Costs associated with the use of highly sensitive axillary ultrasonography in patients with stage ≥T2 invasive breast cancer are evaluated.
Keywords: Axillary ultrasound, Breast cancer, Cost analysis
Learning Objectives
After completing this course, the reader will be able to:
Identify patients likely to benefit from preoperative axillary ultrasound.
Define the clinical implications of a preoperative axillary ultrasound and FNA in patient care.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Purpose.
Preoperative axillary sonography with fine needle aspiration (FNA) in patients with invasive breast cancer identifies patients with nodal metastasis who can be spared further surgery. Indiscriminate use of the diagnostic modality can increase costs and yield inaccurate results. We evaluate the costs associated with the use of highly sensitive axillary ultrasonography in patients with stage ≥T2 tumors.
Patients and Methods.
We constructed a decision analysis tree using TreeAge Pro 2009 software comparing direct hospital charges between patients with and without routine use of axillary ultrasound. Base case estimates were derived from our institutional data and compared with those derived from the literature. One- and two-way sensitivity analyses were performed to check the validity of our inferences.
Results.
We found that, for the base case estimate with 35% lymph node positivity in stage ≥T2 tumors and sensitivity of the axillary ultrasound set at 86% with a specificity of 40%, the strategy to perform preoperative axillary ultrasound yielded rollback costs of $15,215, compared with $15,940 for surgery plus sentinel lymph node biopsy (cost difference, $725 per patient favoring axillary ultrasound). On two-way sensitivity analysis, the cost benefit for axillary ultrasound was not seen in patients with a low risk for nodal metastasis.
Conclusion.
The adoption of routine preoperative axillary sonography with FNA is a lower-cost strategy than conventional strategies in patients with stage ≥T2 invasive breast cancer.
Introduction
The role of axillary ultrasound has been proposed as a valid strategy for preoperative staging of patients with invasive breast cancer [1–7]. Abnormal sonographic morphology may suggest metastatic disease, which can be confirmed by fine needle aspiration (FNA) [8–12]. The identification of such nodes preoperatively has numerous benefits, including avoidance of a sentinel lymph node biopsy (SLNB) procedure and its associated risks, a shorter operative time, and more informed discussions regarding the use of neoadjuvant chemotherapy [13]. In the current era, in which emphasis on costs remains an important consideration in deciding treatment strategies and successive policy implications, the cost-effectiveness of routine axillary ultrasound in decision making has been questioned [1, 11].
We previously reported the use of axillary ultrasound in our cohort of patients with stage ≥T2 invasive breast cancer [14]. The sensitivity of axillary ultrasound in detecting nodal metastasis at our high-volume center was 86.2%, which is generally higher than the previously reported sensitivity rates of 50%–70%; yet the specificity in our cohort was 40%, compared with 91% reported in the literature. We believe that the use of sophisticated ultrasonographic techniques in patients with a nontrivial risk for nodal metastasis (≥18%), such as patients with stage T2 tumors, may enhance the cost benefit of routine axillary ultrasonography [15].
Several sonographic characteristics are used to determine the malignant potential of lymph nodes, including rounding of the normal elliptical shape, obliteration of the normally hypoechoic nodal cortex, irregularities of the cortical or medullary contours, and eccentric compression of the hyperechoic nodal medulla with or without loss of the nodal capsule [16]. We also use color-flow Doppler to enhance the diagnostic sensitivity of axillary ultrasound and incorporate hypervascularity and visualization of multiple feeding vessels as strong indicators of neoplastic activity [17]. This strategy increases the sensitivity of the ultrasound but may contribute to a lower specificity.
The aim of our study was to model the costs associated with a selective application strategy of high-sensitivity axillary ultrasound in patients with stage ≥T2 invasive breast cancer in the setting of experienced axillary ultrasound performance and interpretation, thereby optimizing sensitivity rates. We also compare our cost modeling with modeling done on base case estimates obtained from the literature, and report variance in costs based on the prevalence of node positivity.
Patients and Methods
Institutional review board approval was obtained for the retrospective review of patients with newly diagnosed invasive breast cancer and the role of axillary ultrasound. In this paper, we focus on the economic impact of axillary ultrasound in staging breast cancer by cost modeling. We constructed a decision analysis tree using TreeAge Pro 2009 software (TreeAge Software, Inc., Williamstown, MA) comparing direct hospital charges between patients with and without routine use of axillary ultrasound. Patients undergoing axillary ultrasound underwent further evaluation of abnormal-appearing nodes with FNA if considered suspicious for malignancy, and went on to receive SLNBs if the ultrasound or FNA biopsy failed to demonstrate metastatic disease. Patients with positive nodal disease underwent completion lymph node dissection (CLND) in concordance with standard practices.
Base Case Estimates
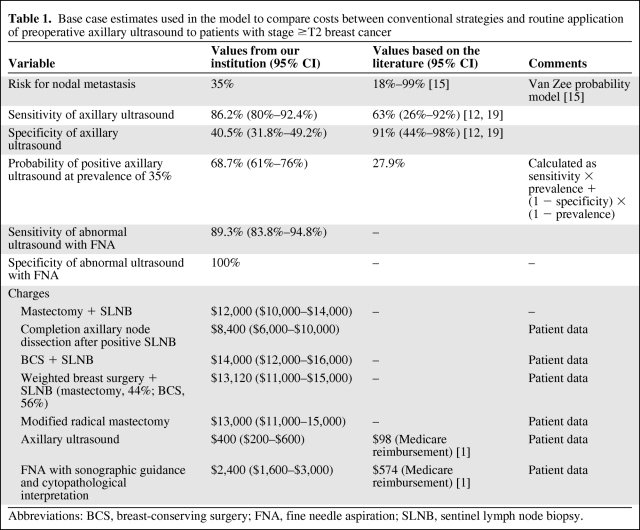
Base case estimates were defined as the baseline probabilities associated with both disease (e.g., probability of nodal metastasis) and testing (e.g., sensitivity and specificity of axillary ultrasound) characteristics. We obtained base case estimates from our data, in which 153 patients underwent an axillary ultrasound at diagnosis (either at the initial visit or concurrently with another visit for other imaging or clinical visit). One hundred twenty (77.9%) patients had a suspicious ultrasound and 33 had a negative ultrasound. Of the 120 patients with a suspicious ultrasound, 85 (70.8%) had pathologic evidence of malignancy on FNA, avoiding SLNB. All 35 remaining patients with a suspicious ultrasound underwent SLNB, of which eight were positive. Of the 33 patients with negative axillary ultrasound, 15 (45%) had a positive SLNB. The sensitivity and specificity of axillary ultrasound were 86.2% and 40.5%, respectively. The sensitivity of axillary ultrasound combined with FNA biopsy was 89.3%, with a 100% specificity (Table 1).
Table 1.
Base case estimates used in the model to compare costs between conventional strategies and routine application of preoperative axillary ultrasound to patients with stage ≥T2 breast cancer
Abbreviations: BCS, breast-conserving surgery; FNA, fine needle aspiration; SLNB, sentinel lymph node biopsy.
We then performed a MEDLINE literature search using the PubMed interface for published literature on the use of axillary ultrasound in invasive breast cancer (keywords, breast cancer OR breast carcinoma AND ultrasound OR sonography OR ultrasonography). In addition, we also hand-searched references of articles identified for the sake of completion. We applied the Memorial Sloan-Kettering Cancer Center (MSKCC) prediction model to estimate the risk for nodal metastasis in patients with stage ≥T2 tumors [15]. Direct hospital charges were obtained from administrative sources after sampling patients undergoing actual procedures in 2008–2010. We calculated weighted charges for patients undergoing simultaneous breast surgery with SLNB procedures. Charges for breast surgery, either mastectomy or breast-conserving surgery, were calculated as a weighted average because, at our institution, 56% of patients underwent breast-conserving surgery and 44% underwent mastectomy in 2004–2007. We believe that inclusion of the cost of the breast surgery procedure is representative of current practice, because lymphatic mapping with SLNB is often performed in combination with a breast procedure, and hence, the charges reflecting the SLNB alone incorrectly reflect additional operating room charges, which may not be the case. We assumed, for purposes of the analysis, that most patients with stage ≥T2 tumors underwent an SLNB at the time of a breast surgery procedure. In addition, we did not separately account for the proportion of patients who underwent prechemotherapy SLNB testing. Charges for ultrasound and image-guided FNA biopsy were obtained from hospital charges but were also compared with the 2007 Medicare reimbursement rates.
Base Case Analysis
Base case analysis was performed using base case probabilities, and incremental models for strategies were created. Confidence intervals (CIs) were calculated for the sensitivity and specificity obtained from our data using the formula CI = p ± SE (√p(1 − p)/n), which is used for binomial distributions, where p is the probability and SE is the standard error. We then obtained 95% CIs for our cost estimates as well. The prevalence of nodal metastasis was changed based on different case estimates from the MSKCC model and this was graphically represented. We performed first-order microsimulation trials with the Monte Carlo technique without prespecifying parameter distributions. This was used to estimate incremental costs after a random sampling distribution.
Sensitivity Analysis
One-way sensitivity analysis was performed using charges by strategy when compared with changing sensitivity, specificity, and probability of nodal metastasis in addition to other factors. We also performed two-way sensitivity analysis for sensitivity and specificity of axillary ultrasound according to the prevalence of node positivity.
Results
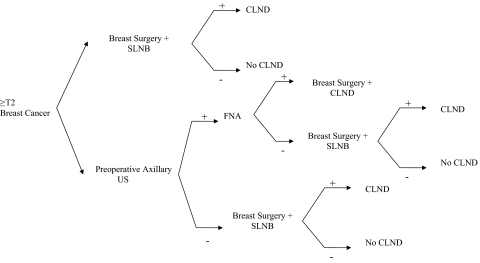
The decision tree used for the analysis is shown in Figure 1. We found that, for the base case estimate with 35% lymph node positivity in stage ≥T2 tumors and sensitivity of the axillary ultrasound set at 86% with a specificity of 40%, the strategy to perform preoperative axillary ultrasound yielded rollback costs of $15,215, compared with $15,940 for surgery plus SLNB (cost difference, $725 per patient favoring axillary ultrasound). Monte Carlo microsimulation trials suggested that the cost of the strategy adopting preoperative routine ultrasound was $15,008 (± $2,623), whereas for the conventional strategy it was $15,974 (± $4,017), with a mean difference of $966 (favoring axillary ultrasound).
Figure 1.
Decision analysis tree used for cost modeling analysis.
Abbreviations: CLND, completion lymph node dissection; FNA, fine needle aspiration; SLNB, sentinel lymph node biopsy; US, ultrasound.
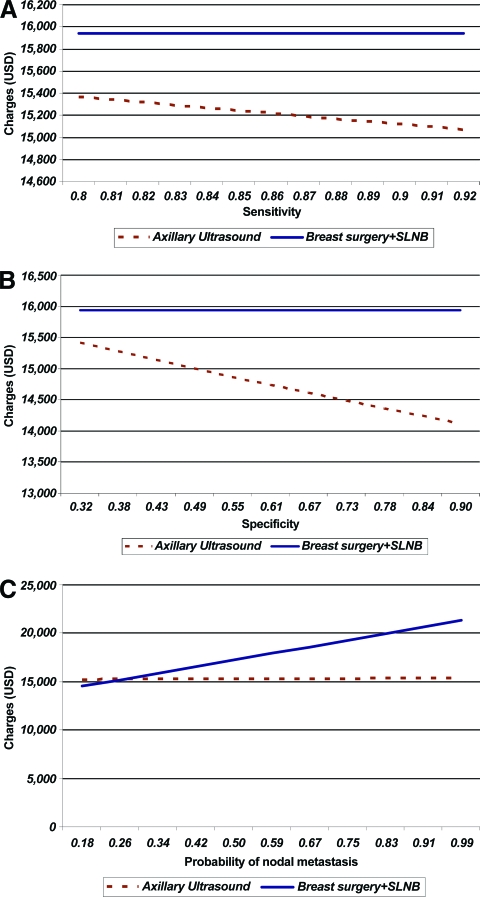
We performed a one-way sensitivity analysis for the sensitivity and specificity of axillary ultrasound to determine the impact on charges of test characteristics at a fixed probability of nodal metastasis. After fixing the base case estimates with a specificity of 40% and risk for nodal metastasis of 35%, the charges for preoperative axillary ultrasonography were compared based on sensitivity (Fig. 2A). When the sensitivity was low (80%), the charges were $15,362, compared with $15,940 for the SLNB arm (difference, −$578). The difference in charges widened with increasing sensitivity—a cost of $15,067 at a sensitivity of 92%, while the cost for the SLNB arm remained at $15,940 (difference, −$873). One-way analysis for specificity was conducted over a range of specificities as obtained from previously published literature and our institutional data. At a specificity of 32%, with a fixed sensitivity of 86% and risk for nodal metastases of 35%, the cost for preoperative axillary sonography was $15,413, compared with $15,940 in the SLNB arm (difference, −$527) (Fig. 2B). However, by increasing specificity with other values fixed, the charge difference widened favoring axillary sonography preoperatively ($14,117 at a specificity of 90%, versus $15,940 for the SLNB arm; difference, −$1,823) We subsequently repeated the one-way analysis for a varied probability of nodal metastasis of 18%–99% based on the MSKCC model for stage ≥T2 tumors with fixed test characteristics (sensitivity and specificity) (Fig. 2C). With a fixed sensitivity and specificity and the probability of nodal metastases at 18%, the charges associated with preoperative axillary sonography were more than those for the SLNB arm ($15,912 versus $14,512; difference, +$1,400). The two charges were almost the same at a 26% probability of nodal disease ($15,202 versus $15,192; difference, +$10). However, as the risk for nodal metastasis was increased, the benefit of preoperative axillary sonography became more apparent (risk, 50%; cost, $15,238 versus $17,233; difference, −$1,995).
Figure 2.
Costs of selective axillary ultrasound strategy. (A): Varying sensitivity of axillary ultrasound (fixed specificity of axillary ultrasound, 40%; probability of nodal metastasis, 35%). (B): Varying specificity of axillary ultrasound (fixed sensitivity of axillary ultrasound, 86%; probability of nodal metastasis, 35%). (C): Varying probability of nodal metastasis (fixed sensitivity of axillary ultrasound, 86%; specificity of axillary ultrasound, 40%).
Abbreviations: SLNB, sentinel lymph node biopsy; USD, U.S. dollars.
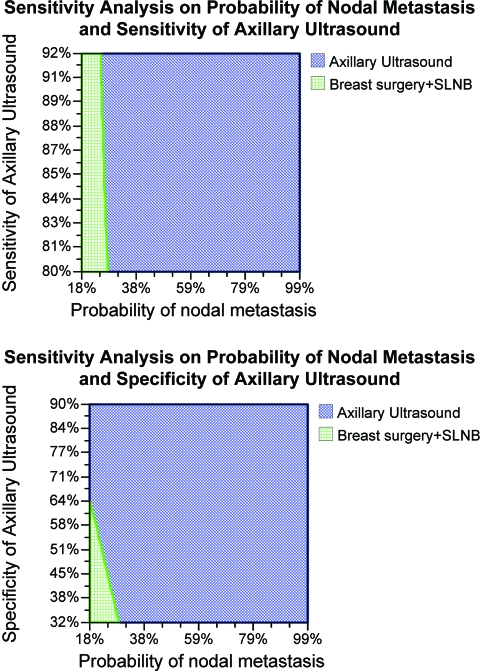
We also performed two-way sensitivity analyses, and found that when the probability of nodal metastasis was low, the costs associated with conventional strategies were comparable with those of a preoperative ultrasonographic exam (Fig. 3). However, for a probability of nodal metastasis >35% and specificity >64%, the charges associated with axillary sonography were significantly lower than with conventional strategies.
Figure 3.
Cost comparison of selective preoperative axillary ultrasound with conventional strategy (shaded area indicates lower cost strategy).
The area on the graph represents the area where the strategy indicated is cheaper. For instance, at a sensitivity of 85%, when the probability of nodal metastasis is <20%, the conventional strategy is cheaper.
Abbreviation: SLNB, sentinel lymph node biopsy.
Discussion
Based on our previous favorable findings with the use of routine breast ultrasound for patients with stage ≥T2 invasive breast cancer, we sought to find the cost burden of employing such a strategy. The benefits of using preoperative ultrasound are obvious: better treatment planning and elimination of the extra SLNB procedure, especially in the presence of a nontrivial risk for lymph node metastasis. We found that, based on our model, which includes the average charge for breast surgery (mastectomy or lumpectomy), the use of routine axillary ultrasound for patients with stage ≥T2 tumors can lead to $725–$966 lower charges per patient.
This strategy has certain unique characteristics that include a highly experienced team of ultrasonographers and surgeons employing high-sensitivity axillary ultrasound to detect metastasis and a high-volume tertiary center. The combination of breast surgery with SLNB is contentious in the setting of neoadjuvant chemotherapy [13]. Practice patterns vary because some physicians prefer to perform SLNB prior to chemotherapy whereas others do not. We chose to work with a model of a single SLNB procedure in combination with definitive breast surgery because the decision analytics of neoadjuvant chemotherapy and inclusion of preoperative SLNB would have made the model significantly more complex and less widely applicable.
Changes in practice patterns based on the recent reporting of the American College of Surgeons Oncology Group (ACOSOG) Z-11 trial were not captured in the decision modeling [18]. However, because the ACOSOG Z-11 study included patients with stage ≤T2 tumors (with a lower probability of nodal metastasis), the cost of the conventional strategy would remain cheaper (by a larger margin) if a CLND was not performed. The results of our analysis remain valid in cases with a high probability of nodal metastasis, for which the standard of care remains CLND. At our institution, we use clinical stage T2 patients for selection for preoperative ultrasound. We do not routinely offer CLND to patients with isolated tumor cells, but we do offer it to patients with micrometastatic disease. This strategy is currently applied to each patient on a case-by-case basis in the current changing environment.
We routinely use axillary ultrasound in stage ≥T2 invasive breast tumors and use experienced ultrasonographers in the interpretation of ultrasounds, because a low nodal metastatic rate or poor test characteristics can significantly impact both the treatment strategy and costs incurred. We reported a fairly low specificity rate, and this may be a result of our highly aggressive inclusion criteria, including the use of color Doppler, contributing to the higher sensitivity of axillary ultrasound. We are currently developing test characteristics to improve the specificity of the test without loss of sensitivity. Our analysis does support a selective approach, with the application of axillary ultrasonography in patients with a nontrivial risk for nodal metastasis. Traditional SLNB without ultrasound costs less in patients with a lower risk for nodal metastasis. In contrast, despite widely varying test characteristics (sensitivity, 80%–92%; specificity, 32%–90%), the performance of a preoperative axillary ultrasound was associated with favorable costs in patients with a probability of nodal metastasis >18%. Our two-way sensitivity analysis showed no cost benefit to preoperative axillary ultrasound when the prevalence of nodal metastasis was low.
Performance of axillary ultrasound is not without risks, however small, especially those caused by the anxiety that an abnormal axillary ultrasound may incur in a patient, and this was not measured in our study. In addition, the risks of FNA, albeit small, include the development of a hematoma that may compromise subsequent dissection planes. In our series, we did not see any instances of hematomas or FNA complications but remain wary of widespread application of FNA without the assistance of an experienced team. In addition, performance of an FNA prior to surgery may lead to a delay in performance of the definitive procedure. However, in our experience we have not observed any difference in time to surgery.
Our study reports on the role of selective high-sensitivity axillary ultrasonography in patients with invasive breast cancer and improves on results from previous studies from Italy and Minnesota that echoed smaller cost benefits [1, 12]. Our study is unique in its selection of a group of patients with a higher risk for nodal metastasis, while applying sophisticated axillary ultrasonographic standards based on our institutional data. The cost modeling in our paper is slightly different from that reported by Boughey et al. [1] because the costs applied in our study were obtained from administrative charges adjusted for the cost to charge ratio, yet the overall conclusions are similar, except for the magnitude of the cost difference. We are also unique in that we included charges incurred at the time of simultaneous breast surgery in our analysis. As we know, hospital charges are defined by hospital operation costs, including the duration of operating room use, preoperative testing, and common costs. These expenses are distributed during the simultaneous performance of SLNB with definitive breast surgery, as opposed to higher costs with performance of an SLNB in isolation. This allows our study widespread applicability and relevance to clinical application.
This study primarily addresses the cost impact of performing preoperative axillary ultrasound on a select breast cancer patient population. However, in addition to economic benefits, preoperative axillary staging can guide breast cancer management including the surgical technique and application of neoadjuvant chemotherapy. Our single-institution experience with axillary ultrasound and the impact on clinical practice have been previously published [14]. In our prior series, preoperative staging significantly affected the number of SLNBs being performed, with fewer SLNB procedures performed in those undergoing preoperative axillary sonography. Furthermore, axillary ultrasound was significantly associated with a greater use of neoadjuvant chemotherapy—49.7% of patients undergoing axillary ultrasound had neoadjuvant chemotherapy, compared with 21.1% of the control group.
One limitation of single-institution data is always the pervasive inferences that can be drawn. Yet the broad ranges included in the sensitivity analysis lend credence to our hypothesis that the routine use of axillary ultrasound for stage ≥T2 breast tumors may be cost favorable on a large scale. The role of core needle biopsy in the diagnosis of nodal metastasis was not addressed in this analysis, but we believe that, given the similar diagnostic yield of core needle biopsies with slightly higher costs, in the range of hundreds of dollars, the strategy of axillary ultrasound may remain cost neutral compared with the conventional strategy. In addition, we do recognize that test characteristics are not the same across centers, yet the validity of the cost benefit across wide ranges of sensitivity and specificity are encouraging. Also, not every patient who undergoes an SLNB ultimately undergoes a CLND for a positive result and this uncertainty is not captured in our modeling.
The public health burden of stage ≥T2 breast cancer patients in the U.S. is extremely high, with almost 50 new cases per 100,000 person-years or approximately 75,000 new cases per year [18]. The cost saving with the routine use of axillary ultrasound with even a modest saving of $700 would amount to a savings of $52 million per year, which could well be used in research in making strides against this disease.
In conclusion, we report cost savings with a selective strategy of application of axillary ultrasound to patients with invasive breast cancer with stage ≥T2 tumors but caution against widespread use without appropriate equipment and training, because the cost benefit is lost with diminishing test characteristics.
Acknowledgment
This study was supported in part by the Don and Erika Wallace Breast Cancer Research Foundation.
Author Contributions
Conception/Design: Kiran K. Turaga
Collection and/or assembly of data: Kiran K. Turaga, Jennifer M. Eatrides, M. Catherine Lee
Data analysis and interpretation: Kiran K. Turaga, Alec Chau
Manuscript writing: Kiran K. Turaga, M. Catherine Lee
Final approval of manuscript: Kiran K. Turaga, Alec Chau, Jennifer M. Eatrides, John V. Kiluk, Nazanin Khakpour, Christine Laronga, M. Catherine Lee
References
- 1.Boughey JC, Moriarty JP, Degnim AC, et al. Cost modeling of preoperative axillary ultrasound and fine-needle aspiration to guide surgery for invasive breast cancer. Ann Surg Oncol. 2010;17:953–958. doi: 10.1245/s10434-010-0919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinson JL, McGrath P, Moore A, et al. The critical role of axillary ultrasound and aspiration biopsy in the management of breast cancer patients with clinically negative axilla. Ann Surg Oncol. 2008;15:250–255. doi: 10.1245/s10434-007-9524-3. [DOI] [PubMed] [Google Scholar]
- 3.Krishnamurthy S, Sneige N, Bedi DG, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–988. doi: 10.1002/cncr.10786. [DOI] [PubMed] [Google Scholar]
- 4.Sapino A, Cassoni P, Zanon E, et al. Ultrasonographically-guided fine-needle aspiration of axillary lymph nodes: Role in breast cancer management. Br J Cancer. 2003;88:702–706. doi: 10.1038/sj.bjc.6600744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahir M, Osman KA, Shabbir J, et al. Preoperative axillary staging in breast cancer-saving time and resources. Breast J. 2008;14:369–371. doi: 10.1111/j.1524-4741.2008.00600.x. [DOI] [PubMed] [Google Scholar]
- 6.Tate JJ, Lewis V, Archer T, et al. Ultrasound detection of axillary lymph node metastases in breast cancer. Eur J Surg Oncol. 1989;15:139–141. [PubMed] [Google Scholar]
- 7.van Rijk MC, Deurloo EE, Nieweg OE, et al. Ultrasonography and fine-needle aspiration cytology can spare breast cancer patients unnecessary sentinel lymph node biopsy. Ann Surg Oncol. 2006;13:31–35. doi: 10.1245/ASO.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Bonnema J, van Geel AN, van Ooijen B, et al. Ultrasound-guided aspiration biopsy for detection of nonpalpable axillary node metastases in breast cancer patients: New diagnostic method. World J Surg. 1997;21:270–274. doi: 10.1007/s002689900227. [DOI] [PubMed] [Google Scholar]
- 9.Brancato B, Zappa M, Bricolo D, et al. Role of ultrasound-guided fine needle cytology of axillary lymph nodes in breast carcinoma staging. Radiol Med. 2004;108:345–355. [PubMed] [Google Scholar]
- 10.Ciatto S, Brancato B, Risso G, et al. Accuracy of fine needle aspiration cytology (FNAC) of axillary lymph nodes as a triage test in breast cancer staging. Breast Cancer Res Treat. 2007;103:85–91. doi: 10.1007/s10549-006-9355-0. [DOI] [PubMed] [Google Scholar]
- 11.Davis JT, Brill YM, Simmons S, et al. Ultrasound-guided fine-needle aspiration of clinically negative lymph nodes versus sentinel node mapping in patients at high risk for axillary metastasis. Ann Surg Oncol. 2006;13:1545–1552. doi: 10.1245/s10434-006-9095-8. [DOI] [PubMed] [Google Scholar]
- 12.Genta F, Zanon E, Camanni M, et al. Cost/accuracy ratio analysis in breast cancer patients undergoing ultrasound-guided fine-needle aspiration cytology, sentinel node biopsy, and frozen section of node. World J Surg. 2007;31:1155–1163. doi: 10.1007/s00268-007-9009-3. [DOI] [PubMed] [Google Scholar]
- 13.Batsis C, Ziogas D, Fatouros M. Neoadjuvant chemotherapy for breast cancer: Does pretreatment axillary nodal staging improve decision making? Ann Surg Oncol. 2009;16:1063–1064. doi: 10.1245/s10434-009-0351-6. [DOI] [PubMed] [Google Scholar]
- 14.Lee MC, Eatrides J, Chau A, et al. Consequences of axillary ultrasound in patients with T2 or greater invasive breast cancers. Ann Surg Oncol. 2011;18:72–77. doi: 10.1245/s10434-010-1171-4. [DOI] [PubMed] [Google Scholar]
- 15.Bevilacqua JL, Kattan MW, Fey JV, et al. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. 2007;25:3670–3679. doi: 10.1200/JCO.2006.08.8013. [DOI] [PubMed] [Google Scholar]
- 16.Bedi D, Krishnamurthy R, Krishnamurthy S, et al. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: In vitro sonographic study. AJR Am J Roentgenol. 2008;191:646–652. doi: 10.2214/AJR.07.2460. [DOI] [PubMed] [Google Scholar]
- 17.Walsh JS, Dixon JM, Chetty U, et al. Colour Doppler studies of axillary node metastases in breast carcinoma. Clin Radiol. 1994;49:189–191. doi: 10.1016/s0009-9260(05)81774-x. [DOI] [PubMed] [Google Scholar]
- 18.Giuliano AE, McCall LM, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: The American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez S, Añorbe E, Alcorta P, et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: A systematic review. AJR Am J Roentgenol. 2006;186:1342–1348. doi: 10.2214/AJR.05.0936. [DOI] [PubMed] [Google Scholar]