18F-fluorodeoxyglucose positron emission tomography/computed tomography was evaluated as a diagnostic tool in patients with extracervical cancer of unknown primary.
Keywords: Carcinoma of unknown primary site, 18F-FDG PET/CT, Extracervical carcinoma of unknown primary, Diagnostic tool
Abstract
Background.
Carcinoma of unknown primary (CUP) represents a heterogeneous group of metastatic malignancies for which no primary tumor site can be identified after extensive diagnostic workup. Failure to identify the primary site may negatively influence patient management. The aim of this review was to evaluate 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) as a diagnostic tool in patients with extracervical CUP.
Materials and Methods.
A comprehensive literature search was performed and four publications were identified (involving 152 patients) evaluating 18F-FDG PET/CT in CUP patients with extracervical metastases. All studies were retrospective and heterogeneous in inclusion criteria, study design, and diagnostic workup prior to 18F-FDG PET/CT.
Results.
18F-FDG PET/CT detected the primary tumor in 39.5% of patients with extracervical CUP. The lung was the most commonly detected primary tumor site (∼50%). The pooled estimates of sensitivity, specificity, and accuracy of 18F-FDG PET/CT in the detection of the primary tumor site were 87%, 88%, and 87.5%, respectively.
Conclusions.
The present review of currently available data indicates that 18F-FDG PET/CT might contribute to the identification of the primary tumor site in extracervical CUP. However, prospective studies with more uniform inclusion criteria are required to evaluate the exact value of this diagnostic tool.
Introduction
Carcinoma of unknown primary (CUP) represents a heterogeneous group of metastatic malignancies for which no primary site of the tumor can be identified following a thorough medical history, careful clinical examination, and extensive diagnostic workup. CUP accounts for approximately 5% of all cancer diagnoses and is characterized by early dissemination, uncommon metastatic sites, and usually a poor prognosis [1, 2].
Although the conventional diagnostic workup has improved over the years, it remains a significant diagnostic challenge to identify the primary tumor site in CUP patients. In <30% of CUP patients, a primary site is identified ante mortem. Postmortem examinations reveal a putative primary site in 60%–80% of CUP patients, most often in the lung (27%), pancreas (24%), and hepatobiliary tree (8%) [3]. Failure to identify the primary tumor site may negatively influence patient management, because tailored chemotherapeutic regimens and targeted agents have been increasingly developed over the last decade for a number of solid tumors.
Although positron emission tomography (PET) using 18F-fluorodeoxyglucose (18F-FDG PET) and 18F-FDG PET/computed tomography (CT) are now recommended as additional diagnostic tools to conventional workup in CUP patients with cervical lymph node metastases [4–7], the value of 18F-FDG PET/CT in CUP patients with extracervical metastases remains to be established. Sève et al. [8] recently provided a thorough review of 18F-FDG PET studies in CUP patients with extracervical metastases. 18F-FDG PET revealed a primary tumor site in 41% of patients (range, 24%–63%).
18F-FDG PET/CT studies in CUP patients with extracervical metastases are mainly retrospective and small. Further, inclusion criteria vary among these studies. Thus, both cervical and extracervical CUP patients are included. In addition, some studies have included patients not fulfilling the generally accepted criteria for a CUP diagnosis (e.g., germ cell tumor, malignant melanoma, sarcoma), or the conventional diagnostic workup before the 18F-FDG PET/CT has been insufficient (e.g., no CT or biopsy performed). Most previous reviews on 18F-FDG PET/CT have included studies using the above rather broad definition of CUP, thus potentially leading to biased conclusions regarding the value of 18F-FDG PET/CT, in particular in CUP patients with extracervical metastases [9–11].
In the present review, we used a more stringent definition of CUP and identified four 18F-FDG PET/CT studies that fulfilled the definition and included CUP patients with extracervical metastases.
Materials and Methods
Search Criteria and Study Selection
A comprehensive literature search of English-language publications in the PubMed online database was performed using the search string (cancer OR carcinoma OR neoplasm OR malignant OR tumour) AND (unknown primary OR unknown origin OR occult primary OR unidentified origin) AND (FDG-PET/CT OR fluorodeoxyglucose-PET/computed tomography OR 18F-FDG PET/CT). The above search string was also used in combination with the thorough search strategy for 18F-FDG PET literature published by Mijnhout et al. [12]. This did not result in additional publications. No date limit was used and the search was updated until May 2010. For completeness, the reference lists of the retrieved articles were reviewed for additional publications.
The following criteria were used to select articles for this review: (a) 18F-FDG PET/CT studies in CUP patients with extracervical metastases, (b) conventional workup that included a thorough history and physical examination and adequate imaging procedures prior to 18F-FDG PET/CT (chest x-ray or CT of the chest and CT of the abdomen and pelvis) but failed to detect the primary site, and (c) data were sufficient to allow calculation of sensitivity and specificity for detection of the primary site. Abstracts presented at congresses, reviews, meta-analyses, editorials, letters, and comments were excluded as well as duplicated studies with overlapping patient populations. In addition, we excluded studies in which (a) results for the subset of CUP patients with extracervical metastases were not extractable, (b) CUP patients had isolated cervical lymph node metastases, (c) results using 18F-FDG PET/CT were not extractable from those using 18F-FDG PET alone, and (d) the diagnosis of malignancy was not histologically confirmed.
Study Quality Assessment
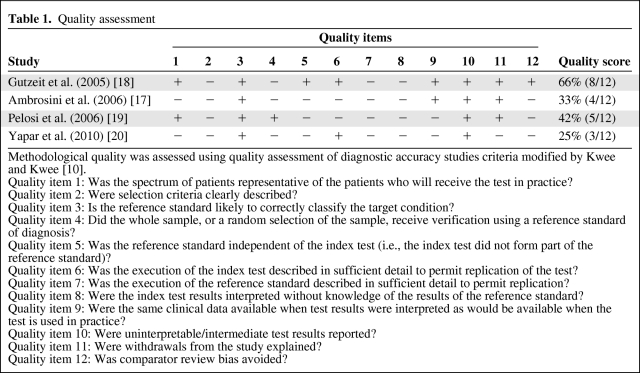
Two authors (K.P. and A.K.H.M.) independently assessed the quality of the included studies using the quality assessment of diagnostic accuracy studies criteria modified by Kwee and Kwee [10]. Twelve methodological quality items were assessed for each study using the scores “yes,” “no,” or “unclear” for each item. No and unclear responses were interpreted as the quality item was not met. Disagreements between the two authors were discussed and resolved by consensus. A quality score for each study is expressed as a percentage of the maximum score of 12. The 12 methodological quality criteria items are specified in Table 1.
Table 1.
Quality assessment
Methodological quality was assessed using quality assessment of diagnostic accuracy studies criteria modified by Kwee and Kwee [10].
Quality item 1: Was the spectrum of patients representative of the patients who will receive the test in practice?
Quality item 2: Were selection criteria clearly described?
Quality item 3: Is the reference standard likely to correctly classify the target condition?
Quality item 4: Did the whole sample, or a random selection of the sample, receive verification using a reference standard of diagnosis?
Quality item 5: Was the reference standard independent of the index test (i.e., the index test did not form part of the reference standard)?
Quality item 6: Was the execution of the index test described in sufficient detail to permit replication of the test?
Quality item 7: Was the execution of the reference standard described in sufficient detail to permit replication?
Quality item 8: Were the index test results interpreted without knowledge of the results of the reference standard?
Quality item 9: Were the same clinical data available when test results were interpreted as would be available when the test is used in practice?
Quality item 10: Were uninterpretable/intermediate test results reported?
Quality item 11: Were withdrawals from the study explained?
Quality item 12: Was comparator review bias avoided?
Data Analysis
To calculate the sensitivity, specificity, and detection rate of the primary site, a true positive (TP) result was considered when 18F-FDG PET/CT suggested the location of the primary site and the location could be confirmed subsequently, whereas a result was considered false positive (FP) when the location of the primary site could not be confirmed. The sites suggested by 18F-FDG PET/CT were confirmed by biopsy and histopathological analysis; however, imaging procedures or clinical follow-up were accepted if no tissue could be obtained. A true negative (TN) result was considered when neither 18F-FDG PET/CT nor other diagnostic procedures (including other imaging tests) detected the primary tumor site in the clinical follow-up period. The finding was classified as false negative (FN) if the primary tumor site was detected by other diagnostic procedures after a negative 18F-FDG PET/CT. Sensitivity, specificity, and accuracy were calculated using the following formulas: sensitivity = TP/(TP + FN), specificity = TN/(TN + FP), and accuracy = TP + TN/(TP + TN + FP + FN).
Results
Literature Search and Study Description
The PubMed search identified eight articles potentially eligible for inclusion. Four articles/studies were excluded for the following reasons: (a) duplicate study [13], (b) data on CUP patients with extracervical metastases were not extractable [14, 15], and (c) part of the study population underwent 18F-FDG PET alone and was not analyzed separately from patients undergoing 18F-FDG PET/CT [16].
Based on the above, four studies [17–20] were identified and included in this systematic review. These studies comprise a total of 225 patients. However, to include only patients who stringently fulfilled the extracervical CUP definition, patients with the following malignancies were excluded: germ cell tumors, malignant melanoma, neuroendocrine tumors, and lymphoma. Likewise, we excluded patients with only cervical lymph node metastases of any histology and patients with only a clinical suspicion of malignancy. In total, 152 of the 225 patients (67.6%) were included in the data analysis.
Study Characteristics
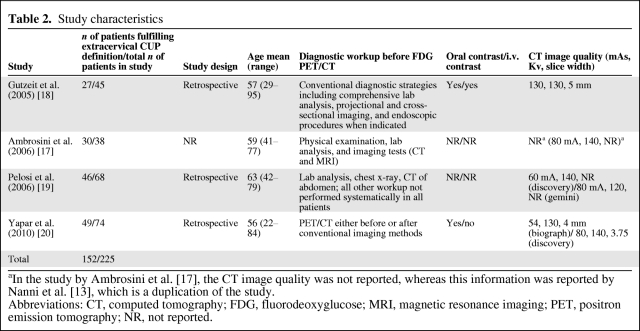
The four studies included in this review are summarized in Table 2. None of the studies were prospective, comprising only retrospective case series of patients referred for 18F-FDG PET/CT scan. In three of the studies, CUP was diagnosed only after a conventional workup failed to identify the primary site. However, the definition of conventional workup varied among the studies (Table 2). In the study by Yapar et al. [20], the 18F-FDG PET/CT scan was performed either before or after the conventional workup.
Table 2.
Study characteristics
aIn the study by Ambrosini et al. [17], the CT image quality was not reported, whereas this information was reported by Nanni et al. [13], which is a duplication of the study.
Abbreviations: CT, computed tomography; FDG, fluorodeoxyglucose; MRI, magnetic resonance imaging; PET, positron emission tomography; NR, not reported.
In all studies, the pathological evaluation included light microscopic evaluation with morphologic descriptions of the tumors; no immunohistochemistry (IHC) or histopathological suggestions of the primary site were reported.
PET/CT Imaging
Only in the study by Gutzeit et al. [18] could the CT in the combined 18F-FDG PET/CT be classified as a diagnostic CT scan, with a radiation dose of 130 mAs and i.v. and oral contrast. In all other studies, CT scans were performed with low radiation doses (54–80 mAs or 60–80 mA), with either no reported use or no use of i.v. contrast (low-dose CT). Therefore, the CT scans in these latter studies were used as a fast transmission source for attenuation correction and approximate anatomical mapping but not for diagnostic purposes.
Nuclear medicine physicians evaluated the combined 18F-FDG PET/CT scans in three of the studies, whereas in the study by Gutzeit et al. [18], nuclear medicine physicians and radiologists evaluated the PET data and the CT data separately. In addition, the PET and CT data were evaluated side by side and the fused PET/CT data were evaluated by both a radiologist and a nuclear medicine physician.
Quality Assessment
The quality scores of the included studies were generally low to moderate, in the range of 25%–67% (Table 1). The study by Gutzeit et al. [18] obtained the highest quality score. All studies were retrospective case series, and the selection criteria (item 2) for the included patients were not well described and may vary in each study as well as between studies. Additionally, in the majority of the studies, the 18F-FDG PET/CT might have been a part of the reference standard (item 5), which was inadequately described in all studies (item 7). It is unclear whether the 18F-FDG PET/CT results were interpreted without knowledge of the results of the reference standard (item 8).
Diagnostic Performance
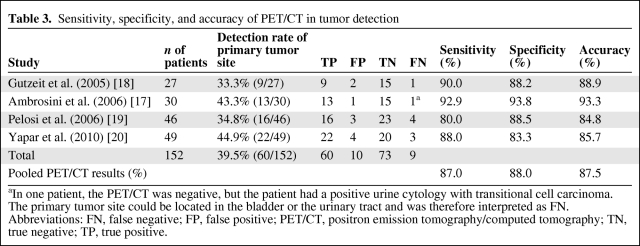
18F-FDG PET/CT detected the primary tumor site in 60 patients with extracervical CUP (39.5%), with a range of 33.3%–44.9% (Table 3). The pooled estimates of sensitivity, specificity, and accuracy of 18F-FDG PET/CT in the detection of the primary tumor site were 87%, 88%, and 87.5%, respectively.
Table 3.
Sensitivity, specificity, and accuracy of PET/CT in tumor detection
aIn one patient, the PET/CT was negative, but the patient had a positive urine cytology with transitional cell carcinoma. The primary tumor site could be located in the bladder or the urinary tract and was therefore interpreted as FN.
Abbreviations: FN, false negative; FP, false positive; PET/CT, positron emission tomography/computed tomography; TN, true negative; TP, true positive.
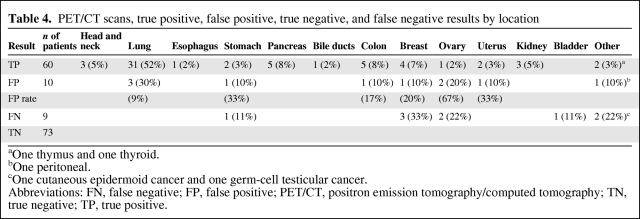
The lung was the most commonly detected primary tumor site, accounting for ∼50% of all cases (n = 31), followed by pancreas (n = 5), colon (n = 5), and breast (n = 4) (Table 4). In total, 10 FP (6.6%) and nine FN (5.9%) 18F-FDG PET/CT cases were reported (Table 4). The lung and ovary were the most commonly reported locations of FP results, whereas the breast and ovary were the most common locations of FN results. Only in the study by Gutzeit et al. [18] were the causes of the two FP results described in detail. The pathological evaluation revealed one case of colitis and one case of pulmonary infarction. In the study by Ambrosini et al. [17], one patient with a negative 18F-FDG PET/CT had a positive urine cytology with transitional cell carcinoma. The primary tumor site could be in the bladder or the urinary tract and was therefore interpreted as FN.
Table 4.
PET/CT scans, true positive, false positive, true negative, and false negative results by location
aOne thymus and one thyroid.
bOne peritoneal.
cOne cutaneous epidermoid cancer and one germ-cell testicular cancer.
Abbreviations: FN, false negative; FP, false positive; PET/CT, positron emission tomography/computed tomography; TN, true negative; TP, true positive.
Gutzeit et al. [18] evaluated and compared the diagnostic performance of PET alone, CT alone, PET and CT side by side, and fused PET/CT [18]. Although more primary tumors were detected on fused PET/CT images (33.3%, nine of 27) than with other modalities (PET: 25.9%, seven of 27; CT: 14.8%, four of 27; PET and CT side by side: 29.6%, eight of 27), the differences were not statistically significant. The authors of that study attributed the rather favorable results when CT scan was used either alone or in combination to their high diagnostic standard achieved with the whole-body CT protocol.
Discussion
For the majority of CUP patients, identification of the primary tumor site remains a significant challenge. The use of 18F-FDG PET/CT scans combines functional and anatomical information, and its use in cancer patient diagnostics and staging has increased very rapidly since it was introduced in 2001. 18F-FDG PET/CT is particularly useful when the CT scan of a combined 18F-FDG PET/CT examination is performed as a high-quality CT scan with i.v. and oral contrast agents. As an example, 18F-FDG PET/CT produced a significantly higher accuracy in staging of non-small cell lung cancer than with PET or CT alone, and positively affected therapeutic management [21]. When interpreting PET/CT, the nuclear physician/radiologist should be aware of misalignment phenomena and artefacts if the chest CT is performed during deep inspiration.
It seems likely that 18F-FDG PET/CT could also be of significant value in detecting the primary tumor site in CUP patients. Indeed, 18F-FDG PET and 18F-FDG PET/CT are of great importance in the detection of the primary tumor site in CUP patients with cervical lymph node metastases, and thus the treatment planning [6, 7]. In contrast, the value of 18F-FDG PET/CT is less well studied in CUP patients with extracervical metastases. We performed a rigorous literature search to identify publications that specifically address this important diagnostic issue. By using a set of defined search and selection criteria we identified eight studies in the PubMed database evaluating 18F-FDG PET/CT in this patient population. Furthermore, analysis of these studies revealed that relevant data could be extracted from only four of these publications [13–16]. Of these four studies, only the study by Gutzeit et al. [18] used a diagnostic CT scan with a standard radiation dose (130 mAs) and i.v. and oral contrast.
The four studies discussed in the present review are all retrospective, representing a total of 225 patients. Patients not fulfilling the definition of extracervical CUP were excluded, leaving a total of 152 patients for our analysis (Table 2). In summary, 18F-FDG PET/CT detected the primary tumor site in 60 patients with extracervical CUP (39.5%) (Table 1).
This is in agreement with the data presented in the study by Sève et al. [8] wherein 18F-FDG PET revealed a primary tumor site in 41% of patients (range, 24%–63%).
The lung was the most commonly detected primary tumor site, accounting for ∼50% of all cases. The pooled estimates of sensitivity, specificity, and accuracy for 18F-FDG PET/CT in the detection of the primary tumor site were 87%, 88%, and 87.5%, respectively. The causes of FP and FN results were described only in the study by Gutzeit et al. [18]. Furthermore, a TN result was considered if the primary tumor site remained unknown using other diagnostic procedures in the clinical follow-up period, but only in the study by Pelosi et al. [19] was the clinical follow-up period defined and described (the minimum follow-up period was 3 months).
Similar to the review by Sève et al. [8] on 18F-FDG PET [8], lung cancer seems to be overrepresented in our review. CT scanning of the chest was not performed in most of these patients before 18F-FDG PET/CT. Thus, it is possible that not all patients fulfilled the stringent CUP definition because of a possible incomplete diagnostic workup prior to 18F-FDG PET/CT. Because minor pulmonary tumors may remain undetected by conventional x-ray, the lack of chest CT in the conventional workup may partly explain the overrepresentation of lung cancer. In accordance with this notion, in the study by Gutzeit et al. [18], CT alone revealed a primary tumor site in four cases (three lung cancers), whereas PET alone and fused PET/CT were used to detect a primary tumor site in seven (three lung cancers) and nine (five lung cancers) cases, respectively.
Selection bias may have been introduced because of the retrospective nature of the studies. As an example, only a few of the CUP patients were reported to have liver metastases, and in general only a few patients had multiple metastases. This is in contrast to findings in recent prospective therapeutic studies in which >50% of patients were diagnosed with multiple metastatic sites, including liver metastases [22, 23].
Current recommendations for CUP diagnostics by the European Society of Medical Oncology emphasize the need for inclusion of IHC in the diagnostic workup [24]. None of the four studies included in this review reported the use of IHC. Furthermore, the performed quality assessment of the included studies resulted in rather low quality scores (Table 1). Three of the studies [17–19] also were quality assessed by Kwee and Kwee [10]. Although there were some specific differences, their overall scores and conclusions were similar. Conclusively, the diagnostic performance of 18F-FDG PET/CT in CUP patients with extracervical metastases might be overestimated in the studies discussed here.
Nonetheless, a multidisciplinary expert panel of oncologists, radiologists, and nuclear physicians with expertise in 18F-PET/CT concluded that 18F-PET/CT would be beneficial in the diagnostic workup of CUP patients [5]. The four studies discussed in this review support the notion that 18F-FDG PET/CT might contribute to the identification of the primary tumor site in extracervical CUP. However, prospective studies with a sufficient number of patients and with more uniform inclusion criteria are required to evaluate the diagnostic value of 18F-FDG PET/CT (high-quality contrast-enhanced CT) in CUP patients with extracervical metastases.
Author Contributions
Conception/Design: Anne Kirstine Hundahl Moller, Bodil Laub Petersen, Anne Kiil Berthelsen, Annika Loft, Jesper Graff, Charlotte Birk Christensen, Katharina Perell, Gedske Daugaard, Karen Damgaard Pedersen
Provision of study material or patients: Anne Kirstine Hundahl Moller, Bodil Laub Petersen, Anne Kiil Berthelsen, Annika Loft, Jesper Graff, Charlotte Birk Christensen, Katharina Perell, Gedske Daugaard, Karen Damgaard Pedersen
Collection and/or assembly of data: Anne Kirstine Hundahl Moller, Bodil Laub Petersen, Anne Kiil Berthelsen, Annika Loft, Jesper Graff, Charlotte Birk Christensen, Katharina Perell, Gedske Daugaard, Karen Damgaard Pedersen
Data analysis and interpretation: Anne Kirstine Hundahl Moller, Bodil Laub Petersen, Anne Kiil Berthelsen, Annika Loft, Jesper Graff, Charlotte Birk Christensen, Katharina Perell, Gedske Daugaard, Karen Damgaard Pedersen
Manuscript writing: Anne Kirstine Hundahl Moller, Bodil Laub Petersen, Anne Kiil Berthelsen, Annika Loft, Jesper Graff, Charlotte Birk Christensen, Katharina Perell, Gedske Daugaard, Karen Damgaard Pedersen
Final approval of manuscript: Anne Kirstine Hundahl Moller, Bodil Laub Petersen, Anne Kiil Berthelsen, Annika Loft, Jesper Graff, Charlotte Birk Christensen, Katharina Perell, Gedske Daugaard, Karen Damgaard Pedersen
References
- 1.Pavlidis N, Briasoulis E, Hainsworth J, et al. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39:1990–2005. doi: 10.1016/s0959-8049(03)00547-1. [DOI] [PubMed] [Google Scholar]
- 2.Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP) Crit Rev Oncol Hematol. 2009;69:271–278. doi: 10.1016/j.critrevonc.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Pentheroudakis G, Greco FA, Pavlidis N. Molecular assignment of tissue of origin in cancer of unknown primary may not predict response to therapy or outcome: A systematic literature review. Cancer Treat Rev. 2009;35:221–227. doi: 10.1016/j.ctrv.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ibraheem A, Buck A, Krause BJ, et al. Clinical applications of FDG PET and PET/CT in head and neck cancer. J Oncol. 2009;2009:208725. doi: 10.1155/2009/208725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 6.Johansen J, Buus S, Loft A, et al. Prospective study of 18FDG-PET in the detection and management of patients with lymph node metastases to the neck from an unknown primary tumor. Results from the DAHANCA-13 study. Head Neck. 2008;30:471–478. doi: 10.1002/hed.20734. [DOI] [PubMed] [Google Scholar]
- 7.Rusthoven KE, Koshy M, Paulino AC. The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer. 2004;101:2641–2649. doi: 10.1002/cncr.20687. [DOI] [PubMed] [Google Scholar]
- 8.Sève P, Billotey C, Broussolle C, et al. The role of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography in disseminated carcinoma of unknown primary site. Cancer. 2007;109:292–299. doi: 10.1002/cncr.22410. [DOI] [PubMed] [Google Scholar]
- 9.Dong MJ, Zhao K, Lin XT, et al. Role of fluorodeoxyglucose-PET versus fluorodeoxyglucose-PET/computed tomography in detection of unknown primary tumor: A meta-analysis of the literature. Nucl Med Commun. 2008;29:791–802. doi: 10.1097/MNM.0b013e328302cd26. [DOI] [PubMed] [Google Scholar]
- 10.Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: Systematic review and meta-analysis. Eur Radiol. 2009;19:731–744. doi: 10.1007/s00330-008-1194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwee TC, Basu S, Cheng G, et al. FDG PET/CT in carcinoma of unknown primary. Eur J Nucl Med Mol Imaging. 2010;37:635–644. doi: 10.1007/s00259-009-1295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mijnhout GS, Riphagen II, Hoekstra OS. Update of the FDG PET search strategy. Nucl Med Commun. 2004;25:1187–1189. doi: 10.1097/00006231-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Nanni C, Rubello D, Castellucci P, et al. Role of 18F-FDG PET-CT imaging for the detection of an unknown primary tumour: Preliminary results in 21 patients. Eur J Nucl Med Mol Imaging. 2005;32:589–592. doi: 10.1007/s00259-004-1734-3. [DOI] [PubMed] [Google Scholar]
- 14.Fencl P, Belohlavek O, Skopalova M, et al. Prognostic and diagnostic accuracy of [18F]FDG-PET/CT in 190 patients with carcinoma of unknown primary. Eur J Nucl Med Mol Imaging. 2007;34:1783–1792. doi: 10.1007/s00259-007-0456-8. [DOI] [PubMed] [Google Scholar]
- 15.Kaya AO, Coskun U, Unlu M, et al. Whole body 18F-FDG PET/CT imaging in the detection of primary tumours in patients with a metastatic carcinoma of unknown origin. Asian Pac J Cancer Prev. 2008;9:683–686. [PubMed] [Google Scholar]
- 16.Garin E, Prigent-Lejeune F, Lesimple T, et al. Impact of PET-FDG in the diagnosis and therapeutic care of patients presenting with metastases of unknown primary. Cancer Invest. 2007;25:232–239. doi: 10.1080/07357900701206331. [DOI] [PubMed] [Google Scholar]
- 17.Ambrosini V, Nanni C, Rubello D, et al. 18F-FDG PET/CT in the assessment of carcinoma of unknown primary origin. Radiol Med (Torino) 2006;111:1146–1155. doi: 10.1007/s11547-006-0112-6. [DOI] [PubMed] [Google Scholar]
- 18.Gutzeit A, Antoch G, Kḧl H, et al. Unknown primary tumors: Detection with dual-modality PET/CT—initial experience. Radiology. 2005;234:227–234. doi: 10.1148/radiol.2341031554. [DOI] [PubMed] [Google Scholar]
- 19.Pelosi E, Pennone M, Deandreis D, et al. Role of whole body positron emission tomography/computed tomography scan with 18F-fluorodeoxyglucose in patients with biopsy proven tumor metastases from unknown primary site. Q J Nucl Med Mol Imaging. 2006;50:15–22. [PubMed] [Google Scholar]
- 20.Yapar Z, Kibar M, Yapar AF, et al. The value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in carcinoma of an unknown primary: Diagnosis and follow-up. Nucl Med Commun. 2010;31:59–66. doi: 10.1097/MNM.0b013e328332b340. [DOI] [PubMed] [Google Scholar]
- 21.Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 22.Hainsworth JD, Spigel DR, Clark BL, et al. Paclitaxel/carboplatin/etoposide versus gemcitabine/irinotecan in the first-line treatment of patients with carcinoma of unknown primary site: A randomized, phase III Sarah Cannon Oncology Research Consortium Trial. Cancer J. 2010;16:70–75. doi: 10.1097/PPO.0b013e3181c6aa89. [DOI] [PubMed] [Google Scholar]
- 23.Moller AK, Pedersen KD, Gothelf A, et al. Paclitaxel, cisplatin and gemcitabine in treatment of carcinomas of unknown primary site, a phase II study. Acta Oncol. 2010;49:423–430. doi: 10.3109/02841860903544592. [DOI] [PubMed] [Google Scholar]
- 24.Briasoulis E, Pavlidis N, Felip E ESMO Guidelines Working Group. Cancers of unknown primary site: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(suppl 4):154–155. doi: 10.1093/annonc/mdp159. [DOI] [PubMed] [Google Scholar]