This article provides clinicians as well as nuclear medicine specialists with a concise summary of the most important and widely available nuclear medicine imaging techniques for infectious and inflammatory diseases in cancer patients with an emphasis on fluorodeoxyglucose positron emission tomography.
Keywords: FDG-PET, Scintigraphy, Infection, Neutropenia, Cancer
Abstract
Infections are a common cause of death and an even more common cause of morbidity in cancer patients. Timely and adequate diagnosis of infection is very important. This article provides clinicians as well as nuclear medicine specialists with a concise summary of the most important and widely available nuclear medicine imaging techniques for infectious and inflammatory diseases in cancer patients with an emphasis on fluorodeoxyglucose positron emission tomography (FDG-PET). 67Ga-citrate has many unfavorable characteristics, and the development of newer radiopharmaceuticals has resulted in the replacement of 67Ga-citrate scintigraphy by scintigraphy with labeled leukocytes or FDG-PET for the majority of conditions. The sensitivity of labeled leukocyte scintigraphy in non-neutropenic cancer patients is comparable with that in patients without malignancy. The specificity, however, is lower because of the uptake of labeled leukocytes in many primary tumors and metastases, most probably as a result of their inflammatory component. In addition, labeled leukocyte scintigraphy cannot be used for febrile neutropenia because of the inability to harvest sufficient peripheral leukocytes for in vitro labeling. FDG-PET has several advantages over these conventional scintigraphic techniques. FDG-PET has shown its usefulness in diagnosing septic thrombophlebitis in cancer patients. It has also been shown that imaging of infectious processes using FDG-PET is possible in patients with severe neutropenia. Although larger prospective studies examining the value of FDG-PET in cancer patients suspected of infection, especially in those with febrile neutropenia, are needed, FDG-PET appears to be the most promising scintigraphic technique for the diagnosis of infection in this patient group.
Introduction
Infections are a common cause of death and an even more common cause of morbidity in patients with a wide variety of neoplasms. Many deaths from acute leukemia and half the deaths from lymphoma are caused directly by infection. With more intensive chemotherapy, patients with solid tumors have also become more likely to die as a result of infection. A physical predisposition to infection in patients with cancer can be the result of the neoplasm itself when the integrity of the mucosa or skin is compromised. More often, it might be a result of obstruction of a normally patent orifice, such as the bronchus, ureter, or bile duct. A similar problem can affect patients whose lymph node integrity has been disrupted by radical surgery or scarring after radiation therapy. A life-threatening problem common to many cancer patients is the loss of the reticuloendothelial capacity to clear microorganisms after splenectomy or functional asplenia. Cancer patients also suffer from potentially life-threatening infectious diseases as a result of neutropenia after chemotherapy or because of immune suppression caused by immunosuppressive drugs or immune therapy. In addition, surgery, drugs, and radiotherapy may cause disruption of the normal barriers to infection. Timely identification and localization of infectious and noninfectious inflammatory lesions are critical in appropriate treatment of cancer patients. Infections may be life threatening if not treated appropriately. Incompletely or inadequately treated or otherwise ongoing infections often lead to postponement of chemotherapy, which in itself can also be life threatening.
In daily clinical practice, several imaging modalities are available for the detection of focal infectious and inflammatory processes. Radiological techniques, including computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography, show anatomical changes, so infectious and inflammatory foci are not detected at an early phase because of the lack of substantial anatomical changes at that time, which is also a major problem in cases of neutropenia when inflammatory infiltrates are minimal or absent. Also, discrimination of active infectious or inflammatory lesions from residual anatomical changes resulting from cured processes or surgery is often difficult. This is even more difficult to discriminate in cases of metastatic disease with obstruction. In contrast, scintigraphic imaging is a noninvasive method that allows identification of both the localization and number of infectious and inflammatory foci in all parts of the body, based on functional (physiological or biochemical) changes in tissues. Furthermore, these nuclear medicine techniques permit whole-body imaging, whereas CT and MRI routinely provide only information on a part of the body. Over the past years, a variety of radiolabeled compounds detected by gamma cameras and single photon emission computed tomography (SPECT) have been used for the visualization of infectious and inflammatory disease. The number of these radiolabeled compounds available for diagnosing infection or inflammation is still increasing. Also, positron emission tomography (PET) using 18F-fluorodeoxyglucose (FDG) has entered the field of clinical infectious and inflammatory diseases. Although PET and SPECT have limited spatial resolutions (PET, 3–5 mm; SPECT, 6–8 mm), when compared with CT and MRI, their asset is high-contrast resolution, offering functional and molecular information with very high sensitivities in the nano- or picomolar range (PET > SPECT). Radiation exposure is mostly low, but depending on the radiotracer used, it may reach the radiation exposure of an abdominal CT. A major problem with FDG-PET used for infection imaging in cancer patients, however, is the difficulty in distinguishing between infection and malignancy.
Many different agents have been used in infection imaging. These agents accumulate in inflamed tissue based on different mechanisms. The first mechanism is uptake into inflamed tissue as a result of increased metabolism, either of inflammatory cells (FDG, as a glucose analog reflecting the energy demand of inflammatory cells) or of tissue-specific cells with increased activity as a reaction to inflammation (hydroxymethane diphosphonate [HDP]/methylene diphosphonate [MDP] reflecting the activity of osteoblasts as an active response of the bone to inflammation). Unfortunately, uptake of these tracers is also present in most malignant cells. The second mechanism is unspecific accumulation in the site of inflammation as a result of increased blood flow and enhanced vascular permeability (radiolabeled albumin or gammaglobulins), which is sometimes also present in malignancy. In the case of labeled activated leukocytes, the uptake mechanism is specific migration to the site of inflammation, which is theoretically more specific for infection and inflammation. Finally, 67Ga binds to transferrin, with the complex being extravasated at sites of inflammation because of increased vascular permeability and then transferred to locally present lactoferrin. Basically, most radiolabeled agents accumulate in sites of infection if the local blood flow and the vascular permeability are increased, but local binding also plays a role, as exemplified by 67Ga-citrate.
The approach for diagnosing a specific infectious disease depends on the type of suspected disease and the clinical presentation. Therefore, the context in which imaging procedures are used varies considerably. For many infectious and inflammatory diseases, no unequivocal guidelines for the use of imaging procedures exist, but in some cases there is sufficient evidence from the literature to advise on imaging procedures for optimal diagnosis or follow-up. In other cases, local factors and specific expertise with the use of a certain diagnostic algorithm may play an important role. Here, we aim to provide clinicians as well as nuclear medicine specialists with a concise summary of the most important and widespread nuclear medicine imaging techniques for infectious and inflammatory diseases in cancer patients with an emphasis on FDG-PET.
The Ideal Radiopharmaceutical for Infection Imaging in Cancer Patients
Radiopharmaceuticals for imaging of inflammatory disease should be highly sensitive, easy to prepare, widely available, and inexpensive. In addition, in an ideal situation they would also be able to distinguish among malignancy, infectious diseases, and sterile inflammation. A wide variety of radiotracers have been tested for imaging of inflammation to achieve the mentioned characteristics. However, only a limited number of agents are currently in general use for inflammation imaging. These agents include FDG, autologous WBCs (leukocytes) labeled with 99mTc or 111In, 99mTc-labeled bisphosphonates such as MDP or HDP, and 67Ga-citrate. All of these are neither specific for inflammation nor do they offer the possibility of directly distinguishing sterile from septic inflammation and malignancy.
67Ga Scintigraphy and 68Ga-Citrate PET-CT
67Ga-citrate has been used extensively in clinical practice for several pathological conditions, demonstrating high sensitivity for both acute and chronic infection and noninfectious inflammation [1]. 67Ga scintigraphy, however, is not specific for diagnosing infectious and inflammatory diseases. Many malignant tumors, such as lymphomas, lung carcinoma, melanoma, hepatocellular carcinoma, sarcomas, testicular tumors, multiple myeloma, head and neck tumors, and neuroblastoma, can also be visualized with this imaging technique [2]. Before the introduction of FDG-PET, diagnosis, staging, and follow-up of lymphoma were major indications for 67Ga scintigraphy [2]. In addition to its inability to differentiate between malignancy and infection, there are several other shortcomings that limit its clinical application. Its specificity is also poor because of physiological bowel excretion and accumulation in areas of bone modeling [3, 4]. This radiopharmaceutical also has unfavorable imaging characteristics, such as a long physical half-life (78 hours) and high-energy γ-radiation (93–889 keV), causing high absorbed radiation doses. Moreover, optimal imaging often requires a delay in imaging for up to 72 hours after the injection. These unfavorable characteristics and the development of newer radiopharmaceuticals have resulted in the replacement of 67Ga-citrate scintigraphy with scintigraphy with labeled leukocytes or FDG-PET in the majority of conditions.
68Ga is the positron-emitting counterpart of 67Ga, and labeled with citrate, it can be used in the detection of infection with better spatial resolution using PET-CT. In a recent study of 31 patients with suspected osteomyelitis or spondylodiscitis, 68Ga-citrate PET-CT achieved a sensitivity of 100% and a specificity of 76% [5]. False-positive results were obtained in patients with tumors. Although 68Ga-citrate PET-CT is also unable to differentiate between infection and malignancy, it may be a useful addition to the existing array of radiopharmaceuticals available for infection imaging.
Labeled Leukocytes
Imaging using ex vivo labeled autologous leukocytes was developed in the 1970s. A blood sample of approximately 50 ml is collected and leukocytes are separated in vitro from RBCs. These leukocytes are then labeled with radioactive isotopes (111In or 99mTc) and reinjected. After i.v. administration, in most cases, uptake in granulocytic infiltrates is high, whereas a substantial portion of the leukocytes accumulate in the spleen and the liver. Autologous leukocytes can be labeled with 111In or 99mTc. The latter has replaced 111In-labeled leukocytes for most indications, because of its more optimal characteristics. However, because of the biodistribution of 99mTc-hexamethylpropyleneamine oxime-labeled leukocytes, the use of 111In-labeled leukocytes is preferred for evaluation of the kidneys, bladder, and gall bladder. 111In-labeled leukocytes are also preferred if late images are needed, as in chronic infection [6]. The preparation of this radiopharmaceutical is laborious: isolating and labeling a patient's WBCs takes a trained technician approximately 3 hours. Isolating enough leukocytes is impossible in patients with neutropenia after chemotherapy or targeted agents. In addition, the need to handle potentially contaminated blood can result in transmission of bloodborne pathogens such as hepatitis virus and HIV to technicians or patients. There has always been concern that chronic infections could be missed by labeled leukocyte scans because these infections generate a smaller granulocyte response than acute infections. However, a study in 155 patients demonstrated that the sensitivity of labeled leukocytes for the detection of acute infections (90%) was not significantly different from its sensitivity for detection of chronic infections (86%) [7].
Uptake of 111In-labeled leukocytes in a metastatic malignancy, such as colon carcinoma, osseous metastases of Ewing's sarcoma, and metastatic melanoma, has been described [8–10]. Of 115 111In-labeled leukocyte scans performed in patients with cancer in order to diagnose localized infectious disease, the overall specificity was 95%, sensitivity was 86%, and accuracy was 91%. In only one case, uptake in an osteolytic metastasis was found [11]. In five cases, donor leukocytes were used, so the remaining 110 scans were presumably performed in patients without neutropenia. A retrospective review of 51 111In-labeled leukocyte scans in patients with a known tumor revealed six false-positive results in which leukocyte uptake by the tumor mimicked an abscess [12]. Five of the cases were primary or metastatic tumors of soft tissue (two carcinomas, two lymphomas, one sarcoma) and the other was a skeletal carcinoma metastasis. The degree of tumor uptake of 111In-labeled leukocytes was variable in these patients and could not reliably be used to distinguish abscess from tumor. In another study, tumor uptake of 111In-labeled leukocytes was observed in 10 of 25 patients with malignant neoplasms (two non-Hodgkin's lymphoma, one colonic carcinoma, one ovarian carcinoma, three cerebral neoplasms, and three liver metastases) [13]. Microscopic investigation following specific granulocyte staining revealed the greatest extent of granulocyte infiltration in tumors that showed 111In-labeled leukocyte targeting, emphasizing the importance of tumor granulocyte infiltration as a factor underlying tumor accumulation of 111In-labeled leukocytes. Of 61 patients with various tumors (33 hematologic and 28 solid tumors) studied for persistent fever, 21 patients (34%) manifested abnormal localization of 111In-labeled leukocytes in neoplasms without clinical evidence of infection [14]. Although data for 99mTc-labeled leukocyte scintigraphy in patients with malignancy are scarce, uptake of 99mTc-labeled leukocytes in non-Hodgkin's lymphoma has been described [15]. A study reviewed 343 99mTc-labeled leukocyte scans in patients suspected of having abdominal infection. There was uptake by malignant abdominal tumors in 10 cases (3%), which represented 63% of known malignancies at the time of the scintigram. The relevant treatment was delayed for 2 weeks to 2 months in four patients with adenocarcinoma of the colon in whom the positive uptake was regarded as confirmation of clinically suspected acute diverticulitis [16].
In conclusion, labeled leukocyte scintigraphy is not specific for infection: uptake in tumors reduces specificity in cancer patients. In addition, labeled autologous leukocyte scintigraphy is impossible in patients with febrile neutropenia. Donor leukocytes can be used, but this is associated with a risk for transmission of infectious diseases.
FDG-PET
FDG-PET has become an established diagnostic tool in oncology in the past decade and the indications for FDG-PET are expanding rapidly. FDG accumulates in all cells with a high rate of glycolysis, which occurs not exclusively in neoplastic cells. FDG uptake is also present in all activated leukocytes (granulocytes, monocytes, as well as lymphocytes), enabling imaging of acute and chronic inflammatory processes. The mechanism of FDG uptake in activated leukocytes is related to the fact that these cells use glucose as an energy source only after activation during the metabolic burst. FDG, like glucose, passes through the cell membrane. Phosphorylated FDG is not further metabolized and remains trapped inside the cell, in contrast to phosphorylated glucose, which enters the glycolytic pathway. Higher uptake and retention of FDG has been shown in lesions with a high concentration of inflammatory cells, such as granulocytes and activated macrophages. In an experimental rat model of turpentine-induced inflammation, FDG uptake was elevated even more in chronic inflammation than in an acute inflammatory process [17]. In another rat model of Escherichia coli infection, the uptake of FDG in the infectious process was higher than that of 67Ga-citrate, radiolabeled thymidine, methionine, and human serum albumin [18].
FDG-PET has several advantages over conventional scintigraphic techniques. The physiological uptake of FDG is low in most organs (except for the brain, heart, kidneys, and bladder), and this provides relatively high target- to-background ratios. Low normal organ uptake is best assured by injecting FDG during a normoglycemic, hypoinsulinemic state created by a 6-hour fasting period [19, 20]. FDG-PET provides high-resolution, three-dimensional images of the whole body. Early imaging after 1 hour is possible, resulting in early reporting. Furthermore, the dosimetry of FDG compares favorably with that of conventional radiopharmaceuticals. Although higher glucose metabolism may correlate with structural anatomical damage, as shown with conventional radiological techniques, FDG-PET depicts functional changes in tissue that may often precede anatomical changes. It may be difficult to detect active disease in scar tissue using conventional radiological techniques whereas one of the strongholds of FDG-PET is the delineation of disease activity. Coregistration of PET and CT in combined PET-CT scanners improves the interpretation of the PET findings by exact localization of lesions [21].
Differentiation Between Infection and Malignancy
In malignant diseases, generally higher standardized uptake values (SUVs) of FDG are expected to be found when compared with FDG accumulation in infectious or inflammatory disorders. The usefulness of SUVs for differentiation between infection and malignancy in solitary pulmonary nodules was studied in 585 patients [22]. Although higher SUVs were related to a higher likelihood of malignancy, the degree of FDG uptake was not sufficiently discriminative to distinguish between infection and cancer. For example, there was still a 24% chance that a suspicious pulmonary nodule with an SUV <2.5 was cancer. Another study investigated glucose metabolism in patients with chronic bacterial osteomyelitis using FDG-PET and a dual time protocol [23]. In patients with chronic osteomyelitis, the median SUV did not change significantly between 30 and 90 minutes, whereas the median SUV increased in patients with malignant bone lesions. Although the authors speculate that this could be useful for differentiation between infection and malignancy, median and maximal SUVs and changes in SUV over time showed considerable overlap in some patients with infection and patients with malignant disease. In most patients, biopsy or microbiology results will still be needed to confirm the diagnosis (Fig. 1).
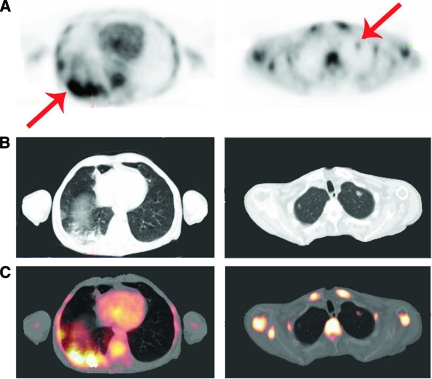
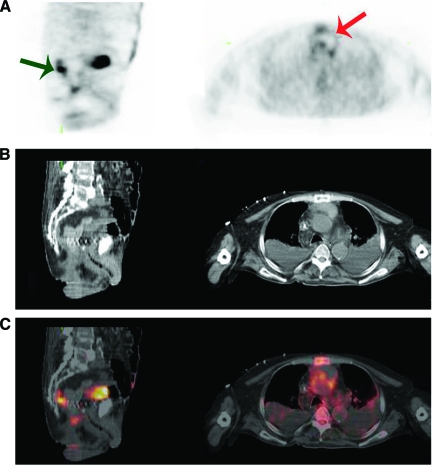
Figure 1.
A 51-year-old man with a history of anal carcinoma treated with radiation therapy, complicated by a fistula, was admitted with candidemia. (A): PET. (B): CT. (C): Fused PET-CT. Left panels show transverse view of the base of the lungs, right panels show transverse view of the top of the lungs. Intense diffuse (left) and focal (right) FDG uptake is seen in pulmonary lesions. Biopsy was performed to differentiate between infection and metastases. Biopsy showed infection caused by C. albicans for which he was treated with fluconazol with complete recovery.
Abbreviations: CT, computed tomography; FDG, fluorodeoxyglucose; PET, positron emission tomography.
Febrile Neutropenia
Although fever during neutropenia after intensive chemotherapy or targeted therapy is not always related to infection, treatment with broad-spectrum antibiotics is promptly begun once fever is observed because infection during neutropenia can be life threatening. Depending on the patient category and treatment given, 30%–50% of febrile episodes are caused by infection [24, 25]. Early recognition of infection contributes to better patient outcome [26]. Starting redundant treatment, on the other hand, is controversial because of possible side effects, development of resistance, and costs. So it is important to attempt to distinguish fever resulting from noninfectious causes, for example, mucosal barrier injury, and fever caused by infection. Early diagnosis and treatment of infectious complications still remain difficult challenges in patients with neutropenia.
It was thought that activated neutrophils play an important role in visualizing localized infection or inflammation using FDG-PET [27]. In cases of severe neutropenia, it has been considered questionable whether FDG-PET can be used for diagnosing infectious diseases because of the markedly lower number of activated neutrophils at the site of infection. In three severely immunocompromised patients (chemotherapy for acute myeloid leukemia, bone marrow transplant for myelodysplastic syndrome, and high-dose salvage chemotherapy for relapsed high-grade non-Hodgkin's lymphoma), however, greater FDG uptake revealed foci of Candida and Aspergillus abscesses [28]. In a retrospective study of 248 PET scans performed in patients with multiple myeloma, either for staging disease progression or for infection workup, FDG-PET identified 165 infectious foci, even in patients with severe neutropenia (in 30 cases) [29]. In 46 patients, infection was not identified by a regular diagnostic workup. In that study, FDG-PET contributed to patient care in 46% of all patients. The authors concluded that FDG-PET is a useful tool for diagnosing and managing infections in patients with multiple myeloma, even in the setting of severe immunosuppression. In that study, however, neutropenia (<1.0 × 109/L) was present in only 27 of 248 (11%) patients. In a partly retrospective and partly prospective observational study, FDG-PET was performed for 36 thrombotic episodes in 27 patients with a hematologic malignancy. PET revealed FDG uptake in all nine episodes of septic thrombophlebitis and none of the 11 acute and 16 chronic deep venous thrombosis cases. Only two patients with septic thrombophlebitis, however, had a neutrophil count <0.5 × 109/L at the time of FDG-PET.
In an ongoing prospective, observational study in patients with chemotherapy-induced neutropenia (peripheral neutrophil count <0.1 × 109/L), we identified infectious foci that were predominantly located in the central venous catheter (CVC) tract (Fig. 2) and lungs, and that were caused by bacteria or fungi. In vivo studies showed uptake of FDG in many activated inflammatory cells, not only neutrophils but also macrophages, natural killer cells, and lymphocytes [30–32]. This is probably the explanation for the visualization of inflammatory foci on FDG-PET scans in the vast majority of patients despite neutrophil counts <0.1 × 109/L in that study. Although larger prospective studies are needed to confirm the value of FDG-PET in patients with febrile neutropenia, these studies have shown that imaging of infectious processes in patients with severe neutropenia using FDG-PET is very well feasible.
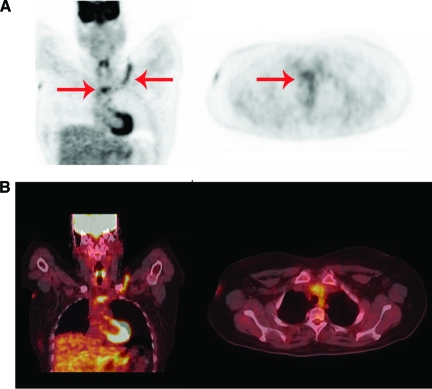
Figure 2.
A 33-year-old woman developed neutropenic fever 1 day after autologous stem cell transplantation for acute myelogenous leukemia. Blood cultures grew coagulase-negative staphylococci. (A): PET. (B): Fused PET-CT. Left panels show coronal view, right panels show transverse view. Septic thrombophlebitis of the left subclavian vein and superior caval vein (red arrows) after removal of CVC is seen. Thrombosis was confirmed by Doppler ultrasound and blood cultures remained positive for 10 days after removal of the CVC despite adequate antibiotic treatment and the absence of another source of infection.
Abbreviations: CT, computed tomography; CVC, central venous catheter; PET, positron emission tomography.
Postoperative Infections
Many cancer patients have to undergo surgery. Many of these patients have an elevated risk for postoperative infections because of previous chemotherapy, radiotherapy, or the underlying malignancy itself. FDG uptake in physiological wound healing is expected to diminish over time. In a German study exploring the value of FDG-PET in 18 patients with suspected postoperative infections, the sensitivity and specificity of infection imaging in areas outside the region of surgical trauma were 86% and 100%, respectively. The sensitivity of infection imaging in the area of the surgical wound was 100%, whereas the specificity was only 56% [33]. The interval between surgery and FDG-PET was significantly shorter in patients with false-positive results. Further studies on the degree, pattern, and duration of physiological FDG uptake after surgery are warranted.
Infection of Metallic Implants and Joint Prostheses
Patients with orthopedic malignancies are sometimes treated with resection and insertion of implants. For patients with metallic implants, FDG-PET has good sensitivity (91%–100%) for the diagnosis of infection [34]. The specificity of FDG-PET in patients with metallic implants, however, is strongly dependent on the criteria used to report infection based on both the localization and intensity of FDG uptake [34]. When using the criterion of uptake at the bone–prosthesis interface (with exclusion of the head and the tip) as positive for infection, the specificity is up to 97% [35]. Specificity is generally higher with hip prostheses than with knee prostheses [36]. The value of FDG-PET was studied in 35 patients with surgery in the previous 2 years who were suspected of harboring a chronic musculoskeletal infection [37]. The accuracy was 94%, which was comparable with that in patients without recent surgery in the same study. In a prospective study, 29 partial-body FDG-PET scans in 22 patients suspected of having metallic implant–associated infections were obtained [38]. The interval between the last surgical intervention and the time of PET scanning was in the range of 6 weeks to 14 months. The sensitivity, specificity, and accuracy were 100%, 93%, and 97%, respectively. FDG-PET appears to be a sensitive and specific method for the detection of infectious foci resulting from metallic implants in patients, even in patients within 1 year after surgery (Fig. 3).
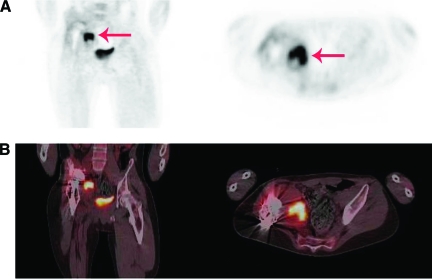
Figure 3.
An 18-year-old man with a megaprosthesis of the right hip after resection of Ewing's sarcoma was admitted with a painful hip 11 months after insertion of the prosthesis. Pseudomonas aeruginosa, Enterococcus faecium, and S. aureus were cultured from several tissue biopsies. (A): PET. (B): Fused PET-CT. Left panels show coronal view, right panels show transverse view. Intense FDG uptake adjacent to the prosthesis (red arrow) caused by infection is seen.
Abbreviations: CT, computed tomography; FDG, fluorodeoxyglucose; PET, positron emission tomography.
Bacteremia and Metastatic Infection
Patients treated with chemotherapy or having CVCs are prone to bacteremia, which can be complicated by metastatic infection. Timely detection of metastatic infectious foci is crucial, because these foci often require prolonged antibiotic treatment or drainage and they often lead to a reduction in the chemotherapy dose intensity, which may compromise long-term disease-free and overall survival times, particularly in patients with responsive and potentially curable malignancies. The diagnosis of metastatic infectious foci is difficult because localizing symptoms are often absent. FDG-PET was assessed for the detection of metastatic infectious foci [39]. In the 40 patients evaluated in that retrospective study, metastatic complications were diagnosed in 75%. It could be demonstrated that, although a median number of four diagnostic procedures had been performed before PET scanning, PET identified clinically relevant new foci in 45% of cases. The positive predictive value (PPV) and negative predictive value (NPV) of FDG-PET were 91% and 99%, respectively [39]. In a recent prospective study, FDG-PET was performed in 115 non-neutropenic patients with Gram+ bacteremia and at least one risk factor for developing infectious complications. The results were compared with those of 230 matched historical controls in whom no FDG-PET was performed. Significantly more patients were diagnosed with metastatic foci in the study group (68% versus 36%). The sensitivity, specificity, NPV, and PPV of FDG-PET–CT were 100%, 87%, 100%, and 89%, respectively. The overall mortality rate after 6 months was lower (19% versus 32%) in the FDG-PET–CT group (p = .014) because of a lower relapse rate [40]. Therefore, FDG-PET is a valuable imaging technique for the detection of metastatic infectious foci in cases of bacteremia and at least one risk factor, such as prolonged fever or persistently positive blood cultures despite adequate antibiotic therapy (Figs 4, 5). Because of its high sensitivity, FDG-PET should be performed in all patients with high-risk Gram+ bacteremia and should be considered in patients with suspected disseminated infection.
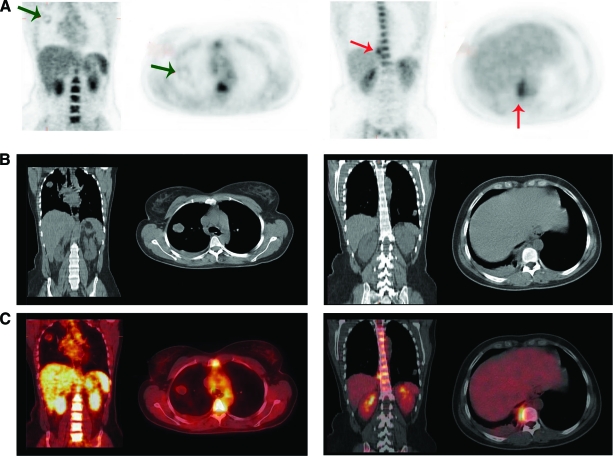
Figure 4.
A 33-year-old woman was admitted with bacteremia with Streptococcus milleri and prolonged fever after chemotherapy for choriocarcinoma with pulmonary metastases despite adequate antibiotic treatment. (A): PET. (B): CT. (C): Fused PET-CT. Left panels show coronal view, right panels show transverse view. (A): Metastatic lesion in the right lung with faint FDG uptake (green arrow) is seen. Intense FDG uptake in paravertebral mass caused by S. milleri (red arrow) is also seen.
Abbreviations: CT, computed tomography; FDG, fluorodeoxyglucose; PET, positron emission tomography.
Figure 5.
A 76-year-old old man with a history of rectum carcinoma and Enterococcus faecalis bacteremia in April 2010 was admitted in June 2010 with E. faecalis bacteremia. Endocarditis was diagnosed and his mitral valve was replaced the next day. His fever continued for >2 weeks despite adequate antibiotic treatment and FDG-PET was performed. (A): PET. (B): CT. (C): Fused PET-CT. Left panels show sagittal view, right panels show transverse view. (A): Greater FDG uptake in a relapse of the rectum carcinoma (green arrow) and FDG uptake in mediastinal infection (red arrow) that was diagnosed as mediastinitis are seen.
Abbreviations: CT, computed tomography; FDG, fluorodeoxyglucose; PET, positron emission tomography.
Infected Vascular Grafts
In cases of extensive tumor resection, vascular grafts need to be placed in some cases. Elderly patients with malignancy sometimes have received prior vascular prostheses because of an aneurysm or stenosis resulting from atherosclerosis. Early diagnosis of vascular graft infection is of utmost importance in the management of all patients with vascular grafts. The rate of infection of vascular grafts is in the range of 0.5%–5%, and it is associated with a high risk for morbidity (such as limb loss) and mortality. On CT scans, air bubbles can be detected around the infected graft in about half of the cases, but these are highly unspecific because in 50% of grafts these bubbles are present for weeks or even months after surgery [41]. Also, it is difficult to differentiate among acute infection, hematoma, and lymphocele on CT scans, and with chronic low-grade infections CT is often false negative [41]. In comparison with CT, PET-CT has shown superior diagnostic performance. PET has shown a sensitivity >90%, in comparison with 64% for CT [42]. When only focal abnormal uptake is interpreted as positive for infection, the specificity is >90% [42]. These data have been confirmed by other studies [43, 44]. Imaging of vascular graft infection by labeled WBCs suffers from a chance of false-positive results caused by the inability to exactly localize the site of inflammation and a lower sensitivity than PET [41, 45]. Therefore, PET-CT imaging seems to have the highest sensitivity. PET should be performed at least 2 months after surgery to avoid false-positive results. Because the majority of vascular graft infections is diagnosed at a later time point after surgery, this limitation plays a minor role [41] (Fig. 6).
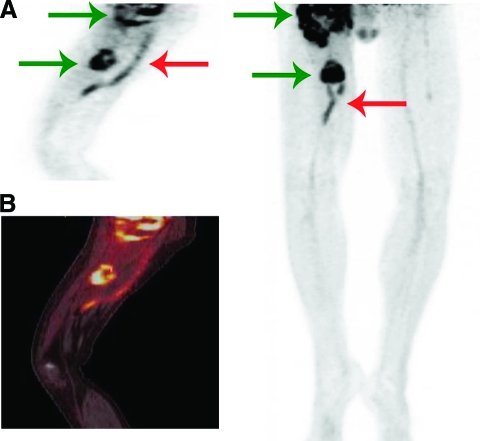
Figure 6.
A 49-year-old man was diagnosed with an extensive relapse of myxofibrosarcoma of the right hip and upper leg. One year earlier, a myxofibrosarcoma of the upper right leg was resected in which a femoropopliteal graft needed to be implanted. He was admitted with fever and cellulitis of the tissue overlying the vascular graft. Pseudomonas aeruginosa was cultured. (A): PET. (B): Fused PET-CT. Left panels show sagittal view, right panel shows maximum intensity projection. Extensive tumor burden (green arrows) and infected vascular graft (red arrow) are seen. He was treated with ciprofloxacin until his death 3 months later.
HIV Patients
The incidence of cancer in patients suffering from HIV/AIDS appears to be rising as a result of longer survival times since the implementation of highly active antiretroviral therapy. Patients with HIV infection are at risk for developing a variety of infections and tumors that may generate a fever, without any localizing features, or nonspecific weight loss. Imaging is often needed to establish a diagnosis. Although Kaposi's sarcoma usually presents with skin lesions, systemic involvement is possible, particularly in the gastrointestinal tract and respiratory system, in which case systemic treatment is warranted to prevent potentially lethal hemorrhage. O'Doherty et al. [46] studied the value of FDG-PET in 80 HIV+ patients with fever, confusion, and/or weight loss without a clinical diagnosis. A half-body FDG-PET scan (n = 57) had a sensitivity of 92% and a specificity of 94% for localization of focal pathology that needed treatment (three patients with pulmonary Kaposi's sarcoma, 13 with lymphoma, eight with various infectious diseases). FDG brain studies (n = 23) were abnormal in all 19 patients with focal space–occupying lesions identified by MRI (six patients with cerebral lymphoma, 13 with various infectious diseases). FDG-PET scans were also abnormal in patients with persistent HIV-related lymphadenopathy without infection or malignancy. In another small retrospective study, nine of 10 FDG-PET–CT scans in HIV+ patients with persistent fever were abnormal [47]. Tuberculosis was diagnosed in six patients and a neoplasm was diagnosed in three (two lymphomas, one Kaposi's sarcoma). FDG-PET–CT directly suggested sites for biopsy in six patients. Unfortunately, no pattern of uptake or cutoff SUV exists in discriminating malignancy from infected nodes in HIV patients [48]. In a small, prospective study, FDG-PET–CT was proven to be effective in detecting clinically occult Kaposi's sarcoma lesions [49].
Conclusions
67Ga-citrate has many unfavorable characteristics, and the development of newer radiopharmaceuticals has resulted in the replacement of 67Ga-citrate scintigraphy with scintigraphy using labeled leukocytes or FDG-PET for the majority of conditions. 68Ga-citrate PET-CT solves some of the drawbacks of 67Ga-citrate, but it cannot differentiate between infection and malignancy either. The sensitivity of labeled leukocyte scintigraphy in non-neutropenic cancer patients is comparable with that in patients without malignancy. The specificity, however, is lower because of uptake of labeled leukocytes in many primary tumors and metastases. In addition, labeled leukocyte scintigraphy cannot be used in patients with febrile neutropenia because of the inability to harvest enough peripheral leukocytes for in vitro labeling.
FDG-PET has several advantages over conventional scintigraphic techniques, but a major drawback is its inability to differentiate between infection and malignancy. When the localization of metastases is known beforehand, this problem is, of course, reduced. FDG-PET has shown its usefulness in diagnosing septic thrombophlebitis in cancer patients. It has also been shown that imaging of infectious processes using FDG-PET is possible in patients with severe neutropenia. This can probably be explained by FDG uptake in many activated inflammatory cells, not only neutrophils but also macrophages, natural killer cells, and lymphocytes. FDG-PET is also useful in patients suspected of infection of metallic implants or vascular grafts. In patients with high-risk Gram+ bacteremia, FDG-PET should always be performed to exclude metastatic infection. It should also be considered in patients suspected of disseminated infection after bacteremia with other microorganisms. Although larger prospective studies examining the value of FDG-PET in cancer patients suspected of infection, especially in those with febrile neutropenia, are needed, FDG-PET appears to be the most promising scintigraphic technique for the diagnosis of infection in this patient group.
Author Contributions
Conception/Design: Chantal P. Bleeker-Rovers, Winette T.A. van der Graaf, Wim J.G. Oyen
Provision of study material or patients: Chantal P. Bleeker-Rovers, Fidel J. Vos, Winette T.A. van der Graaf
Collection and/or assembly of data: Chantal P. Bleeker-Rovers, Fidel J. Vos, Winette T.A. van der Graaf, Wim J.G. Oyen
Data analysis and interpretation: Chantal P. Bleeker-Rovers, Fidel J. Vos, Winette T.A. van der Graaf, Wim J.G. Oyen
Manuscript writing: Chantal P. Bleeker-Rovers, Wim J.G. Oyen
Final approval of manuscript: Chantal P. Bleeker-Rovers, Fidel J. Vos, Winette T.A. van der Graaf, Wim J.G. Oyen
References
- 1.Palestro CJ. The current role of gallium imaging in infection. Semin Nucl Med. 1994;24:128–141. doi: 10.1016/s0001-2998(05)80227-2. [DOI] [PubMed] [Google Scholar]
- 2.Bartold SP, Donohoe KJ, Fletcher JW, et al. Procedure guideline for gallium scintigraphy in the evaluation of malignant disease. Society of Nuclear Medicine. J Nucl Med. 1997;38:990–994. [PubMed] [Google Scholar]
- 3.Perkins PJ. Early gallium-67 abdominal imaging: Pitfalls due to bowel activity. AJR Am J Roentgenol. 1981;136:1016–1017. doi: 10.2214/ajr.136.5.1016. [DOI] [PubMed] [Google Scholar]
- 4.Bekerman C, Hoffer PB, Bitran JD. The role of gallium-67 in the clinical evaluation of cancer. Semin Nucl Med. 1984;14:296–323. doi: 10.1016/s0001-2998(84)80005-7. [DOI] [PubMed] [Google Scholar]
- 5.Nanni C, Errani C, Boriani L, et al. 68Ga-citrate PET/CT for evaluating patients with infections of the bone: Preliminary results. J Nucl Med. 2010;51:1932–1936. doi: 10.2967/jnumed.110.080184. [DOI] [PubMed] [Google Scholar]
- 6.Peters AM. The utility of [99mTc]HMPAO-leukocytes for imaging infection. Semin Nucl Med. 1994;24:110–127. doi: 10.1016/s0001-2998(05)80226-0. [DOI] [PubMed] [Google Scholar]
- 7.Datz FL, Thorne DA. Effect of chronicity of infection on the sensitivity of the In-111-labeled leukocyte scan. AJR Am J Roentgenol. 1986;147:809–812. doi: 10.2214/ajr.147.4.809. [DOI] [PubMed] [Google Scholar]
- 8.Weissman AF, Dayanikli F, Schlesinger AE, et al. Indium-111 leukocyte uptake in a patient with osseous metastatic Ewing's sarcoma. Clin Nucl Med. 1993;18:1063–1066. doi: 10.1097/00003072-199312000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Staudinger KM, Oates E, Naber SP. In-111 labeled leukocyte uptake in metastatic melanoma. Clin Nucl Med. 1989;14:626. doi: 10.1097/00003072-198908000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Becker W, Schaffer R, Börner W. Sigmoid carcinoma mimicking an intra-abdominal abscess in an 111In-labeled white blood cell scan. Eur J Nucl Med. 1985;11:283–284. doi: 10.1007/BF00279085. [DOI] [PubMed] [Google Scholar]
- 11.Schell-Frederick E, Fruhling J, Van der Auwera P, et al. 111Indium-oxine-labeled leukocytes in the diagnosis of localized infection in patients with neoplastic disease. Cancer. 1984;54:817–824. doi: 10.1002/1097-0142(19840901)54:5<817::aid-cncr2820540510>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Fortner A, Datz FL, Taylor A, Jr, et al. Uptake of 111In-labeled leukocytes by tumor. AJR Am J Roentgenol. 1986;146:621–625. doi: 10.2214/ajr.146.3.621. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt KG, Rasmussen JW, Wedebye IM, et al. Accumulation of indium-111-labeled granulocytes in malignant tumors. J Nucl Med. 1988;29:479–484. [PubMed] [Google Scholar]
- 14.Lamki LM, Kasi LP, Haynie TP. Localization of indium-111 leukocytes in noninfected neoplasms. J Nucl Med. 1988;29:1921–1926. [PubMed] [Google Scholar]
- 15.AL-Nahhas AM, Hjiyiannakis P, Hammersley PA, et al. Uptake of technetium-99m hexamethylpropylene amine oxime labelled white cells in lymph nodes involved in non-Hodgkin's lymphoma. Eur J Nucl Med. 1995;22:1453–1456. doi: 10.1007/BF01791154. [DOI] [PubMed] [Google Scholar]
- 16.Lantto E, Järvi K, Lantto T, et al. Accumulation of leucocytes labelled with technetium-99m hexamethylpropylene amine oxime in malignant abdominal tumours. Eur J Nucl Med. 1991;18:824–828. doi: 10.1007/BF00175062. [DOI] [PubMed] [Google Scholar]
- 17.Yamada S, Kubota K, Kubota R, et al. High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J Nucl Med. 1995;36:1301–1306. [PubMed] [Google Scholar]
- 18.Sugawara Y, Gutowski TD, Fisher SJ, et al. Uptake of positron emission tomography tracers in experimental bacterial infections: A comparative biodistribution study of radiolabeled FDG, thymidine, L-methionine, 67Ga-citrate, and 125I-HSA. Eur J Nucl Med. 1999;26:333–341. doi: 10.1007/s002590050395. [DOI] [PubMed] [Google Scholar]
- 19.De Winter F, Vogelaers D, Gemmel F, et al. Promising role of 18-F-fluoro-D-deoxyglucose positron emission tomography in clinical infectious diseases. Eur J Clin Microbiol Infect Dis. 2002;21:247–257. doi: 10.1007/s10096-002-0708-2. [DOI] [PubMed] [Google Scholar]
- 20.de Groot M, Meeuwis AP, Kok PJ, et al. Influence of blood glucose level, age and fasting period on non-pathological FDG uptake in heart and gut. Eur J Nucl Med Mol Imaging. 2005;32:98–101. doi: 10.1007/s00259-004-1670-2. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum SJ, Lind T, Antoch G, et al. False-positive FDG PET uptake—the role of PET/CT. Eur Radiol. 2006;16:1054–1065. doi: 10.1007/s00330-005-0088-y. [DOI] [PubMed] [Google Scholar]
- 22.Bryant AS, Cerfolio RJ. The maximum standardized uptake values on integrated FDG-PET/CT is useful in differentiating benign from malignant pulmonary nodules. Ann Thorac Surg. 2006;82:1016–1020. doi: 10.1016/j.athoracsur.2006.03.095. [DOI] [PubMed] [Google Scholar]
- 23.Sahlmann CO, Siefker U, Lehmann K, et al. Dual time point 2-[18F]fluoro-2′-deoxyglucose positron emission tomography in chronic bacterial osteomyelitis. Nucl Med Commun. 2004;25:819–823. doi: 10.1097/01.mnm.0000135600.23896.9d. [DOI] [PubMed] [Google Scholar]
- 24.Gaytàn-Martínez J, Mateos-García E, Sanchez-Cortés E, et al. Microbiological findings in febrile neutropenia. Arch Med Res. 2000;31:388–392. doi: 10.1016/s0188-4409(00)00080-1. [DOI] [PubMed] [Google Scholar]
- 25.Roongpoovapatr P, Suankratay C. Causative pathogens of fever in neutropenic patients at King Chulalongkorn Memorial Hospital. J Med Assoc Thai. 2010;93:776–783. [PubMed] [Google Scholar]
- 26.Paul M, Yahav D, Fraser A, et al. Empirical antibiotic monotherapy for febrile neutropenia: Systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2006;57:176–189. doi: 10.1093/jac/dki448. [DOI] [PubMed] [Google Scholar]
- 27.Zhuang H, Alavi A. 18-fluorodeoxyglucose positron emission tomographic imaging in the detection and monitoring of infection and inflammation. Semin Nucl Med. 2002;32:47–59. doi: 10.1053/snuc.2002.29278. [DOI] [PubMed] [Google Scholar]
- 28.Ho AY, Pagliuca A, Maisey MN, et al. Positron emission scanning with 18-FDG in the diagnosis of deep fungal infections. Br J Haematol. 1998;101:392–393. doi: 10.1046/j.1365-2141.1998.0738e.x. [DOI] [PubMed] [Google Scholar]
- 29.Mahfouz T, Miceli MH, Saghafifar F, et al. 18F-fluorodeoxyglucose positron emission tomography contributes to the diagnosis and management of infections in patients with multiple myeloma: A study of 165 infectious episodes. J Clin Oncol. 2005;23:7857–7863. doi: 10.1200/JCO.2004.00.8581. [DOI] [PubMed] [Google Scholar]
- 30.Kubota R, Yamada S, Kubota K, et al. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: High accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–1980. [PubMed] [Google Scholar]
- 31.Brown RS, Leung JY, Fisher SJ, et al. Intratumoral distribution of tritiated fluorodeoxyglucose in breast carcinoma: I. Are inflammatory cells important? J Nucl Med. 1995;36:1854–1861. [PubMed] [Google Scholar]
- 32.Brewer S, McPherson M, Fujiwara D, et al. Molecular imaging of murine intestinal inflammation with 2-deoxy-2-[18F]fluoro-D-glucose and positron emission tomography. Gastroenterology. 2008;135:744–755. doi: 10.1053/j.gastro.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meller J, Sahlmann CO, Lehmann K, et al. [F-18-FDG hybrid camera PET in patients with postoperative fever] Nuklearmedizin. 2002;41:22–29. [PubMed] [Google Scholar]
- 34.van der Bruggen W, Bleeker-Rovers CP, Boerman OC, et al. PET and SPECT in osteomyelitis and prosthetic bone and joint infections: A systematic review. Semin Nucl Med. 2010;40:3–15. doi: 10.1053/j.semnuclmed.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Zhuang H, Chacko TK, Hickeson M, et al. Persistent non-specific FDG uptake on PET imaging following hip arthroplasty. Eur J Nucl Med Mol Imaging. 2002;29:1328–1333. doi: 10.1007/s00259-002-0886-2. [DOI] [PubMed] [Google Scholar]
- 36.Chacko TK, Zhuang H, Nakhoda KZ, et al. Applications of fluorodeoxyglucose positron emission tomography in the diagnosis of infection. Nucl Med Commun. 2003;24:615–624. doi: 10.1097/00006231-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 37.de Winter F, van de Wiele C, Vogelaers D, et al. Fluorine-18 fluorodeoxyglucose-position emission tomography: A highly accurate imaging modality for the diagnosis of chronic musculoskeletal infections. J Bone Joint Surg Am. 2001;83:651–660. doi: 10.2106/00004623-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Schiesser M, Stumpe KD, Trentz O, et al. Detection of metallic implant-associated infections with FDG PET in patients with trauma: Correlation with microbiologic results. Radiology. 2003;226:391–398. doi: 10.1148/radiol.2262011939. [DOI] [PubMed] [Google Scholar]
- 39.Bleeker-Rovers CP, Vos FJ, Wanten GJ, et al. 18F-FDG PET in detecting metastatic infectious disease. J Nucl Med. 2005;46:2014–2019. [PubMed] [Google Scholar]
- 40.Vos FJ, Bleeker-Rovers CP, Sturm PD, et al. 18F-FDG PET/CT for detection of metastatic infection in gram-positive bacteremia. J Nucl Med. 2010;51:1234–1240. doi: 10.2967/jnumed.109.072371. [DOI] [PubMed] [Google Scholar]
- 41.Keidar Z, Nitecki S. FDG-PET for the detection of infected vascular grafts. Q J Nucl Med Mol Imaging. 2009;53:35–40. [PubMed] [Google Scholar]
- 42.Fukuchi K, Ishida Y, Higashi M, et al. Detection of aortic graft infection by fluorodeoxyglucose positron emission tomography: Comparison with computed tomographic findings. J Vasc Surg. 2005;42:919–925. doi: 10.1016/j.jvs.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 43.Keidar Z, Engel A, Hoffman A, et al. Prosthetic vascular graft infection: The role of 18F-FDG PET/CT. J Nucl Med. 2007;48:1230–1236. doi: 10.2967/jnumed.107.040253. [DOI] [PubMed] [Google Scholar]
- 44.Spacek M, Belohlavek O, Votrubova J, et al. Diagnostics of “non-acute” vascular prosthesis infection using 18F-FDG PET/CT: Our experience with 96 prostheses. Eur J Nucl Med Mol Imaging. 2009;36:850–858. doi: 10.1007/s00259-008-1002-z. [DOI] [PubMed] [Google Scholar]
- 45.Gardet E, Addas R, Monteil J, et al. Comparison of detection of F-18 fluorodeoxyglucose positron emission tomography and 99mTc-hexamethyl-propylene amine oxime labelled leukocyte scintigraphy for an aortic graft infection. Interact Cardiovasc Thorac Surg. 2010;10:142–143. doi: 10.1510/icvts.2009.215707. [DOI] [PubMed] [Google Scholar]
- 46.O'Doherty MJ, Barrington SF, Campbell M, et al. PET scanning and the human immunodeficiency virus-positive patient. J Nucl Med. 1997;38:1575–1583. [PubMed] [Google Scholar]
- 47.Castaigne C, Tondeur M, de Wit S, et al. Clinical value of FDG-PET/CT for the diagnosis of human immunodeficiency virus-associated fever of unknown origin: A retrospective study. Nucl Med Commun. 2009;30:41–47. doi: 10.1097/mnm.0b013e328310b38d. [DOI] [PubMed] [Google Scholar]
- 48.Sathekge M, Maes A, Kgomo M, et al. FDG uptake in lymph-nodes of HIV+ and tuberculosis patients: Implications for cancer staging. Q J Nucl Med Mol Imaging. 2010;54:698–703. [PubMed] [Google Scholar]
- 49.van de Luijtgaarden A, van der Ven A, Leenders W, et al. Imaging of HIV-associated Kaposi sarcoma; F-18-FDG-PET/CT and In-111-bevacizumab scintigraphy. J Acquir Immune Defic Syndr. 2010;54:444–446. doi: 10.1097/QAI.0b013e3181cdf61f. [DOI] [PubMed] [Google Scholar]