Invasive aspergillosis (IA) continues to be a leading cause of morbidity and mortality in hematologic malignancy (HM) patients. In HM patients, persistent neutropenia and the need for intensive care are associated with failure to respond to antifungal therapy. Use of novel antimold azoles, either as primary or salvage therapy, improves the overall outcome and IA-attributable death of HM patients with IA.
Keywords: Invasive aspergillosis, Hematologic malignancy, Outcome, Azole, Prognostic factor
Abstract
Background.
Invasive aspergillosis (IA) continues to be a leading cause of morbidity and mortality in hematologic malignancy (HM) patients. We evaluated the prognostic factors for IA in HM patients.
Methods.
In this retrospective study, we included all HM patients diagnosed with proven or probable IA between June 1993 and June 2008.
Results.
A total of 449 HM patients were analyzed, the majority of which (75%) had underlying leukemia. Multivariate logistic regression analysis showed that neutropenia for more than two weeks during IA, steroid use, and intensive care admission were independently associated with failure to respond to antifungal therapy, as well as increased IA-attributable mortality (all p-values < .01). Antifungal therapy with an antimold azole-containing regimen (voriconazole or posaconazole) was also independently associated with improved response to treatment, as well as decreased IA-attributable mortality (all p-values < .0001). Survival analysis showed that primary or salvage therapy with a regimen that contained antimold azoles was significantly associated with improved survival (p < .001).
Conclusions.
In HM patients, persistent neutropenia and the need for intensive care are associated with failure to respond to antifungal therapy. Use of novel antimold azoles, either as primary or salvage therapy, improves the overall outcome and IA-attributable death of HM patients with IA.
Introduction
Hematologic malignancy patients are at significantly increased risk of invasive fungal infections for multiple reasons, particularly the use of cytotoxic agents that lead to prolonged neutropenia and dysfunctional cell-mediated immunity [1]. New clinical practices for these immunocompromised patients have significantly changed the epidemiologic characteristics of infectious complications, including invasive fungal infections [2, 3]. However, invasive aspergillosis (IA), one of the most common invasive fungal infections in hematologic malignancy patients, continues to be associated with significant morbidity and mortality, particularly in patients who have undergone hematologic stem cell transplantation (HSCT) [2, 4–6].
Previous studies have identified prognostic factors for mortality among patients with IA, including the severity and dissemination of aspergillosis and degree of host immunosuppression [5–7]. However, most of these findings were from studies of selected HSCT patients and may not be applicable to hematologic malignancy patients (leukemia and lymphoma patients) who have not undergone HSCT because these patients have different host characteristics. Identifying prognostic factors for adverse therapeutic outcomes and mortality could allow us to implement new and intensive strategies in selected high-risk patients. Therefore, in this study, we evaluated the prognostic factors for overall and IA-attributable death as well as treatment response among hematologic malignancy patients (most of whom did not undergo HSCT) who had been diagnosed with IA between 1993 and 2008 at our tertiary cancer center. Furthermore, we assessed the response to various classes of antifungal drugs, given as primary and salvage therapy and the impact of these agents, on overall and IA-attributable mortality.
Patients and Methods
Patient Population
We conducted a retrospective chart review to identify all hematological malignancy patients with documented proven or probable IA diagnosed between June 1993 and June 2008 at The University of Texas MD Anderson Cancer Center (Houston, Texas). IA cases were selected from a database. Patient data had been prospectively collected by institutional infection control personnel. The institutional review board of MD Anderson Cancer Center approved this study. A patient waiver of informed consent was obtained for this study. Standardized forms were used to collect epidemiologic and clinical information for each IA patient. We also collected information pertaining to HSCT within one year prior to IA diagnosis or during treatment and recorded the Aspergillus species and antifungal therapy used.
Definitions
Proven and probable IA were defined according to the European Organization for Research and Treatment of Cancer and Mycoses Study Group criteria [8]. In brief, proven IA was characterized by documented histopathologic and microbiologic evidence of Aspergillus spp. infection on autopsy or biopsy tissue samples or culture samples from a normally sterile site. Probable IA was characterized by the presence of radiologic and microbiologic features supporting an IA diagnosis (the presence of nodules or cavities or a halo or air-crescent sign on chest x-ray or computerized tomography and documentation of Aspergillus spp. infection by direct microscopy or culture) in a patient with an absolute neutrophil count of <500 cells/mm3, who had undergone prolonged steroid therapy, or who had used cytotoxic agents. The day of IA diagnosis was defined as the day on which the first microbiologic or histopathologic evidence of IA was collected. Persistent neutropenia was defined as an absolute neutrophil count of <500 cells/mm3 that lasted for at least two weeks during IA therapy.
Primary antifungal therapy was defined as the first antifungal regimen (single agent or combination) that was administered for at least 7 consecutive days. Salvage therapy was defined as any other regimen administered after primary antifungal therapy.
The therapeutic outcome was categorized as a favorable response or failure to respond. A favorable response was defined as a complete or partial clinical, radiologic, or microbiologic resolution of IA. Failure to respond was characterized as progressive or unchanged clinical or radiologic parameters compared with the baseline. For analysis purposes, antifungal therapy regimens were classified as lipid amphotericin B, echinocandin, or azole containing. An echinocandin-containing regimen included caspofungin, micafungin, or anidulafungin. An azole-containing regimen included posaconazole or voriconazole.
Prognostic factors were compared with overall and IA-attributable mortality rates. Overall mortality included all deaths that had occurred within 12 weeks after the initiation of antifungal therapy. IA-attributable death was defined as death in a patient with documented radiographic, microbiologic, or histologic findings suggestive of active IA antemortem or postmortem at the time of death associated with no sustained favorable response to treatment.
Statistical Analysis
Categorical variables were compared by chi-square or Fisher exact tests and continuous variables compared by Wilcoxon rank-sum tests. Multiple logistic regression analysis was used to identify the independent prognostic factors for the outcomes.
First, univariate analyses were performed to evaluate the predictive effect of each factor alone. Then all factors whose univariate test had a p-value < .25 were included in a full multiple logistic regression model. Finally, the full model was reduced to a final model by backward elimination method so that all factors remaining in the model were statistically significant at a significance level of 5%.
In addition, survival analysis was used to assess the effect of azole on IA-attributable mortality as well as on overall mortality. Patients were divided into three groups: using azole in primary therapy, using azole in salvage therapy only, and not using azole in all therapies. For overall mortality, the survival probability was estimated by the Kaplan-Meier method for each group and compared by log-rank tests. Competing risk analysis was performed for IA-attributable mortality. With mortality due to other causes as a competing event, the cumulative incidence curve of IA-attributable mortality was estimated for each group and their equality was compared. All tests were two-sided tests at a significance level of .05. The competing risk analysis was performed using the statistical software R version 2.8.1, and all other statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
Patients' Demographic and Clinical Characteristics
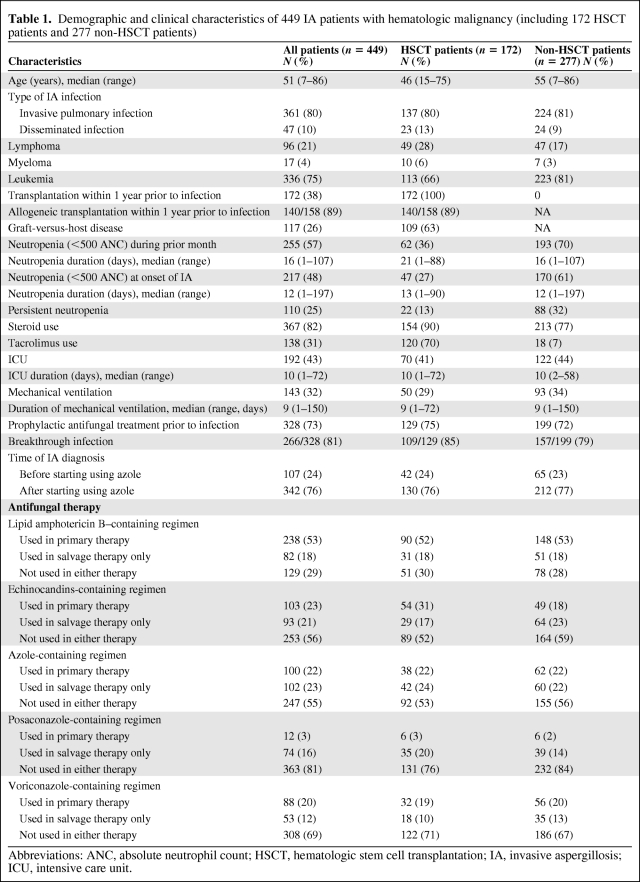
Analyzed in this study were 449 hematologic malignancy patients with proven or probable IA diagnosed between June 1993 and June 2008. Patients' epidemiologic and clinical characteristics are shown in Table 1. The patients' median age was 51 years (range 7–86 years). Most of the patients had leukemia (336 out of 449; 75%), whereas 21% (96 out of 449) had lymphoma and 4% (17 out of 449) had myeloma. Within 1 year before IA diagnosis or during treatment, 172 patients (38%) had undergone HSCT. One hundred ten patients (25%) had persistent neutropenia. The majority of patients (82%) had been treated with steroids during the month before IA diagnosis or during treatment. In addition, prophylactic antifungal therapy was given in 328 patients (73%), and of them, 266 (81%) had breakthrough infection. One hundred ninety-two patients (43%) were admitted to intensive care unit (ICU) for a median duration of 10 days (range 1–72 days), and 143 patients (32%) received mechanical ventilation for a median duration of 9 days (range 1–150 days). Regarding treatment, lipid amphotericin B-containing regimen was mostly used in 71% of the patients either in primary or salvage therapies or in both, followed by azole-containing regimen in 45% and echinocandins-containing regimen in 44%. Data also show that the 172 patients who received HSCT and the 277 patients who did not receive HSCT were comparable on most characteristics.
Table 1.
Demographic and clinical characteristics of 449 IA patients with hematologic malignancy (including 172 HSCT patients and 277 non-HSCT patients)
Abbreviations: ANC, absolute neutrophil count; HSCT, hematologic stem cell transplantation; IA, invasive aspergillosis; ICU, intensive care unit.
Final Response to Therapy
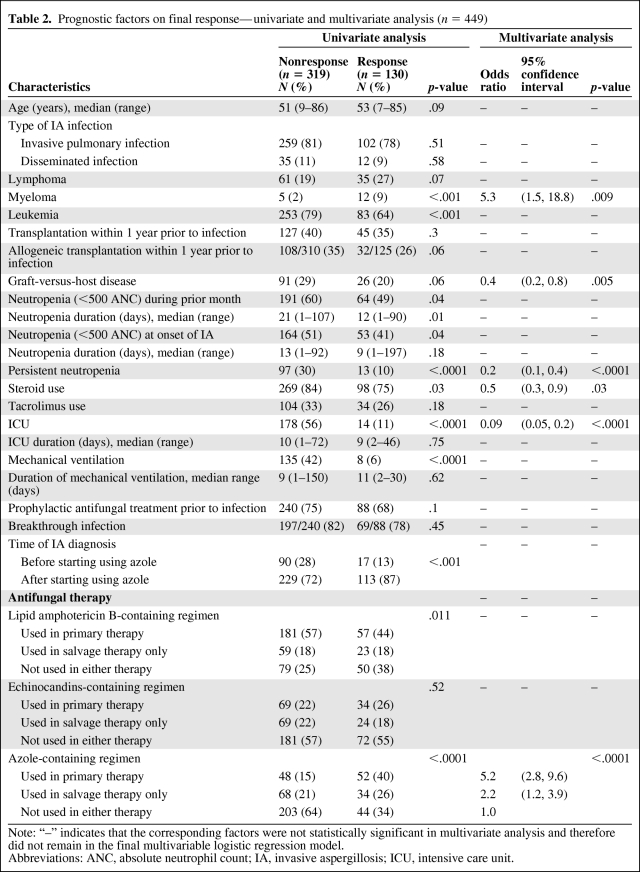
Of the 449 patients, 130 (29%) had final response to their therapy and 319 did not. The potential prognostic factors were evaluated by multivariate analysis as well as univariate analysis (Table 2). The multivariate logistic regression analysis showed that the following factors were independently associated with final response: type of hematologic malignancy, graft-versus-host disease (GVHD), persistent neutropenia, steroids use during the month before IA diagnosis or during treatment, admission to ICU, and azole use in treatment (Table 2). Patients with myeloma were 5.3 times (95% confidence interval [CI], 1.5–18.8) more likely to have final response than those with leukemia or lymphoma (p = .009). Patients with GVHD were 0.4 times (95% CI, 0.2–0.8) less likely to have final response than those without (p = .005). Patients with persistent neutropenia were 0.2 times (95% CI, 0.1–0.4) less likely to have final response than those without (p < .0001). Patients who used steroids were 0.5 times (95% CI, 0.3–0.9) less likely to have final response than those who did not (p = .03). Patients with ICU admission were 0.09 times (95% CI, = 0.05–0.2) less likely to have final response than those without (p < .0001). Compared to patients who never used azole-containing regimen in their therapy, those who used it in primary therapy were 5.2 times (95% CI, 2.8–9.6) more likely to have final response, and those who used it in salvage therapy only were 2.2 times (95% CI, 1.2–3.9) more likely to have final response (p < .0001).
Table 2.
Prognostic factors on final response—univariate and multivariate analysis (n = 449)
Note: “–” indicates that the corresponding factors were not statistically significant in multivariate analysis and therefore did not remain in the final multivariable logistic regression model.
Abbreviations: ANC, absolute neutrophil count; IA, invasive aspergillosis; ICU, intensive care unit.
IA-Attributable Mortality
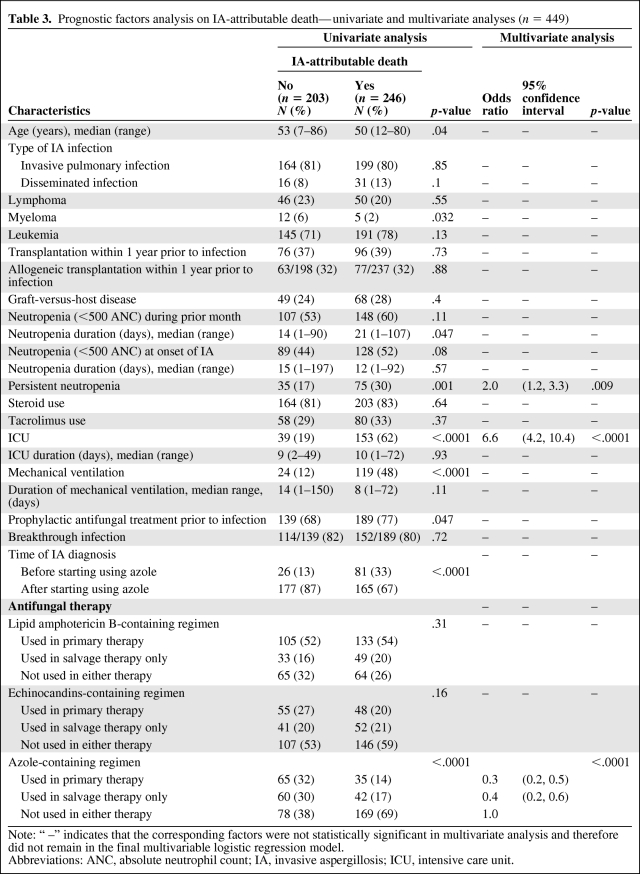
Of the 449 patients, 246 (55%) died of IA-attributable causes. The analysis of the potential prognostic factors by multivariate analysis as well as univariate analysis is shown in Table 3. The multivariate logistic regression analysis showed that 3 factors were independently associated with IA-attributable mortality: persistent neutropenia (OR = 2.0, 95% CI, 1.2–3.3; p = .009), ICU admission (OR = 6.6, 95% CI, 4.2–10.4; p < .0001), and azole use in treatment (primary therapy: OR = 0.3, 95% CI, 0.2–0.5; salvage therapy only: OR = 0.4, 95% CI, 0.2–0.6; p < .0001).
Table 3.
Prognostic factors analysis on IA-attributable death—univariate and multivariate analyses (n = 449)
Note: “ –” indicates that the corresponding factors were not statistically significant in multivariate analysis and therefore did not remain in the final multivariable logistic regression model.
Abbreviations: ANC, absolute neutrophil count; IA, invasive aspergillosis; ICU, intensive care unit.
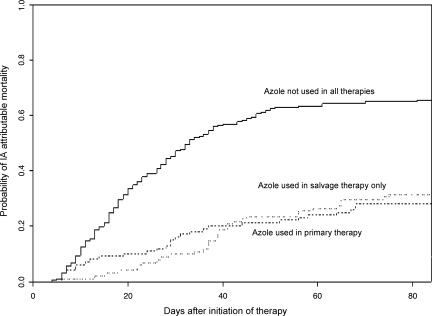
In addition, the effect of azole use on IA-attributable mortality was also evaluated by competing risk analysis using death due to other causes as a competing event (Figure 1). The analysis showed that during the 12 weeks after treatment initiation, the patients without azole use in therapy had significantly higher probability to die due to IA-attributable causes than those who used azole either in primary or in salvage therapy (p < .0001). On the other hand, as Figure 1 shows, there was no significant difference between using azole in primary therapy and in salvage therapy in IA-attributable mortality within 12 weeks after treatment started.
Figure 1.
Estimated cumulative incidence curves of invasive aspergillosis (IA)-attributable mortality for IA patients with different treatment by competing risk analysis with death by other causes as a competing event (p < .0001) (n = 447).
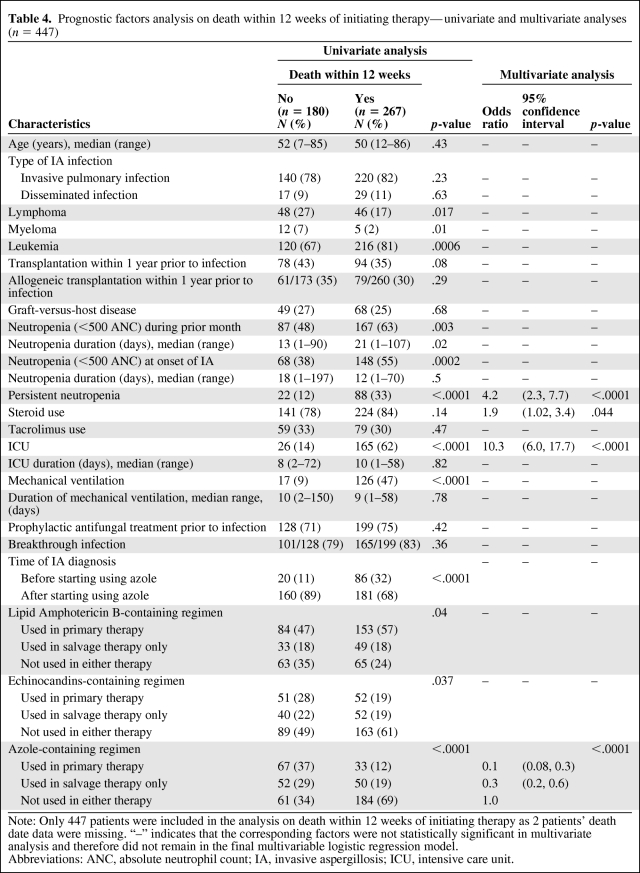
Overall Mortality Within 12 Weeks After Treatment Initiation
Of the 449 patients, 447 were evaluable for mortality within 12 weeks after treatment initiation and, of them, 267 (60%) died. Similar analyses were performed to assess the prognostic factors for it (Table 4). The multivariate logistic regression analysis showed that four factors were independently associated with mortality within 12 weeks after treatment started: persistent neutropenia (OR = 4.2, 95% CI, 2.3–7.7; p < .0001), ICU admission (OR = 10.3, 95% CI, 6.0–17.7; p < .0001), steroids use (OR = 1.9, 95% CI, 1.02–3.4; p = .04), and azole use in treatment (primary therapy: OR = 0.1, 95% CI, 0.08–0.3; salvage therapy only: OR = 0.3, 95% CI, 0.2–0.6; p < .0001).
Table 4.
Prognostic factors analysis on death within 12 weeks of initiating therapy—univariate and multivariate analyses (n = 447)
Note: Only 447 patients were included in the analysis on death within 12 weeks of initiating therapy as 2 patients' death date data were missing. “–” indicates that the corresponding factors were not statistically significant in multivariate analysis and therefore did not remain in the final multivariable logistic regression model.
Abbreviations: ANC, absolute neutrophil count; IA, invasive aspergillosis; ICU, intensive care unit.
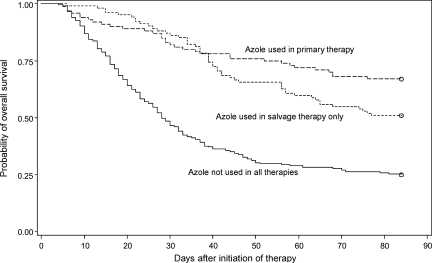
Similarly, we also assessed the effect of azole use on mortality within 12 weeks by survival analysis. Figure 2 shows the Kaplan-Meier survival estimates of the groups with different treatment. The log-rank tests showed that during the 12 weeks after treatment initiation, the patients without azole use in therapy had significantly lower probability to survive than those who used azole either in primary or in salvage therapy (p < .0001).
Figure 2.
Kaplan-Meier survival curves for invasive aspergillosis patients with different treatment (p < .0001 from a log-rank test) (n = 447).
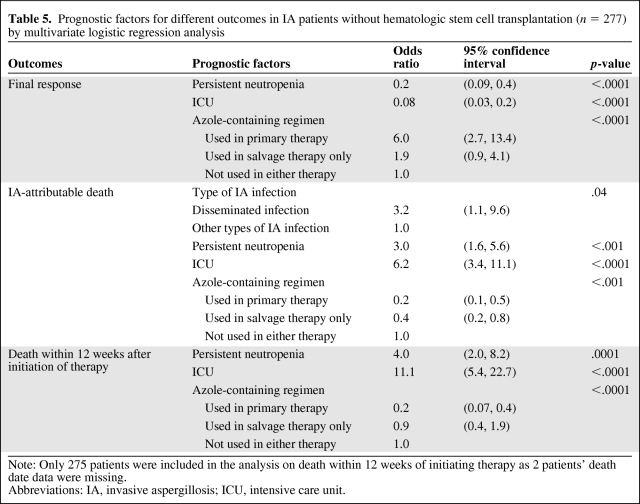
Subset Analyses on Patients Without HSCT
Of the 449 patients, 172 patients (38%) underwent HSCT within 1 year before IA diagnosis or during treatment and 277 (62%) did not. For these 277 patients without HSCT, most (81%) had underlying leukemia. We did similar analyses to evaluate the prognostic factors for final response, IA-attributable mortality, and overall mortality within 12 weeks, respectively, on this subgroup of 277 patients without HSCT (Table 5). As Table 5 shows, three common prognostic factors were independently associated with each of the three outcomes: persistent neutropenia, ICU admission, and azole use in treatment. Similar to the general analyses, the first two factors were associated with unfavorable outcomes, whereas azole use in treatment was associated with favorable outcomes. In addition, one more prognostic factor was associated with IA-attributable mortality—type of IA infection. Data analysis showed that, for patients without HSCT within 1 year, those with disseminated infection were 3.2 times (95% CI, 1.1–9.6) more likely to die due to IA-attributable causes than those with other types of infection (p = .04).
Table 5.
Prognostic factors for different outcomes in IA patients without hematologic stem cell transplantation (n = 277) by multivariate logistic regression analysis
Note: Only 275 patients were included in the analysis on death within 12 weeks of initiating therapy as 2 patients' death date data were missing.
Abbreviations: IA, invasive aspergillosis; ICU, intensive care unit.
Discussion
In our study, we identified prognostic factors for response to antifungal therapy as well as overall and IA-attributable mortality in hematologic malignancy patients with IA who had and had not undergone HSCT. Primary and salvage therapies with voriconazole or posaconazole were independently associated with a favorable therapeutic response and were also independently associated with fewer IA-attributable deaths and overall mortality within 12 weeks after treatment initiation (all p-values < .0001), whereas persistent neutropenia (>2 weeks) and ICU admission during IA were consistently and independently associated with failure to respond to antifungal therapy as well as increased IA mortality and increased death within 12 weeks following initiation of therapy (all p-values < .01). In addition, we would like to point out that the significant effects of voriconazole or posaconazole on the outcomes we found here were independent of the fact that these drugs were just introduced in recent years. Patients treated in recent years may have better outcomes due to new development in diagnosis and treatment (other than using active azoles). In data analysis, we have taken this possible confounding factor into consideration.
Amphotericin B–based therapies have been used for years in the treatment of IA, with different response rates, in a wide range of patient populations [9]. The increased incidence of adverse events related to this drug led to the introduction of less toxic lipid formulations; however, the response rates in hematologic malignancy patients with IA to these newer lipid formulations agents remained poor [10]. Cornely et al. reported on the use of two different doses of liposomal amphotericin B as a primary therapy for invasive aspergillosis. However, they were not able to demonstrate an additional efficacy benefit at a higher dose [11]. Furthermore, in a retrospective study at our institution [12], each of the two available lipid formulations of amphotericin B, used as single agents for primary or salvage antifungal monotherapy, was associated with a poor therapeutic response among hematologic malignancy patients with IA. Consistent with these findings, univariate analysis of our data showed that primary and salvage therapies with lipid formulation-containing regimens were associated with a lower likelihood of a favorable response as well as higher overall mortality within 12 weeks of therapy initiation.
Given the suboptimal therapeutic outcomes reported for amphotericin B and its lipid formulations, particularly in the immunocompromised hosts, new antifungal agents and classes have become available for IA treatment [6, 13]. Herbrecht et al. [13] reported that primary therapy with voriconazole, a broad-spectrum triazole, “led to a better response and improved survival” than did amphotericin B deoxycholate. Similarly, Upton et al. [6] showed that voriconazole was associated with a lower IA-related death rate among HSCT patients, after excluding patients with severe organ involvement. Another study conducted at our center in hematologic malignancy patients demonstrated that posaconazole salvage therapy was significantly more effective against IA than was high-dose lipid amphotericin formulations. In addition, this agent was associated with a significantly lower IA-attributable and overall mortality [14].
Voriconazole was recommended by the Infectious Disease Society of America as first-line primary antifungal therapy for IA because of its proven superiority [15]. In this current study, both primary and salvage therapies containing antimold azoles (voriconazole or posaconazole) were associated with a favorable therapeutic response, a lower IA-attributable death rate, and lower 12-week mortality rates. In addition, subgroup multivariate analysis of hematologic malignancy patients who did not undergo HSCT (277 patients or 62% of the total) revealed a significant association between azole-containing regimens and favorable outcomes. Therefore, novel antimold azoles (voriconazole or posaconazole) should be considered as first-line primary and secondary therapy for IA patients with hematologic malignancy independent of HSCT.
For patients at high risk of IA, such as those with neutropenia and acute myelogenous leukemia and those with GVHD after HSCT, the Infectious Disease Society of America recommends the use of antifungal prophylaxis. Fluconazole was the major antifungal prophylaxis agent used prior to the introduction of posaconazole and voriconazole. On the basis of the results of in vitro and clinical studies, posaconazole has become the preferred first-line prophylactic therapy for these patients [16–19]. However, in the absence of an intravenous formulation, its bioavailability may be limited and is a challenge in high-risk patients. Despite the introduction of posaconazole, voriconazole continues to be commonly used as a prophylactic agent in this high-risk patient population, despite its not being approved by the Food and Drug Administration for prophylactic use. However, the widespread prophylactic use of these novel antimold azoles could lead to the emergence and selection of resistant molds and, hence, diminish their effectiveness as frontline therapeutic agents. Multiple studies have recently reported triazole resistance and triazole cross-resistance among Aspergillus spp. isolates [20–22]. Furthermore, the wide use of voriconazole as a prophylactic agent at our center was associated with an increase in invasive zygomycosis [23].
Consistent with the findings of other investigators [9, 24], our data showed that neutropenia, steroid use, GHVD, and ICU stay during IA were associated with failure to respond to antifungal therapy. Host factors such as the severity and duration of neutropenia and the presence of a dysfunctional cytotoxic immune response, conditioned by the use of steroids and chemotherapy agents, are critical factors that are directly correlated with a predisposition to opportunistic fungal infections such as IA and contribute to patients' outcomes and responses to treatment [5, 6, 25].
Upton et al. [6] reported that steroid use, elevated creatinine and bilirubin levels, and disseminated IA were independently related to IA-attributable death in a cohort of HSCT patients. In a subgroup analysis that excluded patients with severe organ dysfunction, these authors also found that persistent neutropenia was a risk factor for IA-related death. Similarly, in two studies of HSCT patients, the cumulative steroid dose and the presence of active GVHD were significantly associated with IA-related death [5, 25].
The Italian registry studies by Pagano et al. (2001 and 2010) had similar conclusions consistent with our finding that the use of glucocorticoids, recovery from neutropenia, and disease stage are crucial prognostic factors. Another point was addressed by Pagano and colleagues that advances in the diagnosis and the availability of the newer antifungal agents for the treatment of invasive aspergillosis in patients with hematologic malignancies have contributed to reducing fungus-related mortality [26, 27].
We found that persistent neutropenia during IA was a prognostic factor for IA-attributable death in hematologic malignancy patients who had and had not undergone HSCT. Similarly, ICU stay during IA was also strongly associated with IA-attributable and overall mortality.
This study has several limitations. First, our findings are limited to a single institution. Second, this study is retrospective in nature, and finally, low autopsy rate for the confirmation of IA was part of the difficulty in establishing a proven diagnosis.
In summary, persistent neutropenia and clinical deterioration that requires intensive care were consistently and independently associated with failure to respond to antifungal therapy and increased IA-attributable as well as overall death. The use of antimold azole agents (voriconazole or posaconazole) in IA treatment as primary or salvage therapy independently improved the overall clinical outcome in hematologic malignancy patients and particularly reduced the rate of IA-attributable death as well as overall mortality. These agents should be considered as the first-line therapeutic drugs in the treatment of IA in patients with hematologic malignancy, including those who did not undergo HSCT. Further studies are needed to reach a definite conclusion about the cost effectiveness and safety of new azole-containing regimens for IA, used in combination with other agents such as the echinocandins.
Summary
This study represents one of the largest outcome analyses on hematologic malignancy patients (particularly those without hematopoietic stem cell transplant) with invasive aspergillosis. The findings are compelling, novel, and unique in that they consistently and independently demonstrate an improved outcome, consisting of an improved response to therapy with decreased IA-attributable mortality and overall death associated with the use of novel antimold triazoles (i.e., voriconazole or posaconazole).
Worldwide, the current practice is to continue to use amphotericin B-based regimen (including lipid formulations of amphotericin B) in the primary and salvage therapy of IA. Our study shows that amphotericin B regimens on their own could be associated with a poor outcome, whereas the novel antimold azoles (voriconazole and posaconazole) have a definite improved outcome if used during primary and salvage therapy.
Acknowledgments
This study was funded by grants from Astellas and Enzon.
Author Contributions
Conception/Design: Dimitrios P. Kontoyiannis, Issam Raad
Provision of study material or patients: Issam Raad
Collection and/or assembly of data: Ray Hachem, Christelle Kassis
Data analysis and interpretation: Ying Jiang
Manuscript writing: Elizabeth R. Ramos
Final approval of manuscript: Issam Raad
References
- 1.Wingard JR. Fungal infections after bone marrow transplant. Biol Blood Marrow Transplant. 1999;5(2):55–68. doi: 10.1053/bbmt.1999.v5.pm10371357. [DOI] [PubMed] [Google Scholar]
- 2.Marr K, Carter R, Boeckh M, et al. Invasive aspergillosis in allogeneic stem cell transplants recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Vidal K, Upton A, Kirby K, et al. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041–1050. doi: 10.1086/591969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marr K, Carter R, Crippa F, et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 5.Ribaud P, Chastang C, Latgé JP, et al. Survival and prognostic factors of invasive aspergillosis after allogeneic bone marrow transplantation. Clin Infect Dis. 1999;28:322–330. doi: 10.1086/515116. [DOI] [PubMed] [Google Scholar]
- 6.Upton A, Kirby K, Carpenter P, et al. Invasive aspergillosis following hematopoietic transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 7.Chamilos G, Luna M, Lewis RE, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003) Haematologica. 2006;91:986–989. [PubMed] [Google Scholar]
- 8.Ascioglu S, Rex JH, de Pauw B, et al. Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer; Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 9.Denning D. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 10.Walsh TJ, Hiemenz JW, Seibel NL, et al. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis. 1998;26:1383–1396. doi: 10.1086/516353. [DOI] [PubMed] [Google Scholar]
- 11.Cornely OA, Maertens J, Bresnik M, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial) Clin Infect Dis. 2007;44:1289–1297. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- 12.Hachem RY, Boktour MR, Hanna HA, et al. Amphotericin B lipid complex versus liposomal amphotericin B monotherapy for invasive aspergillosis in patients with hematologic malignancy. Cancer. 2008;112:1282–1287. doi: 10.1002/cncr.23311. [DOI] [PubMed] [Google Scholar]
- 13.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 14.Raad II, Hanna HA, Boktour M, et al. Novel antifungal agents as salvage therapy for invasive aspergillosis in patients with hematologic malignancies: posaconazole compared with high-dose lipid formulations of amphotericin B alone or in combination with caspofungin. Leukemia. 2008;22:496–503. doi: 10.1038/sj.leu.2405065. [DOI] [PubMed] [Google Scholar]
- 15.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 16.Kirkpatrick WR, McAtee RK, Fothergill AW, et al. Efficacy of SCH56592 in a rabbit model of invasive aspergillosis. Antimicrob Agents Chemother. 2000;44:780–782. doi: 10.1128/aac.44.3.780-782.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin infect Dis. 2007;44:2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 18.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 19.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;56:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 20.Baddley JW, Marr KA, Andes DR, et al. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the Transplant-Associated Infection Surveillance Network. J Clin Microbiol. 2009;47:3271–3275. doi: 10.1128/JCM.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snelders E, van der Lee HA, Kuijpers J, et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PloS Med. 2008;5:e219. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamaris GA, Ben-Ami R, Lewis RE, et al. Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J Infect Dis. 2009;199:1399–1406. doi: 10.1086/597615. [DOI] [PubMed] [Google Scholar]
- 23.Kontoyiannis DP, Lionakis MS, Lewis RE, et al. Zygomycosis in a tertiary-care center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191:1350–1360. doi: 10.1086/428780. [DOI] [PubMed] [Google Scholar]
- 24.Gallien S, Fournier S, Porcher R, et al. Therapeutic outcome and prognostic factors of invasive aspergillosis in an infectious disease department. A review of 34 cases. Infection. 2008;36:533–538. doi: 10.1007/s15010-008-7375-x. [DOI] [PubMed] [Google Scholar]
- 25.Cordonnier C, Ribaud P, Herbrecht R, et al. Prognostic factors for death due to invasive aspergillosis after hematopoietic stem cell transplantation: a 1-year retrospective study of consecutive patients at French transplantation centers. Clin Infect Dis. 2006;42:955–963. doi: 10.1086/500934. [DOI] [PubMed] [Google Scholar]
- 26.Pagano L, Girmenia C, Mele L, et al. Infections caused by filamentous fungi in patients with hematologic malignancies. A report of 39 cases by GIMEMA Infection Program. Haematologica. 2001;86:862–870. [PubMed] [Google Scholar]
- 27.Pagano L, Caira M, Candoni A, et al. Invasive aspergillosis in patients with acute myeloid leukemia: a SEIFEM-2008 registry study. Haematologica. 2010;95:644–650. doi: 10.3324/haematol.2009.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]