This review is an update to a previous summary of European treatment practices that examines new data that have been published or presented at congresses up to the end of 2010 and assesses their impact on treatment practices.
Abstract
The arrival of the novel agents thalidomide, bortezomib, and lenalidomide has significantly changed our approach to the management of multiple myeloma and, importantly, patient outcomes have improved. These agents have been investigated intensively in different treatment settings, providing us with data to make evidence-based decisions regarding the optimal management of patients. This review is an update to a previous summary of European treatment practices that examines new data that have been published or presented at congresses up to the end of 2010 and assesses their impact on treatment practices.
Introduction
Following an expert meeting in 2009 to examine European multiple myeloma (MM) treatment practices and the resulting summary of the discussions, which was published at the beginning of 2010 [1], an update meeting was held in mid-2010 to review recent data and to assess the impact of the data on clinical practice. Furthermore, new data that were presented and published up to the end of 2010 were discussed and evaluated among the authors. Several important developments were identified, which warrant an update to the existing publication because they are likely to influence treatment practices. These updates are summarized in the present article.
Aiming for Complete Response in MM
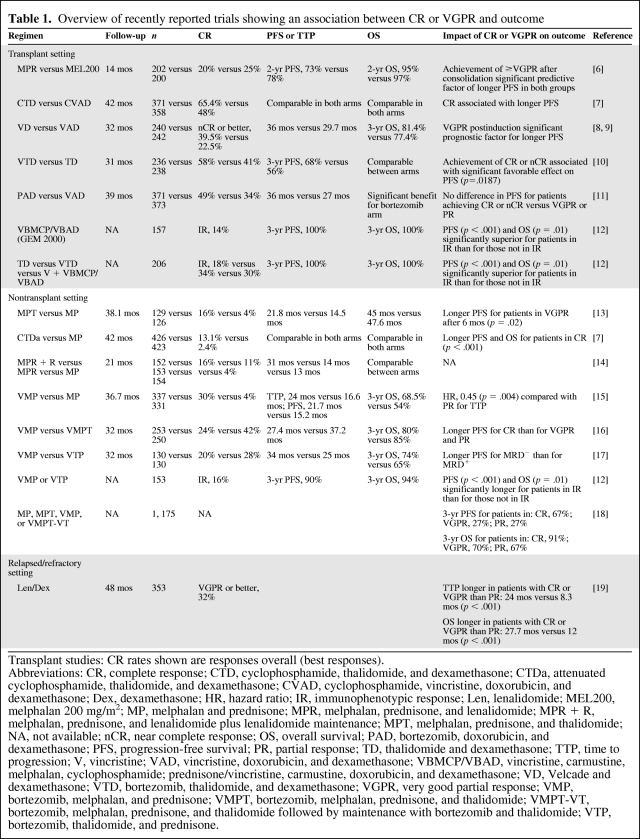
The importance of complete response (CR) is a well-recognized concept in the transplant setting, and a number of publications have addressed this [2–5]. Recent transplant studies and the impact of a CR on outcome are summarized in Table 1 [6–12]. In the nontransplant setting, the role of CR has been less clear, mainly because conventional treatments only resulted in a low rate of CR, making an analysis of the association between different response categories and outcome unfeasible. However, the arrival of novel agents has had an important impact in the nontransplant setting, resulting in CR rates of 7%–30% [13, 14, 20–26]. Analyses directed at elucidating an association between CR and outcome in some of the novel agent trials that have been conducted involving elderly patients have revealed that a maximal response is also associated with a better outcome in that setting. Associations between maximal response and longer progression-free survival (PFS) time and time to progression (TTP) were observed for melphalan, prednisone, and thalidomide (MPT), attenuated cyclophosphamide, thalidomide, and dexamethasone (CTDa), and bortezomib, melphalan, and prednisone (VMP) (Table 1) [7, 13, 15]. In the VISTA trial (Velcade® as Initial Standard Therapy in Multiple Myeloma), achievement of a CR was also associated with a longer time to next therapy and treatment-free interval [15]. Notably, two recent studies add further support to the importance of CR achievement in the nontransplant setting (Table 1). In an analysis based on three randomized European trials conducted by the Gruppo Italiano Malattie EMatologiche Ddell'Adulto (GIMEMA) and the Stichting Hemato-Oncologie voor Volwassenen Nederland (HOVON) in which patients with newly diagnosed MM aged >65 years had received treatment consisting of melphalan and prednisone (MP), MPT, VMP, or bortezomib, melphalan, prednisone, and thalidomide followed by maintenance with bortezomib and thalidomide (VMPT-VT), a significant association between CR and PFS and overall survival (OS) was observed, compared with patients with a very good partial response (VGPR) and a partial response (PR) [18]. Multivariate analysis revealed that response was a major prognostic factor for PFS and OS regardless of International Staging System (ISS) stage, the type of treatment received, or age. Similarly, results of an analysis of three Spanish protocols, which included transplant-eligible and transplant-ineligible patients, showed that achievement of immunophenotypic responses in both the young and elderly patient populations was associated with significant PFS and OS benefits [12]. An association between maximal response and outcome has also been observed in the relapse setting, as recently reported in an analysis of the phase III MM009 and MM010 trials. The analysis showed that those patients who achieved a CR or VGPR following lenalidomide plus dexamethasone treatment experienced a significantly longer PFS interval and OS duration than those in whom treatment resulted in only a PR (Table 1) [19].
Table 1.
Overview of recently reported trials showing an association between CR or VGPR and outcome
Transplant studies: CR rates shown are responses overall (best responses).
Abbreviations: CR, complete response; CTD, cyclophosphamide, thalidomide, and dexamethasone; CTDa, attenuated cyclophosphamide, thalidomide, and dexamethasone; CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; Dex, dexamethasone; HR, hazard ratio; IR, immunophenotypic response; Len, lenalidomide; MEL200, melphalan 200 mg/m2; MP, melphalan and prednisone; MPR, melphalan, prednisone, and lenalidomide; MPR + R, melphalan, prednisone, and lenalidomide plus lenalidomide maintenance; MPT, melphalan, prednisone, and thalidomide; NA, not available; nCR, near complete response; OS, overall survival; PAD, bortezomib, doxorubicin, and dexamethasone; PFS, progression-free survival; PR, partial response; TD, thalidomide and dexamethasone; TTP, time to progression; V, vincristine; VAD, vincristine, doxorubicin, and dexamethasone; VBMCP/VBAD, vincristine, carmustine, melphalan, cyclophosphamide; prednisone/vincristine, carmustine, doxorubicin, and dexamethasone; VD, Velcade and dexamethasone; VTD, bortezomib, thalidomide, and dexamethasone; VGPR, very good partial response; VMP, bortezomib, melphalan, and prednisone; VMPT, bortezomib, melphalan, prednisone, and thalidomide; VMPT-VT, bortezomib, melphalan, prednisone, and thalidomide followed by maintenance with bortezomib and thalidomide; VTP, bortezomib, thalidomide, and prednisone.
These recent results underline the importance of achieving the best quality response possible regardless of the treatment setting. Taken together, the objective of treatment should be the achievement of a sustained CR with a good quality of life.
Given that effective treatments to achieve good quality responses as well as sensitive techniques to assess these responses are available, it remains to be determined which depth of response is relevant with regard to impact on long-term outcomes. In addition, a number of other issues require examination, such as the need for CR achievement in all patients, the appropriate definition of CR, as well as the standardization of techniques used to assess CR (Table 2).
Table 2.
Issues for further research on the role of complete response (CR) and pre-emptive treatment
Frontline Transplant Setting
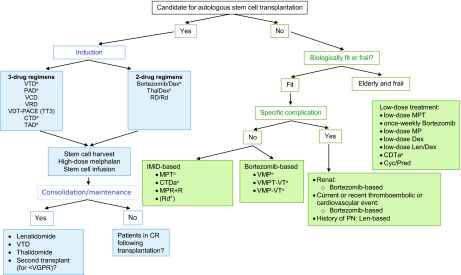
European experts in 2011 recommend autologous stem cell transplantation (ASCT) as the standard of care for young patients (<65 years old) with newly diagnosed MM. The aim of induction is the achievement of the deepest response as quickly as possible prior to transplantation. Induction treatment should consist of a limited number of treatment cycles (three or four). Figure 1 outlines induction treatments that are currently being used and that are recommended. Recent data suggest that three-agent induction regimens, containing at least one novel agent, result in a higher rate of CR or VGPR than two-agent combinations [8, 10, 11, 27–31]. Within the three-drug combinations, bortezomib combined with thalidomide and dexamethasone in the VTD combination appears to be the most active regimen and superior over two-drug regimens incorporating a single novel agent (thalidomide or bortezomib alone), as demonstrated in three separate randomized clinical trials [10, 28, 29, 31]. Trials by the Italian and Spanish myeloma groups were recently updated and confirm the significant superiority of VTD over thalidomide and dexamethasone (TD) in terms of achievement of CR or near CR (nCR) postinduction and posttransplant, as well as PFS. In the Italian study, with a median follow-up of 36 months, the PFS interval was significantly longer for VTD than for TD (p = .0061), with an estimated 3-year PFS rate of 68% versus 56% for the two arms, respectively (p = .0057) [10]. With a median follow-up of 27 months in the Spanish study, the median PFS time was not reached for VTD and was 27 months for TD [31]. In both studies, OS, however, did not differ between treatment arms. In addition to these trials, the French myeloma group presented results of a trial that showed the superiority of a regimen combining bortezomib, thalidomide, and dexamethasone over bortezomib and dexamethasone (VD) [28, 29]. Interestingly, in that trial, bortezomib and thalidomide were used at doses lower than those employed in the previous trials investigating VTD during induction. Bortezomib was used at 1.0 mg/m2 according to the usual administration schedule and thalidomide was used at 100 mg/day. Using this vTD regimen, investigators found that the rates of peripheral neuropathy (PN) were markedly lower than with the standard VD schedule (grade ≥2: VD, 28%; vTD, 15%; grade ≥3: VD, 6%; vTD, 3%). In addition, the rate of CR plus VGPR was significantly superior with vTD than with VD both postinduction (vTD versus VD, 51% versus 35%, respectively; p = .037) and post-transplant (vTD versus VD, 73% versus 59%; p = .037), whereas CR rates after induction, the primary endpoint, were similar in the two arms.
Figure 1.
Multiple myeloma treatment tree outside clinical trials: frontline.
aIndicates data available from phase III randomized trial.
Lenalidomide is currently not EMA approved for the treatment of newly diagnosed multiple myeloma or as consolidation or maintenance treatment. Bortezomib is currently not EMA approved for the treatment of transplant-eligible patients or as consolidation or maintenance treatment. Thalidomide is currently not EMA approved for the treatment of transplant eligible patients or as consolidation or maintenance treatment.
Abbreviations: CR, complete response; CTD, cyclophosphamide, thalidomide, and dexamethasone; CTDa, attenuated cyclophosphamide, thalidomide, and dexamethasone; MP, melphalan and prednisone; MPR + R, melphalan, prednisone, and lenalidomide plus lenalidomide maintenance; MPT, melphalan, prednisone, and thalidomide; PAD, bortezomib, doxorubicin, and dexamethasone; PN, peripheral neuropathy; Pred, prednisone; Rd, lenalidomide plus low-dose dexamethasone; RD, lenalidomide and dexamethasone; TAD, thalidomide, doxorubicin, and dexamethasone; Thal, thalidomide; TT3, Total Therapy 3; VCD, bortezomib, cyclophosphamide, and dexamethasone; VDT-PACE, bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide; VGPR, very good partial response; VMP, bortezomib, melphalan, and prednisone; VMPT-VT, bortezomib, melphalan, prednisone,and thalidomide followed by maintenance with bortezomib and thalidomide; VRD, bortezomib, lenalidomide, and dexamethasone; VTD, bortezomib, thalidomide, and dexamethasone.
At the 2010 American Society of Hematology (ASH) meeting, results of the HOVON-65/German-speaking Myeloma Multicenter Group (GMMG)-HD4 randomized phase III trial comparing bortezomib, doxorubicin, and dexamethasone (PAD) with vincristine, doxorubicin, and dexamethasone (VAD) followed by stem cell transplantation and maintenance with bortezomib or thalidomide were presented with a median follow-up of 39 months [11]. PAD was found to result in significantly higher rates of nCR or better and VGPR or better and PR or better than VAD. Notably, PAD was also significantly superior in terms of PFS (median PFS duration, 36 months versus 27 months for PAD versus VAD, respectively; p = .005) and OS (median not reached in either arm, p = .02), and the study is therefore the first to demonstrate a survival advantage with novel-agent regimens incorporated in the induction and post-transplant setting.
Other effective three-drug regimens include bortezomib, cyclophosphamide, and dexamethasone (VCD), cyclophosphamide, thalidomide, and dexamethasone (CTD), thalidomide, doxorubicin, and dexamethasone (TAD), and bortezomib, lenalidomide, and dexamethasone (VRD), and their use will depend on access to the various agents in different countries, as well as experience in different centers. Most of these regimens include bortezomib and dexamethasone as a backbone onto which cytotoxic agents, such as cyclophosphamide or doxorubicin, or other novel agents, such as thalidomide or lenalidomide, can be added [32].
The utility of adding cyclophosphamide to novel agent regimens is being explored in several studies, for example, the VCD combination is undergoing investigation in an ongoing study by the German myeloma group involving 373 patients, and results of an interim analysis were encouraging [27]. In addition, the ongoing phase II Evaluation of Velcade®, dexamethasone and lenalidomide with or without cyclophosphamide using targeted innovative oncology strategies in the treatment of frontline MM (EVOLUTION) study is investigating the use of cyclophosphamide in combination with bortezomib and dexamethasone with or without lenalidomide [33, 34].
The VRD combination has generated interest based on a study by Richardson et al. [35], who investigated this regimen in the frontline setting in a phase I/II study in which patients received up to eight cycles of induction treatment with VRD and could choose to undergo transplantation. An update of the phase II part of the study showed that at least a PR was achieved in 64% of patients, and after a median follow-up of 26 months, the median TTP and median PFS duration were 9.5 months and the median OS time was 26 months [36]. Based on these results, the VRD regimen is now being investigated as induction therapy in several trials.
In an effort to improve outcomes with novel-agent induction regimens further, four-drug combinations have been examined, for example, in a randomized phase II trial comparing bortezomib, thalidomide, dexamethasone, and cyclophosphamide with bortezomib, thalidomide, and dexamethasone alone [37], as well as in the EVOLUTION study with the combination of bortezomib, lenalidomide, and dexamethasone with or without cyclophosphamide [33, 34]. However, the use of four-drug regimens did not reveal greater efficacy, but was associated with higher toxicity, supporting the use of three-drug regimens.
A recent analysis of the Total Therapy 3 (TT3) protocol investigated the cumulative impact of V, T, and D dosing on outcome [38]. The investigators found that administration of a higher cumulative dose of components of the VTD regimen was associated with significant benefits in terms of long-term outcome, whereas premature discontinuation of any of the agents was found to have a negative impact on OS, event-free survival (EFS), and time to next treatment. Notably, the postrelapse survival duration was not adversely affected by VTD dosing, and moreover, a higher bortezomib dose was found to be associated with a longer postrelapse survival time. Van Rhee et al. [38] concluded that these results support the upfront use of all active agents in a dose-dense and dose-intense fashion.
Induction Treatment in Patients with Cytogenetic Abnormalities
Regarding the use of novel-agent induction regimens in patients with cytogenetic abnormalities, some clearer data have emerged over recent months. Thalidomide-based regimens are associated with a worse outcome in the presence of poor-risk cytogenetics [1], whereas further data are required to elucidate the role of lenalidomide in this setting. Data presented by the French group, who analyzed the impact of the presence of t(4;14) and del(17p) on outcome in patients receiving VD or VAD induction, illustrate that, although bortezomib-based regimens may partially overcome the poor prognostic impact of t(4;14), both t(4;14) and del(17p) remain adverse prognostic factors, because in both treatment arms the presence of either cytogenetic abnormality was associated with significantly shorter EFS and OS times than in patients without the abnormalities [39]. Nevertheless, in patients with t(4;14), bortezomib–dexamethasone induction treatment was associated with significantly longer EFS and OS times than patients receiving VAD induction. In contrast, in patients with del(17p), the EFS and OS times were comparable between the two treatment arms. In the GIMEMA study comparing VTD with TD as induction before and as consolidation therapy after double autologous transplantation, VTD led to a significantly longer PFS duration than TD in the subgroup of t(4;14)+ patients [10]. Remarkably, the PFS curves for patients randomly assigned to VTD were almost superimposable regardless of the presence or absence of patients with t(4;14) [10]. In addition, results of the HOVON-65/GMMG-HD4 study indicate that bortezomib efficacy is maintained in patients with t(4;14), and that outcome is better in patients with del(17p) receiving PAD and bortezomib maintenance over VAD and thalidomide maintenance [11]. However, del(17p) remains a poor prognostic factor [40].
In the recently reported Programa para el Estudio y la Terapéutica de las Hemopatías Malignas y Grupo Español de Mieloma (PETHEMA/GEM) trial, which was designed to compare VMP with bortezomib, thalidomide, and prednisone (VTP) followed by bortezomib and thalidomide (VT) or bortezomib and prednisone (VP) maintenance in elderly patients, t(4;14) and del(17p) remained adverse prognostic factors for PFS and OS [17, 41].
A retrospective analysis of two GIMEMA trials on the impact of upfront bortezomib-based regimens on clinical outcomes according to cytogenetic abnormalities by fluorescence in situ hybridization analysis showed that, in comparison with t(4;14), del(17p) alone did not significantly predict shorter PFS and OS times, whereas the presence of both abnormalities was associated with a significantly shorter PFS interval and a shorter, albeit not statistically significant, OS duration [42]. However, these results require confirmation in a prospective trial.
Richardson et al. [35, 36] reported encouraging results in terms of the VGPR or better rate and PFS interval for the VRD combination in patients with high-risk cytogenetics, in particular those with t(4;14) and del(17p). However, the investigators stressed that no definite conclusions can be drawn from the study regarding the activity of the VRD regimen in patients with adverse cytogenetics because of the small sample size.
Taken together, the results regarding bortezomib-based induction treatment in the transplant setting indicate that the agent is effective not only in patients with standard-risk disease but that the efficacy is retained in the presence of selected cytogenetic abnormalities regarding the overall response rate (ORR), CR rate, and PFS time. Bortezomib-based induction treatment can therefore be considered an appropriate choice for induction treatment across different risk groups.
Increasingly, gene-expression profiling (GEP) is being investigated with the aim of providing prognostic information based on the molecular classification of patients [43–46]. The Arkansas group developed a 70-gene model that allowed identification of a group of patients at high risk for early disease-related death [43]. Using the 70 gene–derived risk score, Nair et al. [47] compared results of the TT3 protocol with those from the successor trial, in which lenalidomide was included as maintenance therapy instead of thalidomide. Overall, results were comparable across GEP-defined risk groups in the two trials. Notably, GEP-defined high risk, which was seen in 17% of patients, was associated with a significantly shorter OS time, EFS time, and CR duration in both trials, demonstrating the utility of the GEP risk model.
Role of ASCT in the Era of Novel Agents
In the era of novel agents, the role of transplantation itself is currently undergoing scrutiny. The question being addressed is whether transplant should be carried out upfront or delayed to relapse. The only prospective data with novel agents in this setting so far were presented by the Italian myeloma group, who are conducting a phase III trial in which patients are randomized to consolidation with six cycles of melphalan, prednisone, and lenalidomide (MPR) or tandem melphalan 200 mg/m2 (MEL200) plus stem cell support following four cycles of induction therapy with lenalidomide plus low-dose dexamethasone (Rd) [6]. The protocol contains a second randomization step: following consolidation, patients are randomized to receive either lenalidomide maintenance treatment until relapse or no maintenance. With a median follow-up of 14 months, there was no difference between the MPR arm and the MEL200 arm regarding the response rate, PFS time, or OS time. Not unexpectedly, grade 3 or 4 hematologic toxicity was significantly greater for patients in the MEL200 arm, as were infections and gastrointestinal toxicity. Currently, follow-up for the study is too short to draw any conclusions regarding the superiority of one approach over the other, and long-term PFS and OS results are eagerly awaited.
In addition, two large multicenter trials have been initiated to investigate the question of transplant upfront versus novel agent combination upfront with transplant reserved for relapse [48, 49].
The results of all these trials will provide important information regarding treatment decisions for specific patient populations and may identify patients in whom transplant upfront is important, versus those in whom transplant could be delayed until later on in the treatment sequence.
However, for MM treatment in 2011, ASCT with novel-agent induction regimens remains the standard of care for young patients.
Post-ASCT Therapy
The overall aim of post-ASCT therapy is to prolong PFS and OS durations, and there are two distinct approaches that are being investigated. On the one hand, post-ASCT therapy can consist of administration of a consolidation treatment applied for a limited period of time following the transplant step, with the aim of increasing the depth of response. On the other hand, maintenance treatment administered for a prolonged time period either following transplantation or following transplant plus consolidation may be chosen with the aim of prolonging the response duration.
A number of recent trials are helping to elucidate the role that novel agents could play in this setting. Employed as consolidation treatments for a limited period of time, all novel agents are able to upgrade the response status in patients with a suboptimal response to ASCT [50–54].
Recently, two studies investigating bortezomib-based consolidation therapy were reported: the Nordic group investigated single-agent bortezomib following ASCT in a randomized phase III trial and found that bortezomib consolidation resulted in a significantly greater CR rate than observation 6 months postrandomization [52]. Regarding tolerability, the investigators reported that grade ≥3 neurologic pain was seen in 5% of patients and sensory neuropathy grade ≥3 was seen in 3% of patients. Ladetto et al. [53] employed VTD consolidation in bortezomib-naive patients who achieved a VGPR or better post-ASCT and observed that this regimen increased the percentage of patients in molecular remission from 3% post-ASCT to 18% postconsolidation. Furthermore, no relapses were seen in patients with molecular remission after 42 months of follow-up. In addition, the phase III GIMEMA trial comparing VTD with TD showed that VTD consolidation treatment was associated with significantly higher CR and CR + nCR rates than TD consolidation (p = .0001 and <.0001, respectively). Unlike consolidation therapy with TD, VTD consolidation significantly upgraded the CR and CR + nCR rate (p = .078 and .012, according to McNemar tests). Overall, the absolute CR upgrade was 11% with VTD consolidation and 6% with TD [55]. In addition, VTD consolidation following double ASCT increased the rate of molecular remission from 39% to 64% (p = .007 according to a McNemar test); in contrast, the upgrade of molecular remissions observed with TD consolidation was not statistically significant. Real-time quantitative polymerase chain reaction analysis confirmed the major reduction in residual tumor burden effected by VTD versus TD consolidation therapy (median, 5 log versus 1 log reduction, respectively [56]. These results are encouraging; however, further data are needed to define the role of bortezomib consolidation therapy in improving PFS and OS times.
In the maintenance setting, thalidomide is the agent that has been studied most extensively and results have been reviewed previously [1]. A recent update to the thalidomide maintenance trials conducted by the Intergroupe Francophone du Myélome (IFM) and the Arkansas group with long-term follow-up revealed that the OS difference observed for the thalidomide-containing maintenance arm in the IFM trial disappeared, whereas in the Arkansas study of TT2 with thalidomide throughout, a survival advantage for the thalidomide arm became apparent [57]. Taken together, all five hitherto published or presented trials evaluating thalidomide maintenance treatment after ASCT [30, 50, 54, 57, 58] reveal a significantly longer PFS duration, but a significantly longer OS time was noted in only two of them [54, 57]. Thus, when considering thalidomide maintenance treatment after ASCT, the benefits in terms of survival parameters have to be balanced against the possible toxicity of this strategy. Several open questions regarding the use of thalidomide in the maintenance setting remain, such as the optimal duration and dose of treatment. Experience to date shows that a dose of 50–100 mg/day is tolerated for prolonged periods. Finally, it has been noted that thalidomide may act as a consolidation, rather than maintenance, treatment.
Two recently reported phase III trials are investigating lenalidomide as a maintenance treatment [51, 59]. In both the Cancer and Leukemia Group B (CALGB) and IFM trials, patients were randomized post-ASCT to either lenalidomide maintenance treatment or placebo (in the French study, all patients also received two cycles of lenalidomide consolidation prior to maintenance randomization). Both studies demonstrated a significantly longer TTP or PFS time for the lenalidomide-containing maintenance arm [51, 59]. With a median follow-up of 34 months from randomization in the IFM study, the median PFS times were 42 months for the lenalidomide arm and 24 months for the placebo arm (p < 10−8) [51]. In the CALGB study, the median TTP was 42.3 months for the lenalidomide arm and 21.8 months for the placebo arm (p < .0001) after a median follow-up of 17.5 months from ASCT [598]. Notably, the longer PFS time was seen regardless of the prespecified stratification criteria of β2-microglobulin level, level of response (VGPR or better versus less than VGPR) and del13 in the French trial [51], and in the CALGB trial, exposure to thalidomide or lenalidomide during induction was not found to influence TTP [59]. In both trials, maintenance treatment with lenalidomide was found to be well tolerated. Although OS data are not yet available, these data are likely to have an impact on treatment practices because of the significantly lower risk for relapse associated with lenalidomide maintenance treatment. Nevertheless, before implementing lenalidomide maintenance therapy in clinical practice, more information regarding its long-term effect on the myeloma clone and the bone marrow stroma as well the optimal dose and duration of therapy should be available. Furthermore, even if the relative risk for progression of disease can be reduced by close to 60%, the demonstration of a survival benefit would greatly promote its acceptance by the medical community.
Bortezomib maintenance treatment is being investigated in ongoing studies, for example, in the HOVON-65 MM/GMMG-HD4 study, which could show that bortezomib maintenance is feasible and induces additional responses in patients, including nCR and CRs [11].
Despite positive results with novel agents in the post-ASCT setting, many open questions remain. These include the optimal dose, schedule, and duration of treatment as well as the impact on survival after relapse. In addition, clarification is needed regarding which patients should receive post-ASCT therapy, that is, whether this should be all patients, including those in CR, or only those with a suboptimal response to ASCT. Finally, longer follow-up is needed to assess the impact of post-ASCT therapy on OS.
Frontline Nontransplant Setting
Regarding the treatment of patients who are not eligible for transplantation, MPT and VMP have shown significant benefit over MP and are recommended treatments (Fig. 1). Following our previous publication [1], three MPT studies that were then only available in abstract format have now been fully published [25, 26, 60]. The HOVON-49 study showed that MPT led to significantly greater ORR, VGPR or better rate, EFS interval, and OS time than with MP [25]. In the trial conducted by the Nordic myeloma group, a significantly higher VGPR rate was observed for the MPT arm than for the MP arm; however, this did not translate into a better outcome and there was no significant difference in terms of the PFS or OS time between the two arms [26]. The Turkish study also showed a beneficial effect of MPT over MP in terms of response and a lower early mortality rate [60]. The discrepancy in efficacy outcomes reported for the various MPT trials may be explained by differences in inclusion criteria, particularly regarding patient age and performance status, greatly differing doses of thalidomide, and differences in the duration of thalidomide treatment, with some trials incorporating thalidomide both during induction and maintenance and other trials using thalidomide only during induction [61]. A recent meta-analysis on the survival of 1,682 individual patients treated with MPT or MP in six randomized studies revealed that the addition of thalidomide to MP led to a significantly longer PFS interval (20.4 months versus 14.9 months; p = .001), and although the OS time was longer (39.3 months versus 32.7 months), the difference was not statistically significant (p = .085) [62]. However, overall, the results support the use of the MPT regimen for the treatment of elderly patients.
Other immunomodulatory drug (IMiD)-based options for the treatment of elderly patients include CTDa and Rd, as previously reviewed [1], whereas in patients aged ≥75, the combination of thalidomide and dexamethasone appears to be inferior to MP regarding OS and toxicity [63].
Recently, prolonged follow-up results of the phase III VISTA trial were fully published and confirm the significant survival advantage of the VMP regimen over MP [23]. VMP treatment was associated with a 35% lower risk for death than with MP (hazard ratio, 0.653; p < .001). With a median follow-up of 36.7 months, the median OS time had not been reached for the VMP arm and was 43 months with MP. The 3-year OS rates were 68.5% and 54%, respectively. The longer OS time with VMP compared with MP was seen in a number of predefined patient subgroups and was found to be independent of age, β2-microglobulin, albumin, ISS stage, and creatinine clearance.
The safety profile was similar to that reported in the initial analysis [21]. Grade 3 PN occurred in 13% of patients and grade 4 PN occurred in <1% of patients. After prolonged follow-up, 79% of PN events had improved or resolved within a median of 1.9 months, whereas 60% completely resolved within a median of 5.7 months. The rate of discontinuation of all treatment was 15% for VMP and 14% for MP. In addition, 19% of VMP patients discontinued bortezomib but remained on MP, resulting in a total discontinuation rate of 34% for bortezomib treatment [23].
The longer follow-up allowed an assessment of the impact of frontline VMP treatment on subsequent therapy in those patients who relapsed. This showed that patients can be successfully treated with thalidomide- or lenalidomide-containing regimens as well as bortezomib-based regimens following upfront treatment with VMP. Among patients initially treated with VMP and MP, the median survival times from the start of subsequent therapy were 30.2 months and 21.9 months, respectively, and there was no difference in the survival time after salvage treatment among patients who received subsequent bortezomib, thalidomide, or lenalidomide, indicating that first-line bortezomib use does not induce a more resistant relapse.
Since our last publication, a study investigating lenalidomide in combination with MP was reported (MM-015 study) [14]. In that trial, three arms were tested: MPR (nine cycles) plus lenalidomide maintenance until progression versus MPR alone for nine cycles versus MP. Although MPR + R was found to be significantly superior to MP in terms of the CR rate, ORR, and PFS time, MPR by itself was not found to result in a longer PFS interval than with MP, demonstrating that lenalidomide maintenance treatment prolongs PFS after 1 year of induction treatment, an improvement that was also seen in a landmark analysis beginning at the time of cycle 10 (approximately 40 weeks after the start of induction therapy). With a median follow-up of 32 months, the median PFS interval was 31 months for MPR + R, it was 14 months for MPR, and it was 13 months for MP (p < .0000001 for MPR + R versus MP, p = .153 for MPR versus MP). No difference in OS among the three arms has been observed yet and longer follow-up is needed. Regarding toxicity, grade 4 neutropenia was the most frequent adverse event (AE) with MPR + R (36%, versus 8% with MP). The rate of discontinuation resulting from AEs was 20% for MPR + R, versus 8% for MP.
Lenalidomide is also being investigated in combination with prednisone as induction treatment followed by MPR consolidation and lenalidomide maintenance in an ongoing phase II study in patients with newly diagnosed MM aged >65 years. Notably, no exclusion criteria were applied in the protocol to prevent the selection of a patient population with a good performance status only [64]. Treatment with four courses of lenalidomide plus prednisone followed by MPR (median, seven cycles overall) resulted in a PR or better in 72% of patients, including a VGPR or better in 22% of patients. Overall, the treatment was manageable, with grade 3 or 4 hematological toxicities being the most frequent AEs.
Treatment Options for Very Elderly Patients
The following novel-agent regimens appear to be feasible in the setting of very elderly patients: MPT, VMP, and Rd. The IFM-01/01 trial investigated MPT versus MP in patients aged >75 years and found that MPT was significantly superior to MP in terms of the ORR, VGPR rate, and CR rate, as well as the PFS and OS times [22]. Notably, in that trial, thalidomide was administered at a dose of 100 mg/day.
A subanalysis of the VISTA trial according to age showed that VMP was significantly superior to MP in terms of the ORR, CR rate, TTP, and OS time in patients aged ≥75 years and <75 years [23, 65]. Nevertheless, a comparison of outcome in the VMP arm for the two age groups showed that patients aged ≥75 years had a shorter survival duration than those aged <75 years [23]. The safety profiles of VMP were generally similar in patients aged ≥75 years and those aged <75 years, as was the rate of discontinuation [65].
The importance of dose reduction in very elderly patients as well as its utility in younger patients is increasingly being recognized, and two phase III trials investigating once weekly administration of bortezomib as part of the VMP regimen in elderly patients with newly diagnosed MM have now been fully published [16, 17, 66, 67]. In the trial conducted by the PETHEMA/GEM group, patients were randomized to receive six cycles of VMP or bortezomib plus thalidomide plus prednisone (VTP) [17]. During cycle 1, bortezomib was administered twice weekly, and in subsequent cycles bortezomib was administered only once weekly at a dose of 1.3 mg/m2. Compared with the results obtained in the VISTA trial, weekly administration resulted in substantially better tolerability. Notably, the incidence of grade 3 or 4 PN was 7% with the reduced-dose VMP regimen and treatment discontinuations were only seen in 12% of patients (compared with 14% and 34% of patients in the VISTA trial, respectively). Efficacy was maintained, with an ORR of 80%, whereas 20% of patients achieved CRs and the OS rate at 2 years was 92%. After a median follow-up of 32 months, the median PFS interval for all patients was 31 months and the median TTP was 35 months.
Similarly, a study conducted by the Italian Myeloma group, which examined bortezomib administered weekly in a trial designed to compare VMPT with VMP in elderly patients (n = 354), found that weekly administration of bortezomib resulted in markedly better tolerability of the VMP regimen [66]. It was initially planned to administer four cycles of twice-weekly bortezomib; however, following a protocol amendment, patients only received once-weekly bortezomib as part of the VMP and VMPT regimens. A comparison of efficacy and toxicity in patients receiving twice-weekly or once-weekly bortezomib revealed that a shift from twice-weekly to once-weekly bortezomib dosing led to a lower CR rate, 30% versus 35%, but it also resulted in a substantially lower incidence of PN (8%, versus 28%) and rate of treatment discontinuation resulting from PN (5%, versus 15%), whereas the OS rates at 3 years were 89% and 88% in the twice-weekly and once-weekly groups, respectively. Of note, in patients aged >75 years, the VMPT regimen followed by VT maintenance was not superior to VMP.
These trials confirm the significant activity of VMP in the nontransplant setting and, notably, they also indicate that weekly bortezomib administration as part of the VMP regimen is a highly effective option for the treatment of elderly patients that results in a better tolerability profile.
At the 2010 ASH meeting, results of a trial in the relapsed/refractory setting investigating an s.c. formulation of bortezomib were reported [68]. Although efficacy was found to be similar to that of the i.v. formulation, s.c. administration of the agent appeared to be associated with a better safety profile. However, further follow-up and analyses in different treatment settings are needed.
Regarding MPR, data from the MM-015 trial indicate that this combination may be less suitable in patients aged >75 years [14]. This was suggested by a higher rate of discontinuation than in the group of patients aged 65–75 years. In addition, the PFS interval was not significantly longer with the MPR combination than with MP. Finally, an analysis of OS at 3 years in an Eastern Cooperative Oncology Group trial that compared RD with Rd showed that, in patients aged ≥70 years, using a lower dose of dexamethasone resulted in better tolerability and a significantly higher OS rate (3-year OS rate, 61% versus 73% for RD versus Rd; p = .03) [69].
Taken together, regarding the treatment of elderly patients with MP-based regimens, the following recommendations can be made. In general, treatment should be selected based on biological age, comorbidities, and overall clinical impression. The following dose schedules are recommended:
Standard schedules for patients requiring quick relief from symptoms and for those aged 65–75 years
A less intense treatment approach for frail patients and those with comorbidities (heart, lung, kidney, liver, diabetes) or aged >75 years
The optimal duration of therapy and, particularly, the utility of maintenance therapy is also being investigated in the nontransplant setting. In the MM-015 study, lenalidomide maintenance treatment provided a significant benefit in terms of PFS over MPR treatment alone, which was similar to that of MP [14]. In addition, the Spanish and Italian myeloma groups investigated maintenance therapy following VMP, VTP [17], or VMPT [16, 66, 67] induction in an elderly population. In the majority of these studies, maintenance treatment resulted in a benefit in terms of PFS; however, longer follow-up is needed to assess the effect on OS. In addition, open questions remain, such as the duration of treatment and the impact of maintenance treatment on survival at relapse.
Treatment of Relapsed/Refractory MM
As in the frontline setting, the goals of treatment at relapse have to be defined individually for each patient, weighing efficacy versus toxicity considerations. Although best response may be the goal in some patients, based on the observation that achieving a CR or VGPR is also associated with longer survival in the relapse setting [19, 70], disease stabilization is the goal for other patients, with the aim of preventing further progression.
Treatment at relapse is significantly influenced by the timing of the relapse, which is linked to the efficacy of the previous treatment. If relapse occurs during treatment, it may be indicative of resistant disease and a change in drug class from that used initially; as well, the use of double or triple combinations should be considered. Similarly, for an early relapse (within 6 months), an alternative treatment from the one used upfront should be contemplated, whereas for late relapses (after >1 year), retreatment with components of the initial therapy is an option.
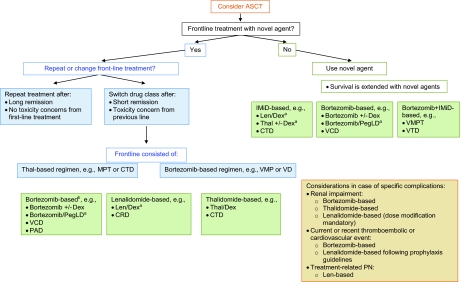
Figure 2 shows a possible decision tree for the treatment of MM at relapse, with a focus on incorporating novel agents into treatment. Transplantation remains a feasible option at relapse and should be considered in those patients in whom ASCT was not performed upfront, and in those with a long remission after the previous transplant. Allogeneic SCT should be performed only in the context of a clinical trial [71].
Figure 2.
Multiple myeloma treatment tree outside clinical trials: relapse.
aIndicates data available from phase III randomized trial.
bRetreatment with bortezomib after frontline bortezomib only if no PN is present, or if PN has recovered and there is no other therapeutic alternative.
Abbreviations: ASCT, autologous stem cell transplantation; CRD, cyclophosphamide, lenalidomide, and dexamethasone; CTD, cyclophosphamide, thalidomide, and dexamethasone; Dex, dexamethasone; IMiD, immunomodulatory drug; Len, lenalidomide; MPT, melphalan, prednisone, and thalidomide; PAD, bortezomib, doxorubicin, and dexamethasone; PegLD, pegylated liposomal doxorubicin; PN, peripheral neuropathy; Thal, thalidomide; VCD, bortezomib, cyclophosphamide, and dexamethasone; VD, bortezomib and dexamethasone; VMP, bortezomib, melphalan, and prednisone; VMPT, bortezomib, melphalan, prednisone, and thalidomide; VTD, bortezomib, thalidomide, and dexamethasone.
The question of sequencing of novel agents as well as retreatment is of substantial interest; however, at present only limited data have been reported. Following a thalidomide-containing regimen, treatment with lenalidomide appears feasible; however, efficacy may be lower [72]. A recent report of a prospective evaluation of the effect of prior therapy on efficacy with the combinations of lenalidomide and dexamethasone or VRD in the relapsed/refractory setting indicates that resistance to previous thalidomide is associated with an inferior response to therapy as well as shorter PFS and OS times [73]. Lenalidomide-based treatment following upfront treatment with a lenalidomide-containing regimen was found to be feasible in a recent report [74].
Regarding bortezomib, results on subsequent treatments from the VISTA trial suggest that retreatment with a bortezomib-containing regimen is feasible following frontline VMP, provided a favorable response was achieved upfront, and that IMiD-containing regimens can also be used following VMP [23]. Retreatment with a bortezomib-containing regimen after a long treatment-free interval may include bortezomib in combination with agents that were not used in the previous line. Regarding further relapses, interim data from the international phase II Retreatment after Initial Response to Velcade® (RETRIEVE) study indicate that bortezomib use is feasible and does not lead to cumulative toxicity in later lines of treatment after prior bortezomib and/or IMiD use [75].
Patients who have received all novel agents, in particular those whose disease has relapsed or has become refractory to novel agents may present a particular challenge [76]. For patients who qualify for inclusion in a clinical trial of novel experimental agents, such as histone deacetylase inhibitors (HDACs), new proteasome inhibitors (e.g., carfilzomib), or IMiDs (e.g., pomalidomide), bendamustine, anti-CD40 monoclonal antibody, etc., this may be the preferred option. If, on the other hand, patients do not qualify for inclusion in a trial, palliative treatment using alkylating agents in combination with corticosteroids, high-dose dexamethasone, or older regimens, such as continuous infusion of dexamethasone, cyclophosphamide, etoposide, and cisplatin (DCEP), could be chosen.
One of the open questions regarding treatment at relapse concerns the management of patients with adverse cytogenetics, which remains an important adverse prognostic factor in this setting [73].
Considerations Regarding Comorbidities and AEs
Renal Impairment
Bortezomib-based regimens result in a fast onset of response, which is important to improve the probability of renal recovery. The benefit of bortezomib in the setting of renal impairment was observed in a number of studies and analyses [77–79]. A thalidomide-based regimen is feasible, and several recent publications have investigated the use of lenalidomide in the setting of renal impairment, suggesting that this agent also presents an option for patients with renal impairment, provided dose modifications are implemented [80].
AEs
PN
PN as a result of disease or treatment presents a challenge; however, as our experience with the use of novel agents has increased, we have also been able to increase our understanding of how best to manage PN. It is crucial that both patients and medical personnel are aware that PN may occur so that prompt intervention can be initiated. For agents that are associated with PN, assessment before every dose is recommended. If PN occurs, prompt intervention according to guidelines is crucial to enable the improvement/reversal of symptoms. In patients with pre-existing PN, the use of drugs without neurotoxic potential such as lenalidomide is preferred [81].
Thromboembolic Complications
In cases with a risk for thromboembolic or cardiovascular events or in the presence of these complications, a bortezomib-containing regimen can be recommended; however, a lenalidomide-based regimen may also be chosen following existing guidelines regarding the use of prophylaxis [82].
Delaying Myeloma Progression in Smoldering Myeloma
Presently, the standard of care for patients with smoldering myeloma is observation, and treatment is delayed until the occurrence of signs of progressive disease. An ongoing phase III trial by the Spanish myeloma group is investigating if treatment with lenalidomide plus dexamethasone for nine cycles followed by continuous lenalidomide maintenance can delay progression to symptomatic myeloma in patients with high-risk smoldering MM [83]. With a median follow-up of 16 months, the TTP was found to be significantly longer in patients receiving treatment with lenalidomide and dexamethasone than in patients in the therapeutic abstention arm; however, the OS duration was not different between the two arms. Overall, treatment-related AEs were manageable, with no grade 4 hematological or nonhematological AEs and a low incidence of grade 3 AEs. Longer follow-up is needed to establish whether an OS benefit will become apparent.
Outlook
The incorporation of novel agents in the different treatment settings has substantially improved the outcome for patients. Long-term follow-up is required to further define the role of novel agents in the various treatment stages based on OS data. Despite substantial progress, many questions remain, such as the role of ASCT in the era of novel agents, the optimal treatment sequence, and the tailoring of therapies to individual cytogenetic risk factors. In this respect, the definition of risk groups using techniques such as GEP and single-nucleotide polymorphism analysis may enable an individualized treatment approach. Furthermore, the role of minimal residual disease detection in the assessment of treatment response requires further investigation.
A number of newer antimyeloma agents are currently undergoing examination in clinical trials, including second-generation proteasome inhibitors and IMiDs, as well as new classes of antimyeloma agents, such as heat-shock protein 90 inhibitors, HDAC inhibitors, and bendamustine. Results from ongoing studies will show how these can be incorporated into the management of multiple myeloma.
Acknowledgments
Advisory Board meeting supported by Janssen Pharmaceutical Companies of Johnson & Johnson in Europe, Middle East, and Africa (EMEA). Unrestricted educational grant provided by Janssen Pharmaceutical Companies of Johnson & Johnson in the EMEA to assist with editorial support.
Author Contributions
Conception/Design: Heinz Ludwig, Meral Beksac, Joan Bladé, Jamie D. Cavenagh, Michele Cavo, Michel Delforge, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Jean-Luc Harousseau, Urs Hess, Martin Kropff, Fernando Leal da Costa, Vernon Louw, Hila Magen-Nativ, Larisa Mendeleeva, Hareth Nahi, Antonio Palumbo, Torben Plesner, Jesús San Miguel, Pieter Sonneveld, Miklos Udvardy, Pia Sondergeld
Data analysis and interpretation: Heinz Ludwig, Meral Beksac, Joan Bladé, Jamie D. Cavenagh, Michele Cavo, Michel Delforge, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Jean-Luc Harousseau, Urs Hess, Martin Kropff, Fernando Leal da Costa, Vernon Louw, Hila Magen-Nativ, Larisa Mendeleeva, Hareth Nahi, Antonio Palumbo, Torben Plesner, Jesús San Miguel, Pieter Sonneveld, Miklos Udvardy, Pia Sondergeld
Manuscript writing: Heinz Ludwig, Meral Beksac, Joan Bladé, Jamie D. Cavenagh, Michele Cavo, Michel Delforge, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Jean-Luc Harousseau, Urs Hess, Martin Kropff, Fernando Leal da Costa, Vernon Louw, Hila Magen-Nativ, Larisa Mendeleeva, Hareth Nahi, Antonio Palumbo, Torben Plesner, Jesús San Miguel, Pieter Sonneveld, Miklos Udvardy, Pia Sondergeld
Final approval of manuscript: Heinz Ludwig, Meral Beksac, Joan Bladé, Jamie D. Cavenagh, Michele Cavo, Michel Delforge, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Jean-Luc Harousseau, Urs Hess, Martin Kropff, Fernando Leal da Costa, Vernon Louw, Hila Magen-Nativ, Larisa Mendeleeva, Hareth Nahi, Antonio Palumbo, Torben Plesner, Jesús San Miguel, Pieter Sonneveld, Miklos Udvardy, Pia Sondergeld
References
- 1.Ludwig H, Beksac M, Bladé J, et al. Current multiple myeloma treatment strategies with novel agents: A European perspective. The Oncologist. 2010;15:6–25. doi: 10.1634/theoncologist.2009-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Velde HJK, Liu X, Chen G, et al. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92:1399–1406. doi: 10.3324/haematol.11534. [DOI] [PubMed] [Google Scholar]
- 3.Lahuerta JJ, Mateos MV, Martínez-López J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: Sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–5782. doi: 10.1200/JCO.2008.17.9721. [DOI] [PubMed] [Google Scholar]
- 4.Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114:3139–3146. doi: 10.1182/blood-2009-03-201053. [DOI] [PubMed] [Google Scholar]
- 5.Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol. 2010;28:2612–2624. doi: 10.1200/JCO.2009.25.4250. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo A, Cavallo F, Hardan I, et al. A phase III study to compare melphalan, prednisone, lenalidomide (MPR) versus melphalan 200 mg/m2 and autologous transplantation (MEL200) in newly diagnosed multiple myeloma patients [abstract 3573] Blood. 2010;116:1471. [Google Scholar]
- 7.Morgan GJ, Davies FE, Gregory WM, et al. The addition of thalidomide to the induction treatment of newly presenting myeloma patients increases the CR rate which is likely to translate into improved PFS and OS [abstract 352] Blood. 2009;114:149. [Google Scholar]
- 8.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: Results of the IFM 2005–01 phase III trial. J Clin Oncol. 2010;28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 9.Moreau P, Attal M, Pégourié B, et al. Achievement of VGPR to induction therapy is an important prognostic factor for longer PFS in the IFM 2005–01 trial. Blood. 2010 Nov 23; doi: 10.1182/blood-2010-08-300863. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: A randomised phase 3 study. Lancet. 2010;376:2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 11.Sonneveld P, Schmidt-Wolf I, van der Holt B, et al. HOVON-65/GMMG-HD4 randomized phase III trial comparing bortezomib, doxorubicin, dexamethasone (PAD) vs VAD followed by high-dose melphalan (HDM) and maintenance with bortezomib or thalidomide in patients with newly diagnosed multiple myeloma (MM) [abstract 40] Blood. 2010;116:23. [Google Scholar]
- 12.Paiva B, Vidriales MB, Montalbàn MA, et al. Analysis of immunophenotypic response (IR) by multiparameter flow cytometry in 516 myeloma patients included in three consecutive Spanish trials [abstract 1910] Blood. 2010;116:796. [Google Scholar]
- 13.Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: Updated results of a randomized, controlled trial. Blood. 2008;112:3107–3114. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo A, Delforge M, Catalano J, et al. A phase 3 study evaluating the efficacy and safety of lenalidomide combined with melphalan and prednisone in patients ≥65 years with newly diagnosed multiple myeloma (NDMM): Continuous use of lenalidomide vs fixed-duration regimens [abstract 622] Blood. 2010;116:273. [Google Scholar]
- 15.Harousseau JL, Palumbo A, Richardson PG, et al. Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with nonintensive therapy: Analysis of the phase 3 VISTA study of bortezomib plus melphalan-prednisone versus melphalan-prednisone. Blood. 2010;116:3743–3750. doi: 10.1182/blood-2010-03-275800. [DOI] [PubMed] [Google Scholar]
- 16.Palumbo A, Bringhen S, Cavalli M, et al. Bortezomib, melphalan, prednisone and thalidomide followed by maintenance with bortezomib and thalidomide (VMPT-VT) for initial treatment of elderly multiple myeloma patients: Updated follow-up and impact of prognostic factors [abstract 620] Blood. 2010;116:272. [Google Scholar]
- 17.Mateos MV, Oriol A, Martínez-López J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: A randomised trial. Lancet Oncol. 2010;11:934–941. doi: 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- 18.Gay F, Larocca A, Wijermans PW, et al. Achievement of complete response is a strong prognostic factor in elderly newly diagnosed myeloma: Retrospective analysis of 1175 patients [abstract 1949] Blood. 2010;116:812. [Google Scholar]
- 19.Harousseau JL, Dimopoulos MA, Wang M, et al. Better quality of response to lenalidomide plus dexamethasone is associated with improved clinical outcomes in patients with relapsed or refractory multiple myeloma. Haematologica. 2010;95:1738–1744. doi: 10.3324/haematol.2009.015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): A randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 21.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 22.Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27:3664–3670. doi: 10.1200/JCO.2008.21.0948. [DOI] [PubMed] [Google Scholar]
- 23.Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: Updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28:2259–2266. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 24.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijermans P, Schaafsma M, Termorshuizen F, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: The HOVON 49 Study. J Clin Oncol. 2010;28:3160–3166. doi: 10.1200/JCO.2009.26.1610. [DOI] [PubMed] [Google Scholar]
- 26.Waage A, Gimsing P, Fayers P, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116:1405–1412. doi: 10.1182/blood-2009-08-237974. [DOI] [PubMed] [Google Scholar]
- 27.Einsele H, Liebisch P, Langer C, et al. Velcade, intravenous cyclophosphamide and dexamethasone (VCD) induction for previously untreated multiple myeloma (German DSMM XIa trial) [abstract 131] Blood. 2009;114:59. [Google Scholar]
- 28.Harousseau JL, Avet-Loiseau H, Mary J, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib plus thalidomide-dexamethasone as induction prior to autologous transplantation in newly diagnosed myeloma [abstract 1097] Haematologica. 2010;95(suppl 2):451. [Google Scholar]
- 29.Moreau P, Facon T, Attal M, et al. Comparison of reduced-dose bortezomib plus thalidomide plus dexamethasone (vTD) to bortezomib plus dexamethasone (VD) as induction treatment prior to ASCT in de novo multiple myeloma (MM): Results of IFM2007–02 study [abstract 8014] J Clin Oncol. 2010;28(15 suppl):576s. [Google Scholar]
- 30.Lokhorst HM, van der Holt B, Zweegman S, et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood. 2010;115:1113–1120. doi: 10.1182/blood-2009-05-222539. [DOI] [PubMed] [Google Scholar]
- 31.Rosiñol L, Cibeira MT, Mateos MV, et al. A phase III PETHEMA/GEM study of induction therapy prior autologous stem cell transplantation (ASCT) in multiple myeloma: Superiority of VTD (bortezomib/thalidomide/dexamethasone) over TD and VBMCP/VBAD plus bortezomib [abstract 307] Blood. 2010;116:139. [Google Scholar]
- 32.Moreau P, Hulin C, Marit G, et al. Stem cell collection in patients with de novo multiple myeloma treated with the combination of bortezomib and dexamethasone before autologous stem cell transplantation according to IFM 2005–01 trial. Leukemia. 2010;24:1233–1235. doi: 10.1038/leu.2010.82. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Flinn IW, Hari PN, et al. Novel three- and four-drug combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide, for newly diagnosed multiple myeloma: Encouraging results from the multi-center, randomized, phase 2 EVOLUTION study [abstract 127] Blood. 2009;114:57. [Google Scholar]
- 34.Kumar SK, Flinn I, Noga SJ, et al. Bortezomib, dexamethasone, cyclophosphamide and lenalidomide combination for newly diagnosed multiple myeloma: Phase 1 results from the multicenter EVOLUTION study. Leukemia. 2010;24:1350–1356. doi: 10.1038/leu.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson PG, Jagannath S, Jakubowiak AJ, et al. Phase II trial of lenalidomide, bortezomib, and dexamethasone in patients (pts) with relapsed and relapsed/refractory multiple myeloma (MM): Updated efficacy and safety data after >2 years of follow-up [abstract 3049] Blood. 2010;116:1257. [Google Scholar]
- 37.Ludwig H, Viterbo L, Greil R, et al. Phase II study of bortezomib, thalidomide and dexamethasone/-cyclophosphamide as induction therapy in previously untreated multiple myeloma (MM): Safety and activity including evaluation of MRD [abstract 371] Haematologica. 2010;95(suppl 2):149–150. [Google Scholar]
- 38.van Rhee F, Szymonifka J, Anaissie E, et al. Total Therapy 3 for multiple myeloma: Prognostic implications of cumulative dosing and premature discontinuation of VTD maintenance components, bortezomib, thalidomide, and dexamethasone, relevant to all phases of therapy. Blood. 2010;116:1220–1227. doi: 10.1182/blood-2010-01-264333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p) J Clin Oncol. 2010;28:4630–4634. doi: 10.1200/JCO.2010.28.3945. [DOI] [PubMed] [Google Scholar]
- 40.Goldschmidt H, Neben K, Bertsch U, et al. Bortezomib-based induction therapy followed by autologous stem cell transplantation and maintenance therapy with bortezomib improves outcome in myeloma patients with fain 1q21 and t(4;14)—a subgroup analysis of the HOVON-65/GMMG-HD4 trial [abstract 305] Blood. 2010;116:138. [Google Scholar]
- 41.Mateos MV, Gutierrez NC, Paiva B, et al. Clinical outcome according to both cytogenetic abnormalities (CA) detected by fluorescence in situ hybridization (FISH) and hyperdiploidy assessed by flow cytometry (FCM) in elderly newly diagnosed myeloma patients treated with a bortezomib-based combination [abstract 309] Blood. 2010;116:140. [Google Scholar]
- 42.Cavo M, Bringhen S, Terragna C, et al. Bortezomib-based induction treatments improve outcomes of newly diagnosed multiple myeloma patients with high-risk cytogenetic abnormalities [abstract 781] Blood. 2010;116:342. [Google Scholar]
- 43.Shaughnessy JD, Jr, Zhan F, Burington BE, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 44.Decaux O, Lodé L, Magrangeas F, et al. Prediction of survival in multiple myeloma based on gene expression profiles reveals cell cycle and chromosomal instability signatures in high-risk patients and hyperdiploid signatures in low-risk patients: A study of the Intergroupe Francophone du Myélome. J Clin Oncol. 2008;26:4798–4805. doi: 10.1200/JCO.2007.13.8545. [DOI] [PubMed] [Google Scholar]
- 45.Broyl A, Hose D, Lokhorst H, et al. Gene expression profiling for molecular classification of multiple myeloma in newly diagnosed patients. Blood. 2010;116:2543–2553. doi: 10.1182/blood-2009-12-261032. [DOI] [PubMed] [Google Scholar]
- 46.Dickens NJ, Walker BA, Leone PE, et al. Homozygous deletion mapping in myeloma samples identifies genes and an expression signature relevant to pathogenesis and outcome. Clin Cancer Res. 2010;16:1856–1864. doi: 10.1158/1078-0432.CCR-09-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nair B, van Rhee F, Shaughnessy JD, Jr, et al. Superior results of Total Therapy 3 (2003–33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006–66 with VRD maintenance. Blood. 2010;115:4168–4173. doi: 10.1182/blood-2009-11-255620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ClinicalTrials.gov. Study to compare VMP with HDM followed by VRD consolidation and lenalidomide maintenance in patients with newly diagnosed multiple myeloma (HO95) [accessed March 10, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01208766.
- 49.ClinicalTrials.gov. Randomized Trial of Lenalidomide, Bortezomib, Dexamethasone vs High-Dose Treatment With SCT in MM Patients up to Age 65 (IFM/DFCI2009) [accessed March 10, 2011]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01208662?term=nct01208662&rank=1.
- 50.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 51.Attal M, Lauwers V, Marit G, et al. Maintenance treatment with lenalidomide after transplantation for myeloma: Final analysis of the IFM 2005–02 [abstract 310] Blood. 2010;116:141. [Google Scholar]
- 52.Mellqvist UH, Westin J, Gimsing P, et al. Improved response rate with bortezomib consolidation after high dose melphalan: First results of a Nordic Myeloma Study Group randomized phase III trial [abstract 530] Blood. 2009;114:221. [Google Scholar]
- 53.Ladetto M, Pagliano G, Ferrero S, et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. J Clin Oncol. 2010;28:2077–2084. doi: 10.1200/JCO.2009.23.7172. [DOI] [PubMed] [Google Scholar]
- 54.Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27:1788–1793. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 55.Cavo M, Perrone G, Buttignol S, et al. Bortezomib-thalidomide-dexamethasone compared with thalidomide-dexamethasone as induction and consolidation therapy before and after double autologous transplantation in newly diagnosed multiple myeloma: Results from a randomized phase 3 study [abstract 42] Blood. 2010;116:25. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 56.Terragna C, Zamagni E, Petrucci MT, et al. Molecular remission after bortezomib-thalidomide-dexamethasone compared with thalidomide-dexamethasone as consolidation therapy following double autologous transplantation for multiple myeloma: Results of a qualitative and quantitative analysis [abstract 861] Blood. 2010;116:376. [Google Scholar]
- 57.Barlogie B, Attal M, Crowley J, et al. Long-term follow-up of autotransplantation trials for multiple myeloma: Update of protocols conducted by the Intergroupe Francophone du Myélome, Southwest Oncology Group, and University of Arkansas for medical sciences. J Clin Oncol. 2010;28:1209–1214. doi: 10.1200/JCO.2009.25.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart AK, Trudel S, Bahlis NJ, et al. A randomized phase III trial of thalidomide and prednisone as maintenance therapy following autologous stem cell transplantation (ASCT) in patients with multiple myeloma (MM): The NCIC CTG MY.10 trial [abstract 39] Blood. 2010;116:23. [Google Scholar]
- 59.McCarthy PL, Owzar K, Anderson KC, et al. Phase III intergroup study of lenalidomide versus placebo maintenance therapy following single autologous hematopoietic stem cell transplantation (AHSCT) for multiple myeloma: CALGB 100104 [abstract 37] Blood. 2010;116:21. [Google Scholar]
- 60.Beksac M, Haznedar R, Firatli-Tuglular T, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: Results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86:16–22. doi: 10.1111/j.1600-0609.2010.01524.x. [DOI] [PubMed] [Google Scholar]
- 61.Palumbo A. Balancing act for elderly myeloma. Blood. 2010;116:1390–1391. doi: 10.1182/blood-2010-05-285155. [DOI] [PubMed] [Google Scholar]
- 62.Waage A, Palumbo A, Hulin C, et al. MP versus MPT for previously untreated elderly patients with multiple myeloma: A meta analysis of survival of 1682 individual patient data from 6 randomized clinical trials [abstract 567] Haematologica. 2010;95(suppl 2):235. [Google Scholar]
- 63.Ludwig H, Hajek R, Tóthov E, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009;113:3435–3442. doi: 10.1182/blood-2008-07-169565. 1269. [DOI] [PubMed] [Google Scholar]
- 64.Palumbo A, Falco P, Benevolo G, et al. A multicenter, open label study of oral lenalidomide and prednisone (RP) followed by oral lenalidomide melphalan and prednisone (MPR) and oral lenalidomide maintenance in newly diagnosed elderly multiple myeloma patients [abstract 1940] Blood. 2010;116:808. [Google Scholar]
- 65.Kropff M, Richardson PG, Schlag R, et al. Similar benefit in patients aged ≥75 vs <75 y with VMP in frontline MM and bortezomib in relapsed MM [abstract 084] Clin Lymphoma Myeloma. 2009;9(1 suppl):512. [Google Scholar]
- 66.Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745–4753. doi: 10.1182/blood-2010-07-294983. [DOI] [PubMed] [Google Scholar]
- 67.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-Melphalan-Prednisone-Thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: A randomized controlled trial. J Clin Oncol. 2010;28:5101–5109. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 68.Moreau P, Pylypenko HV, Grosicki S, et al. A phase 3 prospective randomized International study (MMY-3021) comparing subcutaneous and intravenous administration of bortezomib in patients with relapsed multiple myeloma [abstract 312] Blood. 2010;116:142. [Google Scholar]
- 69.Jacobus S, Callander N, Siegel D, et al. Outcome of elderly patients 70 years and older with newly diagnosed myeloma in the ECOG randomized trial of lenalidomide/high-dose dexamethasone (RD) versus lenalidomide/low-dose dexamethasone (Rd) [abstract 370] Haematologica. 2010;95(suppl 2):149. [Google Scholar]
- 70.Niesvizky R, Richardson PG, Rajkumar SV, et al. The relationship between quality of response and clinical benefit for patients treated on the bortezomib arm of the international, randomized, phase 3 APEX trial in relapsed multiple myeloma. Br J Haematol. 2008;143:46–53. doi: 10.1111/j.1365-2141.2008.07303.x. [DOI] [PubMed] [Google Scholar]
- 71.Lokhorst H, Einsele H, Vesole D, et al. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2010;28:4521–4530. doi: 10.1200/JCO.2010.29.7929. [DOI] [PubMed] [Google Scholar]
- 72.Wang M, Dimopoulos MA, Chen C, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112:4445–4451. doi: 10.1182/blood-2008-02-141614. [DOI] [PubMed] [Google Scholar]
- 73.Dimopoulos MA, Kastritis E, Christoulas D, et al. Treatment of patients with relapsed/refractory multiple myeloma with lenalidomide and dexamethasone with or without bortezomib: Prospective evaluation of the impact of cytogenetic abnormalities and of previous therapies. Leukemia. 2010;24:1769–1778. doi: 10.1038/leu.2010.175. [DOI] [PubMed] [Google Scholar]
- 74.Madan S, Lacy M, Dispenzieri A, et al. Efficacy of retreatment with immunomodulatory compounds in patients receiving initial therapy for newly diagnosed multiple myeloma [abstract 1964] Blood. 2010;116:820. doi: 10.1182/blood-2011-04-350009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petrucci T, Blau I, Corradini P, et al. Efficacy and safety of retreatment with bortezomib in patients with multiple myeloma: Interim results from RETRIEVE, a prospective international phase 2 study [abstract 377] Haematologica. 2010;95(suppl 2):152. [Google Scholar]
- 76.Kumar S, Blade J, Crowley J, et al. Natural history of multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter International Myeloma Working Group study [abstract 2878] Blood. 2009;114:1123. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dimopoulos MA, Richardson PG, Schlag R, et al. VMP (bortezomib, melphalan, and prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: Cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27:6086–6093. doi: 10.1200/JCO.2009.22.2232. [DOI] [PubMed] [Google Scholar]
- 78.Ludwig H, Adam Z, Hajek R, et al. Light chain-induced acute renal failure can be reversed by bortezomib-doxorubicin-dexamethasone in multiple myeloma: Results of a phase II study. J Clin Oncol. 2010;28:4635–4641. doi: 10.1200/JCO.2010.28.1238. [DOI] [PubMed] [Google Scholar]
- 79.Roussou M, Kastritis E, Christoulas D, et al. Reversibility of renal failure in newly diagnosed patients with multiple myeloma and the role of novel agents. Leuk Res. 2010;34:1395–1397. doi: 10.1016/j.leukres.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 80.Dimopoulos M, Alegre A, Stadtmauer EA, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer. 2010;116:3807–3814. doi: 10.1002/cncr.25139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delforge M, Bladé J, Dimopoulos MA, et al. Treatment-related peripheral neuropathy in multiple myeloma: The challenge continues. Lancet Oncol. 2010;11:1086–1095. doi: 10.1016/S1470-2045(10)70068-1. [DOI] [PubMed] [Google Scholar]
- 82.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–423. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 83.Mateos MV, López-Corral L, Hernàndez M, et al. Smoldering multiple myeloma (SMM) at high-risk of progression to symptomatic disease: A phase III, randomized, multicenter trial based on lenalidomide-dexamethasone (Len-Dex) as induction therapy followed by maintenance therapy with Len alone vs so treatment [abstract 1935] Blood. 2010;116:805. [Google Scholar]