Defective function of dendritic cells is examined as a mechanism for myeloma cell escape in multiple myeloma tumor progression.
Keywords: Multiple myeloma, Dendritic cells, Bone disease, Immune system
Learning Objectives
After completing this course, the reader will be able to:
Describe defective immunological features that have been identified in dendritic cells in multiple myeloma and explain how immunologic dendritic cell defects could reduce the clinical efficacy of dendritic cell-based vaccines.
Outline possible therapeutic strategies based on current knowledge of the bone marrow crosstalk between myeloma cells and immature dendritic cells.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
The crosstalk of myeloma cells with accessory cells drives the expansion of malignant plasma cell clones and the hyperactivation of osteoclastogenesis that occurs in multiple myeloma (MM). These reciprocal interactions promote defective dendritic cell (DC) function in terms of antigen processing, clearance of tumor cells, and efficacy of the immune response. Thus, myeloma cells exert immune suppression that explains, at least in part, the failure of therapeutic approaches, including DC vaccination. Impairment of DCs depends on high bone marrow levels of cytokines and adhesion molecules that affect both maturation and expression of costimulatory molecules by DCs. Moreover, DCs share with osteoclasts (OCs) a common ontogenetic derivation from the monocyte lineage, and thus may undergo OC-like transdifferentiation both in vitro and in vivo. Immature DCs (iDCs) induce clonogenic growth of malignant plasma cells while displaying OC-like features, including the ability to resorb bone tissue once cultured with myeloma cells. This OC-like transdifferentiation of iDCs is dependent on the activation of both the receptor activator of nuclear factor κB (RANK)–RANK ligand (RANK-L) and CD47–thrombospondin (TSP)-I axes, although interleukin 17–producing T helper-17 clones within the bone microenvironment may also take part in this function. Therefore, iDCs allied with malignant plasma cells contribute to MM osteoclastogenesis, although other molecules released by tumor cells may independently contribute to the bone-resorbing machinery.
Introduction
The interplay of highly proliferative malignant plasma cells with accessory cells in the marrow microenvironment is typical of multiple myeloma (MM) and results in the formation of lytic lesions of the bone, leading to progressive skeletal devastation [1]. The crosstalk of myeloma cells with osteoblasts, osteoclasts (OCs), stromal cells, and T cells occurs either through cell-to-cell contact or by cytokine, chemokine, adhesion molecule, and metalloprotease overproduction [2]. These factors are usually enriched within the myeloma marrow microenvironment and, following the engagement of specific receptors, trigger myeloma cells to strengthen proliferation, angiogenesis, and osteoclastogenesis [3].
Recent studies highlight the role of effector cells of the immune system in MM tumor progression [4], in that they are susceptible to recruitment into the bone marrow (BM), where they are normally committed to counterbalancing the unrestrained growth of the neoplastic clone [5]. However, the majority of these studies have demonstrated the impairment of several immunological functions in MM and have shown that malignant plasma cells are resistant to the control of immune cells [6]. The interaction of cytokines, chemokines, and growth factors with cognate receptors produces a vicious circle that is primarily responsible for cancer progression as well as for the defective anti-MM immune response [7].
In this context, dendritic cells (DCs) have been demonstrated to be attracted by the tumor environment [8]. They are professional antigen-presenting cells of myeloid origin and are essential for the primary T-cell response via crosspriming that allows the transfer of antigens from neoplastic cells and their presentation through major histocompatibility complex (MHC) class II molecules [9]. DC function depends on both their ontogenetic origin and their stage of maturation and is influenced by signals received from tumor cells, the stromal matrix, and T cells [10]. Maturation has profound effects on DC biology and includes presentation of MHC molecules by the cell surface, increased expression of costimulatory molecules such as CD80 and CD86, changes in motility, and formation of dendrites interacting with lymphocytes [11]. The efficient maturation of DCs is also crucial for their inhibitory effect against MM, but vaccination with patient-specific idiotype-pulsed DCs failed to restrain clonal proliferation in this hematological disorder [12].
An emerging issue is the possibility that the immunological properties of DCs are impaired in MM. Recent studies have, indeed, demonstrated several defective immunological properties in MM-derived DCs, including a lack of CD80 and CD86 molecules, the defective antigen presentation, and major marrow accumulation of both immature DCs (iDCs) and inactivated DCs [13]. Furthermore, other mechanisms lead to tumor escape and immune tolerance, and are apparently dependent on either the high release of interleukin (IL)-6, vascular endothelial growth factor (VEGF), and M-CSF in myeloma milieu or myeloma–stroma interactions that result in DC inability to process and present antigens to T cells [14]. These findings explain, at least in part, the potential role of myeloma cells in defective DC function as well as the poor clinical results obtained in trials using vaccination with idiotypes of the monoclonal component [15].
Additional studies also suggest that DCs may potentially switch toward a new program aimed to: (a) stimulate the proliferation of myeloma cells, (b) activate osteoclastogenic machinery through direct transdifferentiation into OC-like bone-resorbing cells, and (c) enhance the marrow concentration of IL-17 by expanding T helper (Th)-17 clones [16, 17]. iDCs express several chemokine receptors that promote their migration toward high gradients of relative ligands [18]. Enhanced marrow migration of DCs has been demonstrated in MM patients, and a high number of plasmacytoid DCs has been found close to myeloma cells in bone erosive lacunae [19]. Their reciprocal crosstalk apparently promotes the clonogenic proliferation of malignant plasma cells through the activation of transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI)–a proliferation-inducing ligand (APRIL) signaling, whereas iDCs may concurrently undergo OC-like transdifferentiation mostly supported by both receptor activator of nuclear factor κB ligand (RANK-L) and CD47 molecules expressed by myeloma cells [20, 17]. Moreover, it has been reported that marrow iDCs induce the expansion of Th-17 clones leading to IL-17 overproduction, with a final effect of enhancement of osteoclastogenesis by triggering the IL-17 receptor expressed by both OCs and myeloma cells.
Here, we review several events related to the role of DCs in MM tumor progression.
Myeloma BM Microenvironment and DCs
Functional activation of the BM microenvironment drives the expansion of myeloma cells, whereas both neoangiogenesis and defective immunological control by accessory cells contribute to their clonal growth, resulting in tumor progression. Marrow myeloma cell proliferation, however, involves other players, because tumor interplay with stromal cells is also a primary event leading to hyperactive osteoclastogenesis [21].
The BM microenvironment includes both nonhematopoietic and hematopoietic cells as well as an extracellular compartment forming “the niche” within the mineralized bone [22]. Here, quiescence, expansion, and survival as well as migration of hematopoietic cells are consequences of both cell-to-cell and cell–matrix contacts [23]. It has been demonstrated that cell homeostasis within the niche is defective in MM and that BM cells are affected by myeloma cells in terms of immune suppression and upregulation of bone-resorbing functions. On the other hand, myeloma cells are stimulated within the BM by the paracrine and autocrine overproduction of cytokines and growth factors that influence their growth, survival, and migration [24]. Higher levels of IL-6 in the marrow indeed promote the uncontrolled proliferation of myeloma cells, upregulate their drug resistance–related genes, and trigger osteoclastogenesis [25]. The transcriptional pathway stimulated by IL-6 is regulated via the Ras/Raf/mitogen-activated protein kinase (MAPK)–extracellular signal-related kinase kinase/MAPK cascade [26] that results in the activation of the Janus activated kinase (JAK) and signal transducer and activator of transcription (STAT) proteins, as well as the overexpression of both Bcl-xL and Mcl-1 for the inhibition of apoptosis [27].
IL-6 also impairs the efficiency of antigenic presentation by DCs and prevents their differentiation from monocytic precursors, thereby contributing to the immune tolerance that characterizes MM [28]. In addition, IL-6 upregulation is consistent with the prevalence of iDCs, because the BM of IL-6 knockout mice is enriched in mature DCs [14], whereas a peculiar accumulation of iDCs was also demonstrated in the BM of patients with active MM showing high levels of IL-6 [29]. Finally, IL-6 levels parallel the overexpression of stromal cell–derived factor (SDF)-1 [30] and macrophage inhibitory protein 1α in the MM microenvironment [31], which may foster iDC recruitment.
However, the effective role of DCs in MM is still debated. They are numerically greater in BM, although defective in terms of functions, including both antigenic processing and antitumoral activity. On the contrary, these cells apparently promote the clonogenic proliferation of myeloma cells [20] and may transdifferentiate into OC-like bone-resorbing cells [32]. This behavior is dependent on their ontogenic derivation from the monocyte–macrophage lineage, including sensitivity to elevated RANK-L concentrations as occur in the BM of myeloma patients [33].
It is conceivable, therefore, that DCs in concert with other accessory cells deregulate BM homeostasis, thus establishing an active alliance with malignant plasma in driving their clonal expansion and perpetuating OC hyperactivation.
Immunological Features of DCs in MM
DCs are critical for the initiation of the primary T-cell response [34] and exert specific crosspriming that allows processing of tumor antigens, their presentation to T cells through MHC class II molecules, and generation of CD8+ cytotoxic T cells [11]. The effectiveness of the DC-mediated immune response depends on their maturation and the expression of costimulatory molecules [35]. Although iDCs are able to migrate to the tumor bed and capture tumor-derived antigens for presentation to specific T cells, the immune response is frequently inhibited in cancer [36]. In fact, tumors frequently escape immunological surveillance of DCs as a consequence of the suppression of many functions of the immune system [37].
The role of iDCs in MM is controversial with respect to their function and capability of controlling the proliferative extent of malignant plasma cells [38]. Studies have, indeed, failed to demonstrate a major accumulation of DCs within the BM of MM patients, compared with monoclonal gammopathy of uncertain significance (MGUS) patients [39], whereas they have described impaired responsiveness to CD40 as a mechanism for escape of myeloma cells from DC control [13]. Therefore, it is conceivable that a functional, rather than numerical, defect in DCs is prevalent in MM.
In order to define this question, other studies have pointed to the immunological properties of the monoclonal component as effective stimuli priming an efficient immune response by iDCs [40]. Therefore, adjuvant immunotherapy using antigen-loaded DCs has been suggested as an attractive strategy for the treatment of relapsed or resistant MM patients [41, 42]. Preliminary DC-based vaccination strategies used HLA-derived antigens expressed by MM cells. However, this approach was unsuccessful in relation to both HLA restriction [43] and the patient-specific antigen profile. To overcome these limitations, specific idiotypic determinants of the immunoglobulin variable region were used as private antigens of myelomas [44], and vaccination with idiotype-pulsed DCs produced a specific cytotoxic T lymphocyte response, but this treatment achieved a poor clinical response and did not result in longer overall survival [45]. Other approaches used myeloma–DC fused cells that exerted an efficient antitumor effect in vitro with no potential application in vivo [46]. Therefore, the major points for understanding the failure of immunotherapy in MM include: (a) an intrinsic defect in the host immune system, (b) an alteration in the T-cell, B-cell, macrophage, and natural-killer cell repertoire, (c) BM overexpression of immunosuppressive cytokines such as tumor growth factor-β and IL-10, and (d) impaired in vivo crosspriming by T cells [47–52].
Defective Antimyeloma Activity of DCs
Malignant plasma cells show a high proliferation extent and expand within the BM as an effect of their reciprocal interactions with T cells and iDCs that are attracted in response to chemotactic gradients. This interplay may account for the deregulation of the cytokine network that induces defective expression of costimulatory receptors by immune cells and alteration of the T-cell repertoire, thus enabling tumor cells to escape immunological control and resulting in impaired antitumor DC-mediated T-cell activity [53].
Overproduction of IL-10, IL-6, M-CSF, and VEGF occurs in myeloma BM and these interleukins prevent DC differentiation, maturation, and function, both in vitro and in vivo [47]. In particular, high marrow levels of IL-6 in myeloma cells accelerate DC differentiation and maturation [14], because this cytokine inhibits the growth of colony-forming unit-DCs from CD34 precursors, redirecting the differentiation of hematopoietic progenitors toward the monocyte–macrophage lineage [54]. However, treatment with anti–IL-6 antibody does not restore the functional activity of marrow DCs in MM, suggesting a potential influence of other inhibitory factors released by myeloma cells within the BM [55].
Notwithstanding the number of studies concerning tumor-derived factors that affect DC differentiation, little is known about the molecular mechanisms responsible for their dysfunction in cancer. The JAK tyrosines and STAT proteins are crucial for DC maturation and are directly involved in the proliferation, survival, and resistance to apoptosis of cancer cells [56]. A higher transcription rate of JAKs is induced by cytokines and growth factors released within the myeloma BM, primarily including IL-6 [57]. In this regard, it was demonstrated that treatment of DCs with IL-6 abrogates lipopolysaccharide-mediated maturation of DCs through the activation of STAT-3 [58], whereas other factors produced by the myeloma cells enhance both phosphorylation of JAK-2 and activation of STAT-3 in iDCs, preventing their normal maturation. Thus, iDCs in the presence of appropriate stimulators may be reprogrammed to a new transdifferentiative fate [59].
Other studies hypothesize that iDCs directly accelerate the growth of myeloma cells [20] because they control the differentiation and survival of normal B lymphocytes and plasma cells [60]. Cocultures of DCs with autologous myeloma cells prime the formation of myeloma colonies in a fashion similar to that of cocultures with either lymphoma [41] or breast cancer [61] cells, and the TACI–APRIL interaction seems to be pivotal for the proliferation of myeloma cells. Thus, this molecular axis interferes with the antiapoptotic pathways regulated by both Bcl-2 and Bcl-6 molecules in malignant plasma cells [20].
It is conceivable, therefore, that impaired DC function in MM depends on the performance of malignant plasma cells that, through receptor triggers or by soluble factors, abrogate the innate immunological fate of iDCs and create a functional alliance with these cells aimed at progression of the disease rather than its control.
Myeloma Bone Disease and DCs
Skeletal devastation is a hallmark of MM, and the malignant plasma cell clone in BM leads to progressive resorption of the surrounding matrix with formation of lytic lesions. The pathogenetic mechanisms underlying myeloma bone disease (MBD) are undefined, although myeloma cells are believed to play a major osteoclastogenic role through the recruitment, differentiation, and activation of OC precursors within the BM [62]. This event is, at least in part, mediated by stromal cells that are structural components of the marrow niches housing myeloma cells. As a consequence of this chronic interaction, both populations produce several osteoclastogenic factors, including RANK-L and IL-6 [63]. Thus, marrow myeloma cells prime OCs toward resorptive functions by secreting soluble factors.
Bone-resorbing OCs originate from monocyte/macrophage precursors whose terminal differentiation is also regulated by both RANK-L and M-CSF. Once differentiated, precursor cells fuse into tartrate-resistant acid phosphatase (TRAcP)+ and cathepsin-k (CK)+ multinucleated cells. At the site of active bone resorption, OCs form a specialized ruffled border, following the organization of the actin ring operated by αVβ3-integrin–mediated matrix recognition. On attachment to bone, matrix-derived signals polarize intracellular secretory vescicles that deliver H+ATPase to plasma membrane and release proteolytic enzymes into the resorptive lacunae. In this context, ATPase deteriorates the inorganic components of the bone, whereas the organic matrix is digested by CK.
OC hyperactivity is a prominent feature of MBD that occurs as result of the recruitment of OC precursors in response to multiple interactions with malignant plasma cells. This cell-to-cell interplay enhances cytokine concentrations within the marrow milieu and the sensitivity of OCs to many functional ligands, although a major osteoclastogenic effort is exerted by marrow RANK-L and M-CSF [63]. The exposure of OC precursors to high concentrations of these cytokines in the marrow niche induces their differentiation into bone-resorbing cells. The role of RANK-L was clearly demonstrated in RANK-L–deficient mice [64], which develop severe osteopetrosis as a consequence of defective OC function. These mice show impaired expression of transcriptional factors such as MAPK, phosphoinositide 3-kinase, and nuclear factor κB, whose activation is a matter of priority for the nuclear translocation of nuclear factor of activated T cells, cytoplasmic 1 and their functional bone-resorbing activity.
Besides the major regulation by RANK-L, other functional receptors are expressed by bone-resorbing cells and recent evidence has emphasized interactions between the BM stroma and the immune system as a hallmark for the development of MBD [65]. The immune system plays a major role in osteoclastogenesis, in particular through activated T cells that secrete appropriate cytokines. However, DCs have also been emphasized as potential inducers of bone erosion in inflammatory and neoplastic diseases [66]. DCs are derived from the same myeloid precursor as OCs and the differentiation of both cell types is controlled by RANK-L [32]. Also, DCs show high plasticity depending on local factors and stimuli during their maturation [67], and although they are terminally differentiated cells, they may switch into regulatory cells or transdifferentiate into either endothelial cells or OCs in the presence of specific cytokines [32]. Their functional transdifferentiation occurs both in vitro and in vivo because their immature subset actively participates in bone erosion in inflammatory diseases, thus exerting typical functions of OCs in terms of shape, cytoskeleton rearrangement, and erosive capability [32].
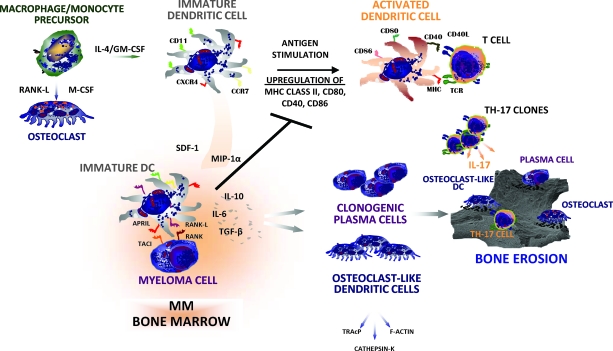
DCs also promote hyperactive osteoclastogenesis in MBD [19, 17] because their number is higher within the erosive lacunae. In addition, they may undergo OC-like transdifferentiation following stimulation by the RANK–RANK-L or TSP-I–CD47 pathways. Thus, hyperactive osteoclastogenesis in MM results from multiple players that show a similar propensity for bone resorption and an ability to activate major osteoclastogenic pathways (Fig. 1).
Figure 1.
Pathophysiology of DC function within the myeloma marrow microenvironment. The fate of DCs is dependent on gradients of cytokines within MM bone marrow. They undergo functional maturation from macrophage/monocyte precursors under the influence of both IL-4 and GM-CSF. This enables a population of mature DCs to process and present antigens to T cells. However, it is conceivable that mature DCs may drive, within the tumor site, the expansion of a Th-17 clone leading to IL-17 overproduction that enhances osteoclastogenesis. On the other hand, DCs may undergo osteoclast-like transdifferentiation as an effect of the increased levels of both soluble and membrane-bound RANK-L produced by stromal cells, osteoblasts, and malignant plasma cells within the marrow microenvironment. This may result in the expansion of an immature subset of DCs that is recruited by malignant plasma cells and participates in bone resorption. Recruited immature DCs enhance the clonogenic growth of myeloma cells by cell-to-cell molecular contacts involving the TACI–APRIL pathway.
Abbreviations: APRIL, a proliferation-inducing ligand; CCR7, CC chemokine receptor 7; CXCR4, CXC chemokine receptor 4; DC, dendritic cell; IL, interleukin; MHC, major histocompatibility complex; MIP-1α, microphage inhibitory protein 1α; MM, multiple myeloma; RANK, receptor activator of nuclear factor κB; RANK-L, RANK ligand; SDF-1, stromal cell–derived factor 1; Th-17, T helper-17; TACI, transmembrane activator and calcium modulator and cyclophilin ligand interactor; TCR, T-cell receptor; TGF-β, transforming growth factor β; TRAcP, tartrate-resistant acid phosphatase.
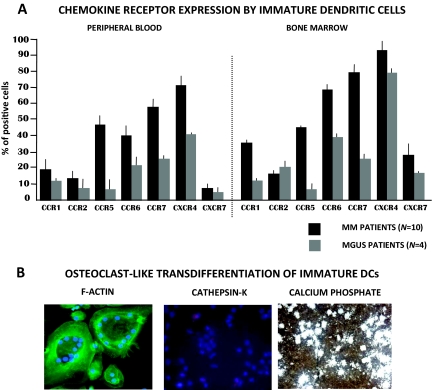
We investigated the role of iDCs in patients with MBD focusing on both their chemotactic features in response to stimulation of marrow myeloma cells and their ability to transdifferentiate into OC-like cells [68]. Ten patients with MM and four with MGUS were analyzed, showing that iDCs from those with MBD are recruited within BM in response to gradients of chemokines promoted by highly proliferating myeloma cells. In this context, CXC chemokine receptor 4 (CXCR4) and CC chemokine receptor 7 (CCR7) apparently play major roles in the migratory behavior of iDCs, in that they were overexpressed in both peripheral blood and BM (Fig. 2A). Moreover, these iDCs showed high migration properties in vitro in response to the CXCR4 ligand, that is, SDF-1. In addition, tight contact of iDCs with myeloma cells through cocultures apparently induced their OC-like transdifferentiation that is mostly mediated by the interaction of RANK with the RANK-L molecules highly expressed by malignant plasma cells. This contact could probably assign iDCs to a new osteoclastogenic program within the myeloma erosive lacunae in line with their morphology, giving them the capacity to rearrange the cytoskeleton with the formation of the ruffled border of F-actin and the expression of proteolytic enzymes of the bone matrix such as TRAcP, ATPase, and CK. iDCs have indeed been demonstrated to promote extensive erosion of experimental substrates (Fig. 2B). These features are not observed in cocultures including plasma cells from MGUS patients with autologous iDCs. Thus, OC-like iDCs may represent an additional population located within the myeloma niche that contributes to MBD.
Figure 2.
Chemokine receptor expression in myeloma and OC-like transdifferentiation of iDCs. (A): Peripheral blood and bone marrow iDCs from 10 MM patients with severe bone disease and four patients with MGUS were investigated by flow cytometry for the expression of chemokine receptors. As shown, the majority of chemokines were overexpressed by iDCs in the bone marrow of MM patients, compared with MGUS patients. In contrast, only CCR7 and CXCR4 were upregulated by peripheral iDCs that are apparently inclined to migrate toward the bone marrow by stimuli of relative ligands expressed by myeloma cells. (B): Bone marrow iDCs cocultured with myeloma cells undergo functional OC-like transdifferentiation in terms of cytoskeleton rearrangement, cathepsin-K expression, and the capability of resorbing substrates of calcium phosphate discs.
Abbreviations: CCR7, CC chemokine receptor; CXCR4, CXC chemokine receptor; DC, dendritic cell; iDC, immature DC; MGUS, monoclonal gammopathy of uncertain significance; MM, multiple myeloma; OC, osteoclast.
Other mechanisms involving DCs, however, participate in the skeletal devastation of MM and include the expansion of T-cell clones polarized toward a Th-17 phenotype [16]. Both DCs and high amounts of IL-6 and interferons in the BM promote the expansion of Th-17 clones [69]. The role of Th-17–polarizing cytokines has been studied in T cells in terms of activation of critical transcription factors such as STAT-3, although these cytokines have been shown to reinforce Th-17 induction via DC activation [70]. The predominant Th-17 polarization in the myeloma microenvironment is concordant with the high amounts of marrow IL-17 found in MM patients, compared with MGUS patients, whereas its potential role in osteoclastogenesis is related to the capability of IL-17 to modulate the RANK-L–dependent pathway [71]. The myeloma tumor bed is greatly infiltrated by DCs, which recruit T cells by CC chemokine ligand 20 upregulation [72]. Moreover, the capability of DCs to promote Th-17 polarization is enhanced by the uptake of apoptotic tumor cells that infiltrate the myeloma niche. Lastly, several pathogens have been postulated as functional inducers of DC-mediated Th-17 polarization, although at present they remain to be identified [73]. It is conceivable, therefore, that Th-17 cells are additional players that affect the worsening of bone disease in MM.
Future Directions to Treat MBD
MBD influences mortality and morbidity in MM patients, although current treatment with bisphosphonates delays the skeletal devastation. Based on recent findings highlighting the marrow crosstalk of myeloma cells with iDCs, future therapeutic strategies might be devoted to: (a) preventing their cellular contact to restrain OC differentiation of myeloid precursors, or (b) directly inhibiting the resorptive functions exerted by both OCs and OC-like cells. In this context, the TACI–APRIL axis is critical for myeloma–iDC interplay, and clinical studies have shown that the neutralization of APRIL by the fusion protein atacicept abrogates the growth of myeloma cells [74]. A similar effect was also produced in vitro by anti-TACI monoclonal antibody (mAb) [19]. Thus, it should be interesting to verify their efficacy in myeloma-induced OC-like differentiation of iDCs. Other antimyeloma strategies include blocking iDC transdifferentiation to OCs by denosumab, the anti–RANK-L mAb used to restrain accelerated OC differentiation in MM [75]. Furthermore, other strategies disabling the resorptive ability of OC-like iDCs include CK inhibitors [76] and tyrosine kinase Src blocking downstream of the RANK receptor [77].
The application of these targeted therapies in preclinical settings should theoretically be successful for future treatments of skeletal colonization in MM.
Conclusions
The aim of this review was to emphasize the defective function of DCs as a mechanism for myeloma cell escape. In fact, the inability of different compartments of the immune system to adequately respond against tumor cells may be ascribed to a functional defect in DCs that appear as faithful allies of malignant plasma cells. A better understanding of the molecular interactions between DCs and myeloma cells will provide new insights into the pathogenetic mechanisms promoting plasma cell proliferation as well as the acceleration of osteoclastogenic derangements. However, it is noteworthy that myeloma clone spread within the marrow also interacts with stromal cells, osteoblasts, OCs, and hematopoietic precursors, resulting in enhanced production of osteoclastogenic soluble factors. However, targeting DCs may be a future therapeutic strategy to control myeloma proliferation and restrain progression of skeletal disease.
Author Contributions
Conception/Design: Marco Tucci, Stefania Stucci
Provision of study material or patients: Marco Tucci
Collection and/or assembly of data: Marco Tucci
Data analysis and interpretation: Sabino Strippoli
Manuscript writing: Marco Tucci
Final approval of manuscript: Franco Dammacco, Franco Silvestris
References
- 1.Anderson KC, Shaughnessy JD, Jr, Barlogie B, et al. Multiple myeloma. Hematology Am Soc Hematol Educ Program. 2002:214–240. doi: 10.1182/asheducation-2002.1.214. [DOI] [PubMed] [Google Scholar]
- 2.Mitsiades CS, McMillin DW, Klippel S, et al. The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol Oncol Clin North Am. 2007;21:1007–1034. vii–viii. doi: 10.1016/j.hoc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Hideshima T, Bergsagel PL, Kuehl WM, et al. Advances in biology of multiple myeloma: Clinical applications. Blood. 2004;104:607–618. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- 4.Munshi NC. Immunoregulatory mechanisms in multiple myeloma. Hematol Oncol Clin North Am. 1997;11:51–69. doi: 10.1016/s0889-8588(05)70415-9. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi T, Hideshima T, Akiyama M, et al. Ex vivo induction of multiple myeloma-specific cytotoxic T lymphocytes. Blood. 2003;102:1435–1442. doi: 10.1182/blood-2002-09-2828. [DOI] [PubMed] [Google Scholar]
- 6.Dhodapkar MV, Dhodapkar KM, Palucka AK. Interactions of tumor cells with dendritic cells: Balancing immunity and tolerance. Cell Death Differ. 2008;15:39–50. doi: 10.1038/sj.cdd.4402247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hideshima T, Mitsiades C, Tonon G, et al. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM. Dendritic cells and the control of immunity: Enhancing the efficiency of antigen presentation. Mt Sinai J Med. 2001;68:160–166. [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Dhodapkar MV, Krasovsky J, Olson K. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc Natl Acad Sci U S A. 2002;99:13009–13013. doi: 10.1073/pnas.202491499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nouri-Shirazi M, Banchereau J, Bell D, et al. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses. J Immunol. 2000;165:3797–3803. doi: 10.4049/jimmunol.165.7.3797. [DOI] [PubMed] [Google Scholar]
- 12.Yi Q, Szmania S, Freeman J, et al. Optimizing dendritic cell-based immunotherapy in multiple myeloma: Intranodal injections of idiotype-pulsed CD40 ligand-matured vaccines led to induction of type-1 and cytotoxic T-cell immune responses in patients. Br J Haematol. 2010;150:554–564. doi: 10.1111/j.1365-2141.2010.08286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RD, Pope B, Murray A, et al. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7–1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-β1 and interleukin-10. Blood. 2001;98:2992–2998. doi: 10.1182/blood.v98.10.2992. [DOI] [PubMed] [Google Scholar]
- 14.Ratta M, Fagnoni F, Curti A, et al. Dendritic cells are functionally defective in multiple myeloma: The role of interleukin-6. Blood. 2002;100:230–237. doi: 10.1182/blood.v100.1.230. [DOI] [PubMed] [Google Scholar]
- 15.Ruffini PA, Biragyn A, Kwak LW. Recent advances in multiple myeloma immunotherapy. Biomed Pharmacother. 2002;56:129–132. doi: 10.1016/s0753-3322(02)00169-5. [DOI] [PubMed] [Google Scholar]
- 16.Dhodapkar KM, Barbuto S, Matthews P, et al. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17–1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112:2878–2885. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kukreja A, Radfar S, Sun BH, et al. Dominant role of CD47-thrombospondin-1 interactions in myeloma-induced fusion of human dendritic cells: Implications for bone disease. Blood. 2009;114:3413–3421. doi: 10.1182/blood-2009-03-211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cravens PD, Lipsky PE. Dendritic cells, chemokine receptors and autoimmune inflammatory diseases. Immunol Cell Biol. 2002;80:497–505. doi: 10.1046/j.1440-1711.2002.01118.x. [DOI] [PubMed] [Google Scholar]
- 19.Chauhan D, Singh AV, Brahmandam M, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: A therapeutic target. Cancer Cell. 2009;16:309–323. doi: 10.1016/j.ccr.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kukreja A, Hutchinson A, Dhodapkar K, et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silvestris F, Ciavarella S, De Matteo M, et al. Bone-resorbing cells in multiple myeloma: Osteoclasts, myeloma cell polykaryons, or both? The Oncologist. 2009;14:264–275. doi: 10.1634/theoncologist.2008-0087. [DOI] [PubMed] [Google Scholar]
- 22.Basak GW, Srivastava AS, Malhotra R, et al. Multiple myeloma bone marrow niche. Curr Pharm Biotechnol. 2009;10:345–346. doi: 10.2174/138920109787847493. [DOI] [PubMed] [Google Scholar]
- 23.De Raeve HR, Vanderkerken K. The role of the bone marrow microenvironment in multiple myeloma. Histol Histopathol. 2005;20:1227–1250. doi: 10.14670/HH-20.1227. [DOI] [PubMed] [Google Scholar]
- 24.Mitsiades CS, Mitsiades NS, Munshi NC, et al. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: Interplay of growth factors, their receptors and stromal interactions. Eur J Cancer. 2006;42:1564–1573. doi: 10.1016/j.ejca.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Bataille R, Jourdan M, Zhang XG, et al. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J Clin Invest. 1989;84:2008–2011. doi: 10.1172/JCI114392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogata A, Chauhan D, Teoh G, et al. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–2221. [PubMed] [Google Scholar]
- 27.Jourdan M, Veyrune JL, De Vos J, et al. A major role for Mcl-1 antiapoptotic protein in the IL-6-induced survival of human myeloma cells. Oncogene. 2003;22:2950–2959. doi: 10.1038/sj.onc.1206423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chomarat P, Banchereau J, Davoust J, et al. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 29.Bataille R, Chappard D, Alexandre C, et al. Importance of quantitative histology of bone changes in monoclonal gammopathy. Br J Cancer. 1986;53:805–810. doi: 10.1038/bjc.1986.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsayed Y, Ngo H, Runnels J, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109:2708–2717. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivollier A, Mazzorana M, Tebib J, et al. Immature dendritic cell transdifferentiation into osteoclasts: A novel pathway sustained by the rheumatoid arthritis microenvironment. Blood. 2004;104:4029–4037. doi: 10.1182/blood-2004-01-0041. [DOI] [PubMed] [Google Scholar]
- 33.Maitra R, Follenzi A, Yaghoobian A, et al. Dendritic cell-mediated in vivo bone resorption. J Immunol. 2010;185:1485–1491. doi: 10.4049/jimmunol.0903560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinman RM. Dendritic cells and immune-based therapies. Exp Hematol. 1996;24:859–862. [PubMed] [Google Scholar]
- 35.O'Doherty U, Peng M, Gezelter S, et al. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 36.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 37.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 38.Hàjek R, Butch AW. Dendritic cell biology and the application of dendritic cells to immunotherapy of multiple myeloma. Med Oncol. 2000;17:2–15. doi: 10.1007/BF02826210. [DOI] [PubMed] [Google Scholar]
- 39.Raje N, Gong J, Chauhan D, et al. Bone marrow and peripheral blood dendritic cells from patients with multiple myeloma are phenotypically and functionally normal despite the detection of Kaposi's sarcoma herpesvirus gene sequences. Blood. 1999;93:1487–1495. [PubMed] [Google Scholar]
- 40.Lim SH, Wen YJ, Ling M. Malignancy: Idiotypic immune targeting of multiple myeloma. Hematology. 2000;4:471–477. [PubMed] [Google Scholar]
- 41.Wen YJ, Ling M, Bailey-Wood R, et al. Idiotypic protein-pulsed adherent peripheral blood mononuclear cell-derived dendritic cells prime immune system in multiple myeloma. Clin Cancer Res. 1998;4:957–962. [PubMed] [Google Scholar]
- 42.Wen YJ, Ling M, Lim SH. Immunogenicity and cross-reactivity with idiotypic IgA of VH CDR3 peptide in multiple myeloma. Br J Haematol. 1998;100:464–468. doi: 10.1046/j.1365-2141.1998.00592.x. [DOI] [PubMed] [Google Scholar]
- 43.Campoli M, Ferrone S. HLA antigen changes in malignant cells: Epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curti A, Tosi P, Comoli P, et al. Phase I/II clinical trial of sequential subcutaneous and intravenous delivery of dendritic cell vaccination for refractory multiple myeloma using patient-specific tumour idiotype protein or idiotype (VDJ)-derived class I-restricted peptides. Br J Haematol. 2007;139:415–424. doi: 10.1111/j.1365-2141.2007.06832.x. [DOI] [PubMed] [Google Scholar]
- 45.Liso A, Stockerl-Goldstein KE, Auffermann-Gretzinger S, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood progenitor cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2000;6:621–627. doi: 10.1016/s1083-8791(00)70027-9. [DOI] [PubMed] [Google Scholar]
- 46.Raje N, Hideshima T, Davies FE, et al. Tumour cell/dendritic cell fusions as a vaccination strategy for multiple myeloma. Br J Haematol. 2004;125:343–352. doi: 10.1111/j.1365-2141.2004.04929.x. [DOI] [PubMed] [Google Scholar]
- 47.Cook G, Campbell JD. Immune regulation in multiple myeloma: The host-tumour conflict. Blood Rev. 1999;13:151–162. doi: 10.1054/blre.1999.0111. [DOI] [PubMed] [Google Scholar]
- 48.Dhodapkar MV, Geller MD, Chang DH, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lauria F, Foa R, Cavo M, et al. Membrane phenotype and functional behaviour of T lymphocytes in multiple myeloma: Correlation with clinical stages of the disease. Clin Exp Immunol. 1984;56:653–658. [PMC free article] [PubMed] [Google Scholar]
- 50.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 51.Schultze JL, Nadler LM. T cell mediated immunotherapy for B cell lymphoma. J Mol Med. 1999;77:322–331. doi: 10.1007/s001090050358. [DOI] [PubMed] [Google Scholar]
- 52.Steinbrink K, Wölfl M, Jonuleit H, et al. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 53.Podar K, Richardson PG, Hideshima T, et al. The malignant clone and the bone-marrow environment. Best Pract Res Clin Haematol. 2007;20:597–612. doi: 10.1016/j.beha.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Menetrier-Caux C, Montmain G, Dieu MC, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: Role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- 55.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 56.Nefedova Y, Nagaraj S, Rosenbauer A, et al. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the Janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65:9525–9535. doi: 10.1158/0008-5472.CAN-05-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nosaka T, Kitamura T. Janus kinases (JAKs) and signal transducers and activators of transcription (STATs) in hematopoietic cells. Int J Hematol. 2000;71:309–319. [PubMed] [Google Scholar]
- 58.Fricke I, Gabrilovich DI. Dendritic cells and tumor microenvironment: A dangerous liaison. Immunol Invest. 2006;35:459–483. doi: 10.1080/08820130600803429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park SJ, Nakagawa T, Kitamura H, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 60.García De Vinuesa C, Gulbranson-Judge A, Khan M, et al. Dendritic cells associated with plasmablast survival. Eur J Immunol. 1999;29:3712–3721. doi: 10.1002/(SICI)1521-4141(199911)29:11<3712::AID-IMMU3712>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 61.Della Bella S, Gennaro M, Vaccari M, et al. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89:1463–1472. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mundy GR. Myeloma bone disease. Eur J Cancer. 1998;34:246–251. doi: 10.1016/s0959-8049(97)10133-2. [DOI] [PubMed] [Google Scholar]
- 63.Abe M, Hiura K, Wilde J, et al. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: A vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104:2484–2491. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- 64.Farrugia AN, Atkins GJ, To LB, et al. Receptor activator of nuclear factor-κB ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res. 2003;63:5438–5445. [PubMed] [Google Scholar]
- 65.Takayanagi H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 66.Alnaeeli M, Park J, Mahamed D, et al. Dendritic cells at the osteo-immune interface: Implications for inflammation-induced bone loss. J Bone Miner Res. 2007;22:775–780. doi: 10.1359/jbmr.070314. [DOI] [PubMed] [Google Scholar]
- 67.Wakkach A, Mansour A, Dacquin R, et al. Bone marrow microenvironment controls the in vivo differentiation of murine dendritic cells into osteoclasts. Blood. 2008;112:5074–5083. doi: 10.1182/blood-2008-01-132787. [DOI] [PubMed] [Google Scholar]
- 68.Tucci M, Stucci S, Strippoli S, et al. Cytokine overproduction, T-cell activation, and defective T-regulatory functions promote nephritis in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:457146. doi: 10.1155/2010/457146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Beaucoudrey L, Puel A, Filipe-Santos O, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giuliani N, Lisignoli G, Colla S, et al. CC-Chemokine ligand 20/macrophage inflammatory protein-3α and CC-chemokine receptor 6 are overexpressed in myeloma microenvironment related to osteolytic bone lesions. Cancer Res. 2008;68:6840–6850. doi: 10.1158/0008-5472.CAN-08-0402. [DOI] [PubMed] [Google Scholar]
- 73.Ouyang W, Filvaroff E, Hu Y, et al. Novel therapeutic targets along the Th17 pathway. Eur J Immunol. 2009;39:670–675. doi: 10.1002/eji.200839105. [DOI] [PubMed] [Google Scholar]
- 74.Rossi JF, Moreaux J, Hose D, et al. Atacicept in relapsed/refractory multiple myeloma or active Waldenstrom's macroglobulinemia: A phase I study. Br J Cancer. 2009;101:1051–1058. doi: 10.1038/sj.bjc.6605241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 76.Eisman JA, Bone HG, Hosking DJ, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: Three-year continued therapy and resolution of effect. J Bone Miner Res. 2011;26:242–251. doi: 10.1002/jbmr.212. [DOI] [PubMed] [Google Scholar]
- 77.Araujo JC, Poblenz A, Corn P, et al. Dasatinib inhibits both osteoclast activation and prostate cancer PC-3-cell-induced osteoclast formation. Cancer Biol Ther. 2009;8:2153–2159. doi: 10.4161/cbt.8.22.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]