [5]. In addition, one series found that 19% of women age >70 had “luminal B” type tumors, which, despite being hormone receptor positive, are associated with higher grade and proliferation, larger size, and greater propensity toward nodal invasion [7]. The diagnosis of breast malignancies in older women should be approached similar to that of younger women. Clinically apparent abnormal breast lumps should be imaged (mammography with adjunctive ultrasound or magnetic resonance imaging [MRI] as required) and biopsied (core biopsy preferred). Breast tissue becomes less dense as women age, usually making clinical breast exams easier, and the positive predictive value of an abnormality on mammogram higher [8]. However, even older women (aged >50) were found in one study to have mammographically occult tumors that were only picked up by MRI (26% of tumors, versus 38% in women age <50) [9].
Elderly Women and Breast Cancer Surgery
There is a paucity of data specifically looking at how to manage the elderly patient with breast cancer from a surgical perspective. The general consensus remains that an older woman with a life expectancy >5 years should be offered the same surgical treatment options as a younger patient [4]. This includes breast-conserving surgery (BCS), mastectomy, and breast reconstruction, as appropriate. One recent retrospective study showed that 95% of women aged >70 received primary surgery, with 72% receiving BCS (62% of those aged >80) and 89% receiving axillary dissection (77% of those aged >80). Radiation was given to 100% of patients treated with BCS, and small numbers received endocrine therapy or chemotherapy (57% and 4%, respectively). Nonetheless, at 7 years, the survival duration of patients treated with adequate surgery (and radiation when indicated) was similar to that of younger patients [10]. In women who may have a limited life expectancy (2–5 years) as a result of comorbidities or extreme age, surgical management generally may not be appropriate. In these women, primary surgery is unlikely to impact overall survival (OS) because of competing causes of death. Regardless, surgery may still be offered to alleviate symptoms from locally progressive disease. Surgical morbidity from breast surgery remains low even in the frail elderly, and those deemed unfit to undergo general anesthesia can often be managed with local anesthetic approaches [4].
In the 1980s, trials in older women comparing surgery with primary endocrine therapy using tamoxifen showed lower local relapse rates with surgery [4]. The benefit of surgery over tamoxifen in improving OS in these women remains unclear, but based on a systematic review of such trials, surgery does improve progression-free survival [11]. It is thus suggested that only women deemed unfit to undergo surgery be offered primary endocrine therapy with either tamoxifen or aromatase inhibitors (AIs) [4, 11]. Trials are ongoing to further establish the utility of AIs in this setting. One such trial in the U.K. included formal geriatric assessment and collected quality of life, patient preference, and cost-effectiveness data; however, that trial was closed early because of poor accrual [12]. This type of evidence is greatly needed; it remains clear that assessment of medical fitness for breast cancer surgery (and adjuvant therapies) in older women is generally not thorough or standardized, despite the emergence of dedicated geriatric assessment tools for this purpose [13]. Such tools are imperative in determining whether a woman will benefit overall from surgical approaches.
There also remains debate as to whether or not axillary surgery should also be offered to older women. Axillary nodal dissection can be associated with lymphedema, which in one study, was found in 40% of patients aged 50–79 years, and in 26% of those aged >80 (compared with 50% of patients aged <50) [14]. One should note that the lower rate of lymphedema in the older cohort may be partially explained by lower rates of level 1 and level 2 axillary dissections. Nonetheless, although usually mild or moderate in degree, lymphedema can cause significant morbidity. In addition, lymph node–positive breast cancer is most often treated with adjuvant systemic chemotherapy, and the true benefit of these treatments in older women can be unclear. In addition, women with low-grade, strongly hormone receptor–positive tumors with only one or two involved lymph nodes may be treated successfully with adjuvant endocrine therapy. Therefore, those women who are unlikely candidates for adjuvant chemotherapy may not require axillary assessment, unless there are indications to prevent symptomatic progression of axillary disease [4]. When axillary assessment is warranted, the sentinel node approach is preferred to minimize complications from lymphedema. Whether sentinel node–positive disease is then treated with full surgical dissection or primary radiotherapy appears to be center specific, and no clear guidelines exist for this in the elderly population [4]. Finally, one study illustrated that older women aged 65–80 with clinically small, lymph node negative (T1N0) tumors may not require even sentinel lymph node biopsy; patients in that trial were randomized to breast surgery with or without axillary dissection and there was no difference in survival between the two groups at 60 months, and <2% of patients in the no axillary dissection arm developed axillary disease [15]. It is important to note, however, that most patients in that study had other favorable tumor characteristics (low grade, strongly hormone sensitive).
Elderly Women and Adjuvant Radiation
Women offered BCS for primary breast tumors should also be considered for adjuvant radiation to the breast. Local control rates with BCS are equivalent to those of mastectomy with adjuvant radiation, and the omission of adjuvant radiation after BCS in elderly women has been shown to decrease breast cancer–specific survival [16]. Therefore, those women who are unable or unwilling to undergo adjuvant radiation may benefit from mastectomy instead of BCS, in order to minimize their risk for local relapse.
In addition, adjuvant radiation to the breast after BCS, and to the chest wall and/or axillae in lymph node–positive disease after both mastectomy and BCS, has been shown to have a modest impact on the OS rate at 15 years [17]. Therefore, an older woman who is expected to live >10–15 years may be offered postmastectomy and/or axillary radiation as would be offered to a younger woman [4, 18]. In general, the impact of adjuvant radiation on OS is likely less pronounced in older women, and the decision for this therapy should largely be based on improving the local control rate [18]. There is some evidence that toxicity from radiation is no greater in elderly women [19, 20]. However, unique factors in older women that should be addressed when considering radiation therapy include significant pulmonary, skin, or cardiac disease, dementia, and decreased mobility, making regular visits or manipulation of upper limbs for treatment administration difficult. There are both retrospective and ongoing prospective trials examining whether adjuvant radiation can be omitted after BCS in elderly women. One such study found that women with adequate margins and hormone receptor–positive disease treated with adjuvant tamoxifen had low rates of local recurrence and thus could likely forgo adjuvant radiation [21]. Those women with poor prognostic factors, such as hormone receptor–negative disease, had a higher rate of relapse and would likely still benefit from radiation [21]. Recently, another group reported 10-year follow-up data on women aged >70 with stage 1 hormone receptor–positive breast cancer who were randomized to adjuvant tamoxifen with or without radiation. There was a 6% lower absolute local recurrence rate with the addition of radiation (2%, versus 8%), but no difference in distant relapse or survival endpoints [22]. Once again, this represents women with low-risk disease, and whether or not one can omit radiation safely from higher risk patients is less clear and awaits data from further trials [23].
Elderly Women and Adjuvant Endocrine Treatment
The goal of adjuvant therapy in general is to improve relapse and cure rates after definitive (usually surgical) treatment for cancer at an early (nonmetastatic) stage. For decades, women with hormone receptor–positive breast cancer have been known to benefit from adjuvant endocrine treatment in the form of tamoxifen, with a lower relapse risk and longer survival time; this benefit was independent of age in a systematic review of many trials [24]. In the last decade, the AIs have led to a longer disease-free survival (DFS) interval and lower distant relapse rates than with tamoxifen. The large pivotal trials illustrating these results did include women age >70 [25, 26]; in general, benefit was found with AIs regardless of age [27]. Thus, there is evidence that older women should be offered 5 years of endocrine treatment for hormone receptor–positive breast cancer, and AIs are preferable. However, the optimal strategy for AIs (upfront for 5 years, sequencing after 2–3 years of tamoxifen, or for an additional 5 years after completing 5 years of tamoxifen) remains to be established [25, 26]. The toxicity profiles of tamoxifen and AIs differ. Both can cause menopausal symptoms, but tamoxifen is associated with thromboembolic disease, and this may be of concern in the older woman at higher vascular risk (which may in part be a result of lower mobility in this population). Tamoxifen is also associated with a small risk for endometrial cancer, with a latency of 5–10 years [28]; this may or may not be a concern for older women depending on their projected lifespan. In contrast, AIs are associated with osteoporosis and arthralgias. These may be of concern for the older woman with baseline osteoporosis or significant arthritis. AIs may marginally increase the risk for grade 3–4 cardiovascular events, but with still a very low absolute risk, compared with tamoxifen; it has been suggested that the latter is somewhat cardioprotective [28, 29]. Overall, it is imperative to individualize the choice of endocrine treatment, particularly in older women. One must particularly consider competing comorbidities, tolerance of side effects, and adherence to medication in this population. In addition, the potential interaction of endocrine therapies with other medications is important to consider; this is most notable for tamoxifen and cytochrome P450 (CYP)2D6 inhibitors (such as the selective serotonin reuptake inhibitors). Several studies have suggested that CYP2D6 inhibitors may decrease the effectiveness of tamoxifen, presumably by reducing conversion to its active metabolite, endoxifen [30]. However, data are conflicting and still accumulating regarding the tamoxifen, endoxifen, and CYP2D6 association, including the role of endogenous CYP2D6 polymorphisms [31, 32].
In general, regular follow-up for women on adjuvant endocrine therapy is particularly important. On AIs, baseline and annual or biannual bone mineral density measurements are recommended, and concomitant calcium and vitamin D administration is warranted. The addition of bisphosphonate therapy is advocated for those with osteoporosis or significant osteopenia (T score < −1.5) in concert with other risk factors for osteoporosis [33]. These risk factors are generally more common in the elderly woman.
Elderly Women and Adjuvant Chemotherapy and Targeted Treatment
Adjuvant chemotherapy improves survival from breast cancer, particularly in patients with high-risk disease (such as node-positive or hormone receptor–negative patients). Most modern (third-generation) adjuvant chemotherapy regimens usually include anthracylines and taxanes (in combination or in sequence.) For patients with HER-2+ tumors, the addition of the targeted agent trastuzumab to adjuvant chemotherapy has further improved breast cancer outcomes [34]. In general, women aged >70 have been excluded from adjuvant trials demonstrating these benefits, particularly in trials of trastuzumab. Nonetheless, older women do seem to derive benefit from adjuvant chemotherapy, as found by meta-analysis of adjuvant treatment trials [24], although absolute benefits may decrease as a function of increasing age. There have been a few adjuvant chemotherapy studies specifically examining older women. One of these recently published trials demonstrated that, in women aged >65, a single-agent chemotherapy (capecitabine) regimen was inferior to standard polychemotherapy with either doxorubicin and cyclophosphamide (AC) or cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) [35]. It is thus recommended that candidate elderly women be considered for adjuvant chemotherapy as indicated for younger patients [5]. However, several important caveats exist to this stipulation. First, certain toxicities are much more common in elderly women, and anthracycline-based regimens can cause significant cardiotoxicity, especially in older women. Also, the elderly are at particular risk for developing myelosuppression, and this can significantly increase the risk for febrile neutropenia. Given these potential risks associated with anthracyclines, some elderly patients may be candidates for nonanthracycline chemotherapy regimens such as CMF or docetaxel and cyclophosphamide (TC). The TC regimen was compared with AC in the adjuvant setting and found to be superior in terms of the 5-year DFS at the first analysis [36]. A follow-up analysis at 6 years documented ongoing improvements, not only in terms of DFS but also an OS benefit in patients receiving TC versus AC (88% versus 84%) [37]. Furthermore, patients aged >65 still had OS and DFS benefits, with toxicities similar to those of patients aged <65. As such, TC became recommended as a standard, nonanthracycline-containing adjuvant regimen that may be particularly suitable for those patients in whom cardiac toxicity is a concern (such as some elderly patients) [37].
Trastuzumab therapy must be prescribed with caution in elderly women because cardiotoxicity from this drug can also be significant. Age >50, hypertension, and baseline cardiac dysfunction are important risk factors for trastuzumab cardiotoxicity [38]; these same factors, in addition to cumulative chemotherapy dose, also contribute to anthracycline-induced heart failure [39]. Once again, if cardiac dysfunction is a concern, a nonanthracycline-based chemotherapy regimen is preferable, particularly if trastuzumab therapy is being considered [40]. An ongoing study, the RESPECT trial, is randomizing patients aged 70–80 with HER-2+ early breast cancer to adjuvant therapy with either trastuzumab alone or trastuzumab and chemotherapy [41]. This may help address whether or not this subgroup of elderly breast cancer patients with HER-2+ disease can forgo adjuvant chemotherapy and be treated successfully with trastuzumab monotherapy.
Other long-term side effects of chemotherapy (such as secondary malignancies like leukemia) may be of less relevance in older women because latencies of up to 5–10 years [42, 43]. The dementia risk may not be higher in elderly breast cancer patients after chemotherapy [44], although cognitive dysfunction after chemotherapy has been described in breast cancer patients in many studies, and older women are generally underrepresented in these analyses [45, 46].
In general, the International Society on Geriatric Oncology (SIOG) suggests considering anthracycline-based chemotherapy in high-risk older women (with node-positive or hormone receptor–negative breast cancer). Elderly women who are fit can be considered for anthracycline- and taxane-based regimens [5]. In all cases, comprehensive geriatric assessments are imperative, and screening for comorbidities (especially cardiac) is crucial. The SIOG also recommends using the Adjuvant! Online risk modeling system to estimate benefit from chemotherapy in older women, as is often done in younger patients [5]. However, this tool has not been validated for specific populations based on age; furthermore, physician estimation of comorbidities for input into this program is subjective, and likely does not take into account a complex geriatric assessment. The program may therefore overestimate the benefit of chemotherapy in unfit older women, whose benefit may be substantially negated by competing health risks and/or treatment toxicity [47]. Functional status and patient comorbid illness have been found to be strong independent predictors of adverse outcomes from chemotherapy [48]. It is thus imperative that a complex geriatric assessment be done in establishing whether an older woman with breast cancer will tolerate and benefit from adjuvant systemic therapy, including targeted agents. Finally, the use of molecular profiling for prognostic and predictive modeling is promising to become a key element in therapeutic decision making for patients with breast cancer. In general, studies validating these molecular assays—such as MammaPrint® (Agendia Inc., Irvine, CA) and Oncotype DX® (Genomic Health, Inc., Redwood City, CA)—and testing their utility in predicting chemotherapy benefit have stratified women by age <50 versus >50 [49, 50]. However, a recent study looking at Oncotype DX® in node-positive women did delineate a subgroup of women aged >65 [51]. In general, the utility of the assay in predicting chemotherapy benefit was independent of age, even when looking at this age group. Several of these molecular assays are emerging, and as they become better validated prospectively it will be imperative to ensure that older women are included in these trials and analyses. It was recently shown that a score combining molecular profiling with standard histopathologic and clinical parameters may be better able to prognosticate relapse risk for patients; as mentioned above, these types of findings need to be prospectively validated. Nonetheless, clinicians and elderly patients may find this information vital in helping to make adjuvant chemotherapy and novel targeted therapy decisions in the near future.
Follow-Up of Elderly women
Guidelines on the surveillance of women treated for early breast cancer vary. There do not appear to be any specific recommendations for elderly women. The American Society of Clinical Oncology recommends clinical assessment (history and physical) every 3–6 months for the first 3 years, every 6–12 months for years 4 and 5, and then annually [52]. More recent recommendations (including from Canadian societies) recommend an individualized, patient-tailored approach, with no specific intervals for clinical assessment outlined [53, 54]. All guidelines do recommend ongoing annual mammograms starting 1 year after the initial diagnostic mammogram [49–51]. Follow-up of elderly breast cancer patients should be based on patient preference, general health status, and projected longevity [55]. All breast cancer patients should be counseled on symptoms of disease recurrence and any long-term toxicities of treatment [50, 51].
Conclusions
The older woman with early breast cancer needs to be treated in an individualized manner. Complex geriatric assessment and consideration of patient comorbidities, lifespan, and preferences are crucial elements in decision making for primary surgical and adjuvant treatments. Most previous trials have underrepresented older women, and systematic assessments of frailty are generally lacking. Tumor molecular profiling may be increasingly useful in decision making for chemotherapy and specific targeted agents in all breast cancer patients, particularly older women, if treatments with significant toxicities can be spared. Nonetheless, age in itself should not be a contraindication to providing standard treatments; however, better representation of elderly women in clinical studies, with thorough and systematic assessment of frailty, is imperative in the future. Finally, multidisciplinary collaboration should be always be emphasized in treating women aged >65–70 with early breast cancer.
References
- 1.Canadian Cancer Society. Canadian Cancer Statistics 2009. [accessed January 5, 2010]. Available at http://www.cancer.ca/Canada-wide/About%20cancer/Types%20of%20cancer/Causes%20of%20breast%20cancer.aspx?sc_lang=en.
- 2.SEER database. SEER Stat Fact Sheets: Breast. [accessed January 6, 2010]. Available http://seer.cancer.gov/statfacts/html/breast.html.
- 3.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 4.Reed MWR, Audisio RA, Wyld L. The role of surgery in the treatment of older women with breast cancer. Clin Oncol (R Coll Radiol) 2009;21:103–110. doi: 10.1016/j.clon.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Wildiers H, Kunkler I, Biganzoli L, et al. Management of breast cancer in elderly individuals: Recommendations of the International Society of Geriatric Oncology. Lancet Oncol. 2007;8:1101–1115. doi: 10.1016/S1470-2045(07)70378-9. [DOI] [PubMed] [Google Scholar]
- 6.Galit W, Green MS, Lital KB. Routine screening mammography in women older than 74 years: A review of the available data. Maturitas. 2007;57:109–119. doi: 10.1016/j.maturitas.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Durbecq V, Ameye L, Veys I, et al. A significant proportion of elderly patients develop hormone-dependant “luminal-B” tumours associated with aggressive characteristics. Crit Rev Oncol Hematol. 2008;67:80–92. doi: 10.1016/j.critrevonc.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Holmes CE, Muss HB. Diagnosis and treatment of breast cancer in the elderly. CA Cancer J Clin. 2003;53:227–244. doi: 10.3322/canjclin.53.4.227. [DOI] [PubMed] [Google Scholar]
- 9.Sardanelli F, Podo F, Santoro F, et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the High Breast Cancer Risk Italian 1 Study): Final results. Invest Radiol. 2011;46:94–105. doi: 10.1097/RLI.0b013e3181f3fcdf. [DOI] [PubMed] [Google Scholar]
- 10.Laki F, Kirova YM, Savignoni A, et al. Management of operable invasive breast cancer in women over the age of 70: long-term results of a large-scale single-institution experience. Ann Surg Oncol. 2010;17:1530–1538. doi: 10.1245/s10434-010-0967-6. [DOI] [PubMed] [Google Scholar]
- 11.Hind D, Wyld L, Beverley C, et al. Surgery versus primary endocrine therapy for operable primary breast cancer in elderly women (70 years plus) Cochrane Database Syst Rev. 2006;(1):CD004272. doi: 10.1002/14651858.CD004272.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Reed MWR, Wyld L, Ellis P, et al. Breast cancer in older women: Trials and tribulations. Clin Oncol (R Coll Radiol) 2009;21:99–102. doi: 10.1016/j.clon.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Albrand G, Terret C. Early breast cancer in the elderly: assessment and management considerations. Drugs Aging. 2008;25:35–45. doi: 10.2165/00002512-200825010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: Incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27:390–397. doi: 10.1200/JCO.2008.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martelli G, Boracchi P, De Palo M, et al. A randomized trial comparing axillary dissection to no axillary dissection in older patients with T1N0 breast cancer: Results after 5 years of follow-up. Ann Surg. 2005;242:1–6. doi: 10.1097/01.sla.0000167759.15670.14. discussion 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truong PT, Bernstein V, Lesperance M, et al. Radiotherapy omission after breast-conserving surgery is associated with reduced breast cancer-specific survival in elderly women with breast cancer. Am J Surg. 2006;191:749–755. doi: 10.1016/j.amjsurg.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 17.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 18.Wildiers H. Challenges in treating older cancer patients: breast cancer. Ann Oncol. 2008;19(suppl 7):vii99–vii103. doi: 10.1093/annonc/mdn481. [DOI] [PubMed] [Google Scholar]
- 19.Huguenin P, Glanzmann C, Lutolf UM. Acute toxicity of curative radiotherapy in elderly patients. Strahlenther Onkol. 1996;172:658–663. [PubMed] [Google Scholar]
- 20.Wyckoff J, Greenberg H, Sanderson R, et al. Breast irradiation in the older woman: A toxicity study. J Am Geriatr Soc. 1994;42:150–152. doi: 10.1111/j.1532-5415.1994.tb04943.x. [DOI] [PubMed] [Google Scholar]
- 21.Valassiadou K, Morgan DA, Robertson JF, et al. Successful management of elderly breast cancer patients treated without radiotherapy. World J Surg Oncol. 2007;5:62. doi: 10.1186/1477-7819-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes KS, Schnaper LA, Cirrincione C, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 or older with early breast cancer. J Clin Oncol. 2010;28(15 suppl) doi: 10.1200/JCO.2012.45.2615. Abstract 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunkler IH, Williams LJ, King CC, et al. Breast radiotherapy: Considerations in older patients. Clin Oncol (R Coll Radiol) 2009;21:111–117. doi: 10.1016/j.clon.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 25.Chlebowski R, Cuzick J, Amakye D, et al. Clinical perspectives on the utility of aromatase inhibitors for the adjuvant treatment of breast cancer. Breast. 2009;18(suppl 2):S1–S11. doi: 10.1016/S0960-9776(09)70002-5. [DOI] [PubMed] [Google Scholar]
- 26.Iwase H. Current topics and perspectives on the use of aromatase inhibitors in the treatment of breast cancer. Breast Cancer. 2008;15:278–290. doi: 10.1007/s12282-008-0071-y. [DOI] [PubMed] [Google Scholar]
- 27.Tipples K, Robinson A. Optimising care of elderly breast cancer patients: A challenging priority. Clin Oncol (R Coll Radiol) 2009;2:118–130. doi: 10.1016/j.clon.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Braithwaite RS, Chlebowski RT, Lau J, et al. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18:937–947. doi: 10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuppone F, Bria E, Verma S, et al. Do adjuvant aromatase inhibitors increase the cardiovascular risk in postmenopausal women with early breast cancer? Meta-analysis of randomized trials. Cancer. 2007;112:260–267. doi: 10.1002/cncr.23171. [DOI] [PubMed] [Google Scholar]
- 30.Singh MS, Francis PA, Michael M. Tamoxifen, cytochrome P450 genes and breast cancer clinical outcomes. Breast. 2010 Dec 23; doi: 10.1016/j.breast.2010.11.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Rae JM, Drury S, Hayes DF, et al. Lack of Correlation Between Gene Variants in Tamoxifen Metabolizing Enzymes with Primary Endpoints in the ATAC Trial. Presented at the San Antonio Breast Symposium; December 9, 2010; San Antonio, Texas. [accessed January 20, 2011]. Available at http://www.abstracts2view.com/sabcs10/view.php?nu=SABCS10L_1093&terms= [Google Scholar]
- 32.Zaman K, Dahmane E, Csajka C, et al. Prospective Assessment of CYP2D6 by Genotyping, Phenotyping and Measurement of Tamoxifen, 4-Hydroxy-Tamoxifen and Endoxifen in Breast Cancer Patients Treated with Tamoxifen. Presented at the San Antonio Breast Cancer Symposium; December 10, 2010; San Antonio, Texas. [accessed January 20, 2011]. http://www.abstracts2view.com/sabcs10/view.php?nu=SABCS10L_1285&terms= [Google Scholar]
- 33.Hadji P, Body JJ, Aapro MS, et al. Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann Oncol. 2008;19:1407–1416. doi: 10.1093/annonc/mdn164. [DOI] [PubMed] [Google Scholar]
- 34.Goldhirsch A, Wood WC, Gelber RD, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 35.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 37.Jones S, Holmes F, O'Shaughnessy J, et al. Extended follow-up and analysis by age of the US Oncology Adjuvant trial 9735: docetaxel/cyclophosphamide is associated with an overall survival benefit compared to doxorubicin/cyclophosphamide and is well-tolerated in women 65 or older. Breast Cancer Res Treat. 2007;106(suppl 1):S5. [Google Scholar]
- 38.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 40.Slamon D, Eiermann W, Robert N, et al. BCIRG 006 Phase III Trial Comparing AC→T with AC→TH and with TCH in the Adjuvant Treatment of HER2-Amplified Early Breast Cancer Patients: Third Planned Efficacy Analysis. Presented at the 32nd Annual San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, Texas. [Google Scholar]
- 41.Sawaki M, Iwata H, Kashiwaba M, et al. Evaluation of Trastuzumab Without Chemotherapy as a Postoperative Adjuvant Therapy in HER2-Positive Elderly Breast Cancer Patients: Randomized Controlled Trial (RESPECT [N-SAS BC07]). Presented at the American Society of Clinical Oncology Annual Breast Cancer Symposium; October 1–3, 2010; Washington, D.C.. [accessed January 20, 2011]. Available at http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=100&abstractID=60159. [Google Scholar]
- 42.Smith RE, Bryant J, DeCillis A, et al. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: The National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21:1195–1204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 43.Schaapveld M, Visser O, Louwman MJ, et al. Risk of new primary nonbreast cancers after breast cancer treatment: A Dutch population-based study. J Clin Oncol. 2008;26:1239–1246. doi: 10.1200/JCO.2007.11.9081. [DOI] [PubMed] [Google Scholar]
- 44.Baxter NN, Durham SB, Phillips KA, et al. Risk of dementia in older breast cancer survivors: A population-based cohort study of the association with adjuvant chemotherapy. J Am Geratr Soc. 2009;57:403–411. doi: 10.1111/j.1532-5415.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- 45.Schagen SB, van Dam FS, Muller MJ, et al. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 46.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J Clin Oncol. 2007;25:2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 47.Aapro M, Monfardini S, Jirillo A, et al. Management of primary and advanced breast cancer in older unfit patients (medical treatment) Cancer Treat Rev. 2009;35:503–508. doi: 10.1016/j.ctrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Marinello R, Marenco D, Roglia D, et al. Predictors of treatment failures during chemotherapy: A prospective study on 110 older cancer patients. Arch Gerontol Geriatr. 2009;48:222–226. doi: 10.1016/j.archger.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor–positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 50.van de Vijver MJ, He YD, van't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 51.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang G, Cuzick J, Wale C, et al. Recurrence risk of node-negative and ER-positive early-stage breast cancer patients by combining recurrence score, pathologic, and clinical information: A meta-analysis approach [abstract 509]. Presented at the American Society of Clinical Oncology 2010 Annual Scientific Meeting; June 4–8, 2010; Chicago, IL. [Google Scholar]
- 53.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 54.Grunfeld E, Dhesy-Thind S, Levine M Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: Follow-up after treatment for breast cancer (summary of the 2005 update) CMAJ. 2005;172:1319–1320. doi: 10.1503/cmaj.045062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grunfeld E. Optimizing follow-up after breast cancer treatment. Curr Opin Obstet Gynecol. 2009;21:92–96. doi: 10.1097/gco.0b013e328321e437. [DOI] [PubMed] [Google Scholar]