The prognostic value of the expression level of tyrosine 1248–phosphorylated human epidermal growth factor receptor (HER)-2 in patients with HER-2+ primary breast cancer is examined.
Keywords: Breast cancer, HER-2, Reverse-phase protein array, Metastases, Phosphorylation
Abstract
Purpose.
Tyrosine 1248 is one of the autophosphorylation sites of human epidermal growth factor receptor (HER)-2. We determined the prognostic value of the expression level of tyrosine 1248–phosphorylated HER-2 (pHER-2) in patients with HER-2+ primary breast cancer using a reverse-phase protein array.
Patients and Methods.
The optimal cutoff value of pHER-2 for segregating disease-free survival (DFS) was determined by receiver operating characteristic (ROC) curve analysis. Five-year DFS for pHER-2 expression level was estimated with the Kaplan-Meier method using both derivation (n = 162) and validation (n = 227) cohorts.
Results.
Of the 162 patients in the derivation cohort, 26 had high HER-2 expression levels. The area under the ROC curve for pHER-2 level and DFS was 0.662. Nineteen of the 162 patients (11.7%) had high pHER-2 expression levels (pHER-2high); 143 patients (88.3%) had low pHER-2 expression levels (pHER-2low). Among the 26 patients with high HER-2 expression levels, the 17 pHER-2high patients had a significantly lower 5-year DFS rate than the nine pHER-2low patients (23.5% versus 77.8%). On multivariate analysis, only pHER-2high independently predicted DFS in the Cox proportional hazards model. In the validation cohort, among 61 patients with high HER-2 expression, the difference in 5-year DFS rates between pHER-2high (n = 7) and pHER-2low (n = 54) patients was marginal (57.1% versus 81.5%).
Conclusion.
In patients with HER-2+ primary breast cancer, pHER-2high patients had a lower 5-year DFS rate than pHER-2low patients. Quantification of pHER-2 expression level may provide prognostic information beyond the current standard HER-2 status.
Introduction
Human epidermal growth factor receptor (HER)-2 is overexpressed or amplified in approximately 15%–20% of breast cancers, and HER-2 expression is a prognostic factor for breast cancer [1–3]. In patients with HER-2+ breast cancer, the combination of standard chemotherapy plus trastuzumab, a monoclonal antibody targeting HER-2, has resulted in higher progression-free and overall survival rates than with standard chemotherapy alone [4–6]. However, only 30% of patients with HER-2+ metastatic breast cancer respond to trastuzumab [7–19].
Phosphorylation of HER-2 induces downstream intracellular signaling and activates genes related to cell growth. HER-2 has several autophosphorylation sites, including tyrosine (Tyr)1248, 1221/1222, and 877, and others, and phosphorylation at these sites may reflect HER-2 activity [20–30]. To date, the most widely studied phosphorylation site is Tyr1248, the only site for which there is an antibody established to be stable in human tissues [25, 26, 28–30]. Some studies have reported that Tyr1248-phosphorylated HER-2 (pHER-2) assessed by immunohistochemistry (IHC) is related to resistance to trastuzumab and poor prognosis in patients with HER-2+ breast cancer [25, 26, 28–30]. However, we are aware of no studies to date that have identified specific levels of pHER-2 that can be used as cutoffs to stratify patients with respect to prognosis. The ability to stratify the prognosis of patients with HER-2+ breast cancer on the basis of pHER-2 expression level could allow testing of novel agents targeting pHER-2.
Reverse-phase protein array (RPPA) is an antibody-based quantitative assay that has advantages over IHC in assessing the phosphorylation of proteins. Although IHC provides information about the presence or absence of protein phosphorylation, it does not permit precise quantification of the amount of phosphorylated protein. RPPA, in contrast, permits, with just a small amount of material, quantification of the expression level and modification (e.g., phosphorylation) of proteins as a continuous value for a large number of patients.
We hypothesized that the HER-2 expression level by RPPA is concordant with HER-2 status by IHC or fluorescence in situ hybridization (FISH) and that in patients with HER-2+ breast cancer, high expression of pHER-2 by RPPA is associated with shorter disease-free survival (DFS) than low expression of pHER-2 by RPPA. We evaluated this hypothesis in a large derivation set and in a separate validation set from another institution.
Materials and Methods
Human Tumor Samples
Tumor samples from 162 patients with primary invasive ductal or invasive lobular breast carcinoma surgically resected from June 1992 to March 2007 were obtained from the breast tissue frozen tumor bank at The University of Texas MD Anderson Cancer Center. Patients with ductal carcinoma in situ, metaplastic carcinoma, or sarcoma were excluded. Samples were not collected consecutively but mainly based on tumor availability. No patients received a HER-2–targeting agent for pre- or postoperative chemotherapy because the use of trastuzumab for adjuvant therapy was not approved by the U.S. Food and Drug Administration until November 2006. Clinical data were collected from the Breast Cancer Management System database of MD Anderson. A validation set of tumor samples from 227 postmenopausal patients who had primary invasive ductal or invasive lobular breast carcinoma surgically resected from August 1988 to December 1998 and who received hormone therapy only was obtained from collaborators at the Hospital Clínico Universitario de Valencia, Spain. Samples were collected based on tumor availability. The validation set was used to validate the findings from the MD Anderson population. All specimens were collected under institutional review board approval.
Pathology Methods
Samples were considered positive for estrogen receptor (ER) or progesterone receptor (PR) if ≥10% of the cells had nuclear staining for the receptor. In the derivation set, HER-2 status was evaluated by IHC or FISH. HER-2 positivity was defined as 3+ receptor overexpression on IHC staining or gene amplification on FISH, defined as a gene copy–chromosome enumeration probe 17 ratio >2.0. In the validation set, HER-2 status by IHC or FISH was not available. Therefore, to stratify these patients with respect to HER-2 level, we used the cutoff value of HER-2 positivity that was developed using RPPA data from the derivation set (see Statistical Methods below).
RPPA
RPPA analysis was performed in our laboratory as described previously [31–35]. Briefly, lysis buffer was used to lyse frozen tumors by homogenization. Tumor lysates were normalized to 1 μg/μl concentration using a bicinchoninic acid assay. The lysates were then boiled with 1% sodium dodecyl sulfate, and the supernatants were manually diluted in six or eight twofold serial dilutions with lysis buffer. From the serial dilutions, an Aushon Biosystems 2470 arrayer was used to create sample arrays on nitrocellulose-coated FAST™ slides (Schleicher & Schuell Bio-Science, Inc., Keene, NH). Each slide was probed with a validated primary HER-2 and pHER-2 antibody, and the signal was amplified with a DakoCytomation catalyzed system (Dako, Glostrup, Denmark). The slides were analyzed and quantitated with the use of MicroVigene software (VigeneTech Inc., Carlisle, MA). The levels of HER-2 and pHER-2 in each sample were expressed as log-mean centered as previously described. We used the p-Neu 2A (PN2A) antibody to detect phosphorylation of Tyr1248. This antibody specifically recognizes phosphorylation of the major autophosphorylation site Tyr1248 and has no crossreactivity with other related receptors, including epidermal growth factor receptor, HER-3, and HER-4, or unphosphorylated HER-2, as demonstrated in a previous study [36]. Antibodies for other phosphorylation sites of HER-2 have not been validated for RPPA analysis. Therefore, we determined Tyr1248 phosphorylation only.
Statistical Methods
Correlations between the expression levels of HER-2 and pHER-2 in tumors and the 5-year DFS rate were determined using both the derivation (n = 162) and validation (n = 227) cohorts. Receiver operating characteristic (ROC) curve analyses were used to determine the optimal cutoff values for segregating 5-year DFS. The concordance of HER-2 expression level by RPPA and HER-2 status by IHC or FISH was analyzed using Cohen's κ coefficient.
Baseline patient characteristics by HER-2 and pHER-2 expression are summarized with medians and ranges for age and follow-up time and with frequencies and percentages for all other characteristics. Time to recurrence was measured from the date of diagnosis to the date of first detection of local disease recurrence or distant metastasis or to the date of last follow-up. Because the at-risk proportion of patients became very small at 5 years and because most of the recurrences had occurred by then, the follow-up time was truncated at 5 years. Time to recurrence was estimated with the Kaplan–Meier method and was compared between groups using the log-rank statistic. Cox proportional hazards models were fit to determine the association of pHER-2 status and the risk for recurrence after adjustment for other patient and disease characteristics. Each model contained terms for age at diagnosis, menopausal status, presence of lymph node metastasis at diagnosis, stage, hormone receptor status, and receipt of pre- or postoperative chemotherapy. p-values <.05 were considered statistically significant. All statistical analyses were done using SPSS version 17 (SPSS Inc., Chicago, IL).
Results
Clinicopathologic Characteristics of the Derivation Cohort
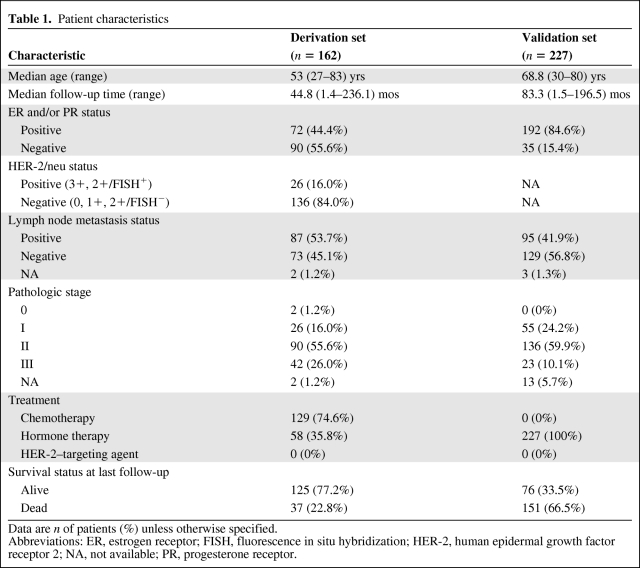
The clinicopathologic characteristics of the derivation cohort are listed in Table 1. The median age was 53 years (range, 27–83 years). The 162 breast cancers included 72 cancers (44.4%) that were hormone receptor positive (ER or PR) and 26 cancers (16.0%) that were positive for HER-2 by IHC or FISH. At 5 years of follow-up, 37 patients (22.8%) in the derivation cohort had died.
Table 1.
Patient characteristics
Data are n of patients (%) unless otherwise specified.
Abbreviations: ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER-2, human epidermal growth factor receptor 2; NA, not available; PR, progesterone receptor.
Concordance Between HER-2 Expression Level by RPPA and HER-2 Status by IHC or FISH
The area under the ROC curve for analysis of the relationship between HER-2 expression level by RPPA and HER-2 status by IHC and/or FISH was 0.873. An optimal cutoff value of HER-2 expression level was developed at 0.272. Using this optimal cutoff value, 26 (16.0%) of the 162 patients in the derivation cohort had high HER-2 expression levels (HER-2+ breast cancer). HER-2 expression level by RPPA was concordant with HER-2 status by IHC or FISH (κ coefficient p = .679; Fisher's exact test p = .000; sensitivity, 73.8%; specificity, 94.9%).
Prognostic Value of HER-2 in the Entire Derivation Cohort
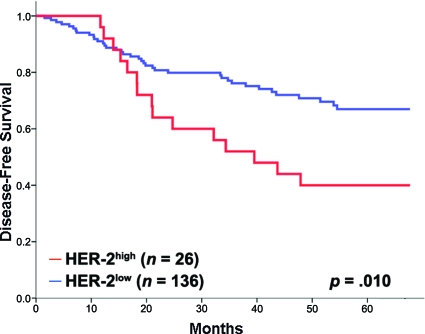
The prognostic role of HER-2 expression level by RPPA was determined using the aforementioned cutoff values. In the derivation set, the 5-year DFS rate was significantly lower in the 26 patients with a high HER-2 expression level than in the 136 patients with a low HER-2 expression level (42.3% versus 72.1%; p = .010) (Fig. 1).
Figure 1.
Kaplan–Meier functions of 5-year disease-free survival by HER-2 status in the derivation cohort.
Abbreviation: HER-2, human epidermal growth factor receptor 2.
Optimal Cutoff Value of pHER-2 Expression
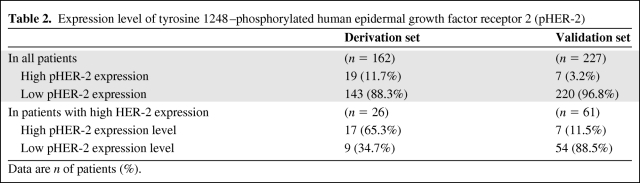
The area under the ROC curve for the analysis of the relationship between the pHER-2 expression level and 5-year DFS rate in the 26 patients with breast cancer with a high HER-2 expression level was 0.662. An optimal cutoff value of pHER-2 expression level was developed at 1.536. Using this optimal cutoff value, 19 (11.7%) of the 162 patients had a high pHER-2 expression level and 143 (88.3%) had a low pHER-2 expression level (Table 2). In the 26 patients with a high HER-2 expression level, 17 (65.3%) had a high pHER-2 expression level and nine (34.7%) had a low pHER-2 expression level (Table 2).
Table 2.
Expression level of tyrosine 1248–phosphorylated human epidermal growth factor receptor 2 (pHER-2)
Data are n of patients (%).
Prognostic Value of pHER-2 in the Entire Derivation Cohort
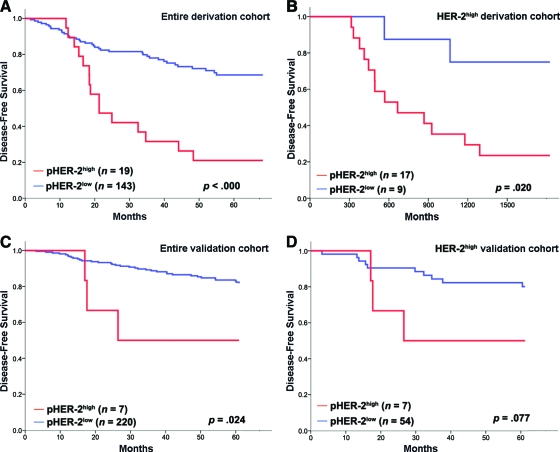
The prognostic role of the pHER-2 expression level was determined using the aforementioned cutoff values in a log-rank analysis. In the derivation cohort, patients with a high pHER-2 expression level had a significantly lower 5-year DFS rate than patients with a low pHER-2 expression level (p < .000) (Fig. 2A).
Figure 2.
Kaplan–Meier functions of 5-year disease-free survival by Tyr1248-phosphorylated HER-2 (pHER-2) status in the entire derivation cohort (A), derivation-cohort patients with high HER-2 expression (B), the entire validation cohort (C), and validation-cohort patients with high HER-2 expression (D).
Abbreviation: HER-2, human epidermal growth factor receptor 2.
Prognostic Value of pHER-2 in Derivation-Cohort Patients With a High HER-2 Expression Level
Among the 26 patients who had a high HER-2 expression level, patients with a high pHER-2 expression level had a significantly lower 5-year DFS rate than patients with a low pHER-2 expression level (23.5% versus 77.8%; p = .020) (Fig. 2B).
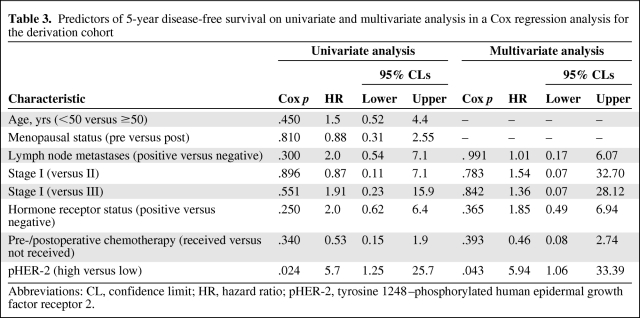
On univariate analysis, other factors, including age, menopausal status, lymph node status, pathological stage, hormone receptor status, and pre- or postoperative chemotherapy, were not associated with 5-year DFS (Table 3). On multivariate analysis, only the pHER-2 expression level was an independent prognostic factor (hazard ratio, 5.94; 95% confidence interval, 1.057–33.387; p = .043) (Table 3).
Table 3.
Predictors of 5-year disease-free survival on univariate and multivariate analysis in a Cox regression analysis for the derivation cohort
Abbreviations: CL, confidence limit; HR, hazard ratio; pHER-2, tyrosine 1248–phosphorylated human epidermal growth factor receptor 2.
Clinicopathologic Characteristics of the Validation Cohort
Clinicopathologic characteristics of the validation cohort are listed in Table 1. The median age was 68.8 years (range, 30–80 years). The 227 breast cancers included 192 cancers (84.6%) that were hormone receptor positive. All patients received hormone therapy; none received chemotherapy. Ninety-five of the patients (41.9%) had lymph node metastases; 55 (24.2%) had stage I, 136 (59.9%) had stage II, and 23 (10.1%) had stage III disease.
Prognostic Value of pHER-2 in the Validation Cohort
The reproducibility of the prognostic value of pHER-2 by RPPA was determined in the validation cohort. In this cohort, HER-2 status was not available by IHC or FISH. Therefore, we applied a cutoff value of HER-2 positivity of 0.272, which was estimated using the derivation set. Using this cutoff value, 61 (26.9%) of the 227 patients in the validation cohort had a high HER-2 expression level by RPPA (Table 2).
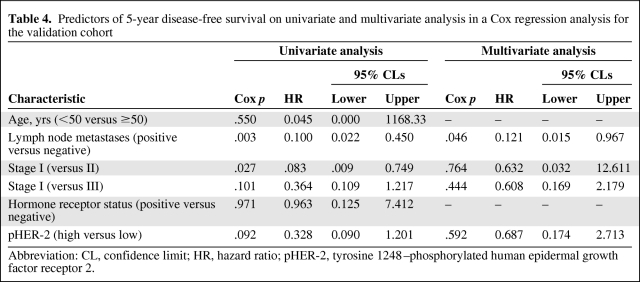
Patients with a high pHER-2 expression level (n = 7) had a significantly lower 5-year DFS rate than patients with a low pHER-2 expression level (n = 220; p = .024) (Fig. 2C). In the 61 patients with a high HER-2 expression level, there was a trend toward a lower 5-year DFS rate in patients with a high pHER-2 expression level (n = 7) than in patients with a low pHER-2 expression level (n = 54) (57.1% versus 81.5%; p = .077) (Fig. 2D). Only lymph node metastasis was an independent prognostic factor on multivariate analysis (hazard ratio, 0.121; 95% confidence interval, 0.015–0.967; p = 0.046) (Table 4).
Table 4.
Predictors of 5-year disease-free survival on univariate and multivariate analysis in a Cox regression analysis for the validation cohort
Abbreviation: CL, confidence limit; HR, hazard ratio; pHER-2, tyrosine 1248–phosphorylated human epidermal growth factor receptor 2.
Discussion
We have provided the first clinical evidence that the expression level of pHER-2 by RPPA, which can quantify phosphorylated proteins, may have a prognostic role in patients with HER-2+ breast cancer. In the derivation cohort, among patients with HER-2+ breast cancer, the expression level of pHER-2 was the only factor associated with the 5-year DFS rate on both univariate and multivariate analysis. There was a similar trend in the validation cohort using data from another institution. These results indicate that pHER-2 expression level may have potential as an additional prognostic marker in patients with HER-2+ breast cancer.
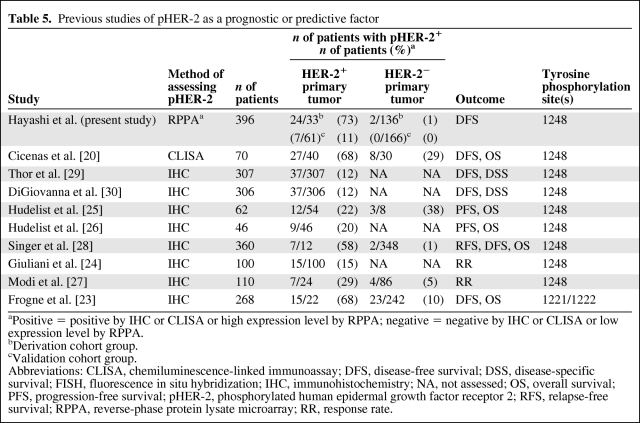
The prognostic and predictive roles of pHER-2 in breast cancer patients have previously been reported (Table 5) [20, 23–28, 30]. Our results concur with those of previous studies, although in most previous studies phosphorylated HER-2 was examined using IHC with PN2A [22, 29, 30], which is an antibody against HER-2 with phosphorylation at Tyr1248. Tyr1248 is one of the C-terminal sites that are phosphorylated before downstream signaling occurs [21, 37–39]. Tyr1248 phosphorylation contributes to induce activation of the HER-2 oncoprotein [20–29]. Frogne et al. [23] reported the clinical relevance of Tyr1221/1222-phosphorylated HER-2, because pHER-2 using the antibody against Tyr1248 was only weakly stained in their positive control. Other phosphorylation sites of HER-2 (Tyr877, Tyr1005, Tyr1127, Tyr1144, Tyr1201, Tyr1226/1227, and Tyr1253) have not been examined in human specimens, and their accuracies and prognostic values have not been shown [21, 40, 41].
Table 5.
Previous studies of pHER-2 as a prognostic or predictive factor
aPositive = positive by IHC or CLISA or high expression level by RPPA; negative = negative by IHC or CLISA or low expression level by RPPA.
bDerivation cohort group.
cValidation cohort group.
Abbreviations: CLISA, chemiluminescence-linked immunoassay; DFS, disease-free survival; DSS, disease-specific survival; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NA, not assessed; OS, overall survival; PFS, progression-free survival; pHER-2, phosphorylated human epidermal growth factor receptor 2; RFS, relapse-free survival; RPPA, reverse-phase protein lysate microarray; RR, response rate.
In this study, we assessed the expression level of pHER-2 by RPPA, which permits quantification of phosphorylated proteins [31–35]. RPPA is a highly reliable, reproducible, and high-throughput system for quantitative proteomic analysis of a large number of protein expression and protein activation sites [31, 32, 35, 42]. Expression levels of proteins by RPPA have been confirmed to be correlated with the results of traditional Western blotting [35]. In addition, the concurrent comparison of a large number of samples, which RPPA makes possible, can reduce experimental bias [32, 33, 35]. RPPA also has the advantage of permitting objective assessment of the expression levels of proteins because they are shown as continuous values. These characteristics of RPPA allowed us to use the technique to develop optimal cutoff values of protein expression for each analysis. RPPA may identify optimal molecular markers for the detection of response to treatment and may have potential for developing novel agents targeting pHER-2 and for therapeutic monitoring. In the past, issues related to fixation and stability of proteins were a concern with RPPA. However, recent advances in technology have proven the reproducibility and high sensitivity of RPPA [31–33, 35]. We previously assessed the technical utility of RPPA analysis and confirmed the stability of pHER-2 and other proteins in frozen samples within 24 hours after sample collection for RPPA analysis [43]. We also checked the reproducibility of RPPA by validating the results of our initial, derivation-set analysis with an analysis of samples from a different institution.
Our comparison of RPPA findings and findings from traditional pathologic analysis, including IHC and FISH, indicated that expression levels of HER-2 by RPPA were remarkably concordant with the standard HER-2 status determined by traditional pathologic analysis. On the basis of this reliability, we were able to justify the optimal cutoff values related to the 5-year DFS rate that were developed using the ROC curve analysis.
In previous studies, pHER-2 was found in 12%–68% of HER-2+ patients [31–35]. Our study showed high pHER-2 expression levels in 65.3% of the patients with a high HER-2 expression level in the derivation cohort and in 11.5% of the patients with a high HER-2 expression level in the validation cohort. Although both rates of high expression of pHER-2 are within the range of rates reported in previous studies (Table 4), the rate of high expression of pHER-2 was lower in the validation cohort. This lower rate in the validation cohort may be explained by differences in other clinicopathologic characteristics between the derivation and validation cohorts—specifically, all the patients in the validation cohort were postmenopausal, most of the patients were hormone receptor positive, and all the patients received hormone therapy but not chemotherapy. Therefore, the number of pHER-2+ patients in the validation cohort was small, and the prognostic role of pHER-2 was statistically marginal in the validation cohort despite the significance proven with multivariate analysis in the derivation cohort.
In this study, none of the patients with HER-2+ breast cancer received any HER-2–targeting agent. Thus, we were able to assess the prognostic significance of pHER-2 without interference by a HER-2–targeting agent. Assessment of the correlation between the pHER-2 expression level and the effect of HER-2–targeting agents is a necessary next step. Furthermore, recently, several mechanisms have been revealed for resistance to trastuzumab [10–19]. Phosphatase and tension homolog deleted on chromosome 10 (PTEN) suppresses the phosphatidylinositol-3-kinase (PI3K)/Akt pathway. Activation of the PI3K pathway by PIK3CA mutations or low PTEN expression has been associated with a poor response to trastuzumab and poor prognosis after trastuzumab therapy [10–13]. Assessment of the correlation between phosphorylation of HER-2 and activation of these downstream pathways is also needed.
A limitation of this study is that, if the normalization process for the RPPA dataset changes, the optimal cutoff values for each dataset will change as well. If our optimal cutoff values of HER-2 and pHER-2 are used with other datasets, the normalization process needs to be the same as the one used in our study.
In summary, in HER-2+ breast cancer patients, high expression of pHER-2 was associated with a lower 5-year DFS rate than with low expression of pHER-2 in the derivation cohort, and a similar trend was observed in the validation cohort. High expression of pHER-2 was rare in HER-2− breast cancer patients. Quantification of the expression level of pHER-2 may provide further prognostic information in HER-2+ breast cancer patients in addition to the information gained from the current standard pathologic studies. Definitive prospective studies are warranted to further confirm this relationship.
Acknowledgments
The project was supported by the National Institutes of Health through MD Anderson's Cancer Center Support Grant, CA016672; the Kleberg Center for Molecular Markers at MD Anderson Cancer Center; an ASCO Career Development Award; NCI grants 1K23CA121994–01 and NCI 1R21CA120248–01; Susan G. Komen Foundation grant FAS0703849 (AMG); NIH grant R01 CA123318–01; a donation from Mr. and Mrs. Sidney J. Jansma, Jr.; a grant from the I.C.N. Foundation; and the Nellie B. Connally Breast Cancer Research Fund (NTU).
This study was invited for an oral presentation at the General Session of the 2010 ASCO Breast Cancer Symposium, held in October 2010, Washington, DC.
We thank Ping Liu, from the Department of Biostatistics, MD Anderson Cancer Center, and Yasuharu Tokuda, from the Institute of Clinical Medicine, Graduate School of Comprehensive Human Sciences, University of Tsukuba, for data analyses.
The authors take full responsibility for the content of the paper but thank Stephanie Deming from the Department of Scientific Publication, University of Texas, for editorial review.
Author Contributions
Conception/Design: Naoki Hayashi, Takayuki Iwamoto, Ana M. Gonzalez- Angulo, Seigo Nakamura, Gabriel N. Hortobagyi, Naoto T. Ueno
Provision of study material or patients: Naoki Hayashi, Takayuki Iwamoto, Ana M. Gonzalez-Angulo, Jaime Ferrer-Lozano, Ana Lluch, Naoto T. Ueno
Collection and/or assembly of data: Naoki Hayashi, Takayuki Iwamoto, Ana M. Gonzalez-Angulo, Jaime Ferrer-Lozano, Ana Lluch, Chandra Bartholomeusz
Data analysis and interpretation: Naoki Hayashi, Takayuki Iwamoto, Ana M. Gonzalez-Angulo, Jaime Ferrer-Lozano, Ana Lluch, Seigo Nakamura, Gabriel N. Hortobagyi, Naoto T. Ueno
Manuscript writing: Naoki Hayashi, Takayuki Iwamoto, Naoto T. Ueno
Final approval of manuscript: Naoki Hayashi, Takayuki Iwamoto, Ana M. Gonzalez-Angulo, Jaime Ferrer-Lozano, Ana Lluch, Chandra Bartholomeusz, Seigo Nakamura, Gabriel N. Hortobagyi, Naoto T. Ueno, Naoki Niikura
References
- 1.Clark GM, McGuire WL. Follow-up study of HER-2/neu amplification in primary breast cancer. Cancer Res. 1991;51:944–948. [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Vogel C, Cobleigh MA, Tripathy D, et al. First-line, single-agent Herceptin® (trastuzumab) in metastatic breast cancer. A preliminary report. Eur J Cancer. 2001;37(suppl 1):S25–S29. [PubMed] [Google Scholar]
- 4.Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 5.Seidman AD, Fornier MN, Esteva FJ, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19:2587–2595. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 8.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 9.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 10.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Fujita T, Doihara H, Kawasaki K, et al. PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer. 2006;94:247–252. doi: 10.1038/sj.bjc.6602926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita T, Doihara H, Washio K, et al. Proteasome inhibitor bortezomib increases PTEN expression and enhances trastuzumab-induced growth inhibition in trastuzumab-resistant cells. Anticancer Drugs. 2006;17:455–462. doi: 10.1097/01.cad.0000198910.90819.06. [DOI] [PubMed] [Google Scholar]
- 13.Lu CH, Wyszomierski SL, Tseng LM, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 14.Motoyama AB, Hynes NE, Lane HA. The efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res. 2002;62:3151–3158. [PubMed] [Google Scholar]
- 15.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Nagy P, Friedländer E, Tanner M, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a Herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–482. [PubMed] [Google Scholar]
- 17.Nahta R, Esteva FJ. HER2 therapy: Molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nahta R, Yu D, Hung MC, et al. Mechanisms of disease: Understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 19.Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27:5838–5847. doi: 10.1200/JCO.2009.22.1507. [DOI] [PubMed] [Google Scholar]
- 20.Cicenas J, Urban P, Kn̈g W, et al. Phosphorylation of tyrosine 1248-ERBB2 measured by chemiluminescence-linked immunoassay is an independent predictor of poor prognosis in primary breast cancer patients. Eur J Cancer. 2006;42:636–645. doi: 10.1016/j.ejca.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Dankort D, Jeyabalan N, Jones N, et al. Multiple ErbB-2/Neu phosphorylation sites mediate transformation through distinct effector proteins. J Biol Chem. 2001;276:38921–38928. doi: 10.1074/jbc.M106239200. [DOI] [PubMed] [Google Scholar]
- 22.DiGiovanna MP, Carter D, Flynn SD, et al. Functional assay for HER-2/neu demonstrates active signalling in a minority of HER-2/neu-overexpressing invasive human breast tumours. Br J Cancer. 1996;74:802–806. doi: 10.1038/bjc.1996.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frogne T, Laenkholm AV, Lyng MB, et al. Determination of HER2 phosphorylation at tyrosine 1221/1222 improves prediction of poor survival for breast cancer patients with hormone receptor-positive tumors. Breast Cancer Res. 2009;11:R11. doi: 10.1186/bcr2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuliani R, Durbecq V, Di Leo A, et al. Phosphorylated HER-2 tyrosine kinase and Her-2/neu gene amplification as predictive factors of response to trastuzumab in patients with HER-2 overexpressing metastatic breast cancer (MBC) Eur J Cancer. 2007;43:725–735. doi: 10.1016/j.ejca.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Hudelist G, Köstler WJ, Attems J, et al. Her-2/neu-triggered intracellular tyrosine kinase activation: In vivo relevance of ligand-independent activation mechanisms and impact upon the efficacy of trastuzumab-based treatment. Br J Cancer. 2003;89:983–991. doi: 10.1038/sj.bjc.6601160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudelist G, Köstler WJ, Czerwenka K, et al. Her-2/neu and EGFR tyrosine kinase activation predict the efficacy of trastuzumab-based therapy in patients with metastatic breast cancer. Int J Cancer. 2006;118:1126–1134. doi: 10.1002/ijc.21492. [DOI] [PubMed] [Google Scholar]
- 27.Modi S, DiGiovanna MP, Lu Z, et al. Phosphorylated/activated HER2 as a marker of clinical resistance to single agent taxane chemotherapy for metastatic breast cancer. Cancer Invest. 2005;23:483–487. doi: 10.1080/07357900500201301. [DOI] [PubMed] [Google Scholar]
- 28.Singer CF, Gschwantler-Kaulich D, Fink-Retter A, et al. HER2 overexpression and activation, and tamoxifen efficacy in receptor-positive early breast cancer. J Cancer Res Clin Oncol. 2009;135:807–813. doi: 10.1007/s00432-008-0516-x. [DOI] [PubMed] [Google Scholar]
- 29.Thor AD, Liu S, Edgerton S, et al. Activation (tyrosine phosphorylation) of ErbB-2 (HER-2/neu): A study of incidence and correlation with outcome in breast cancer. J Clin Oncol. 2000;18:3230–3239. doi: 10.1200/JCO.2000.18.18.3230. [DOI] [PubMed] [Google Scholar]
- 30.DiGiovanna MP, Stern DF, Edgerton SM, et al. Relationship of epidermal growth factor receptor expression to ErbB-2 signaling activity and prognosis in breast cancer patients. J Clin Oncol. 2005;23:1152–1160. doi: 10.1200/JCO.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–2478. doi: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]
- 32.Hennessy BT, Lu Y, Poradosu E, et al. Pharmacodynamic markers of perifosine efficacy. Clin Cancer Res. 2007;13:7421–7431. doi: 10.1158/1078-0432.CCR-07-0760. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, He X, Baggerly KA, et al. Non-parametric quantification of protein lysate arrays. Bioinformatics. 2007;23:1986–1994. doi: 10.1093/bioinformatics/btm283. [DOI] [PubMed] [Google Scholar]
- 34.Liang J, Shao SH, Xu ZX, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 35.Tibes R, Qiu Y, Lu Y, et al. Reverse phase protein array: Validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 36.DiGiovanna MP, Stern DF. Activation state-specific monoclonal antibody detects tyrosine phosphorylated p185neu/erbB-2 in a subset of human breast tumors overexpressing this receptor. Cancer Res. 1995;55:1946–1955. [PubMed] [Google Scholar]
- 37.Harder KW, Owen P, Wong LK, et al. Characterization and kinetic analysis of the intracellular domain of human protein tyrosine phosphatase beta (HPTP beta) using synthetic phosphopeptides. Biochem J. 1994;298:395–401. doi: 10.1042/bj2980395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 39.Akiyama T, Matsuda S, Namba Y, et al. The transforming potential of the c-erbB-2 protein is regulated by its autophosphorylation at the carboxyl-terminal domain. Mol Cell Biol. 1991;11:833–842. doi: 10.1128/mcb.11.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berezov A, Greene MI. Towards comprehensive characterization of HER2 overexpression. Mol Syst Biol. 2006;2:55. doi: 10.1038/msb4100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Telesco SE, Radhakrishnan R. Atomistic insights into regulatory mechanisms of the HER2 tyrosine kinase domain: A molecular dynamics study. Biophys J. 2009;96:2321–2334. doi: 10.1016/j.bpj.2008.12.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramaswamy A, Lin E, Chen I, et al. Application of protein lysate microarrays to molecular marker verification and quantification. Proteome Sci. 2005;3:9. doi: 10.1186/1477-5956-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hennessy BT, Lu Y, Gonzalez-Angulo AM, et al. A Technical Assessment of the Utility of Reverse Phase Protein Arrays for the Study of the Functional Proteome in Non-microdissected Human Breast Cancers. Clin Proteomics. 2010;6:129–151. doi: 10.1007/s12014-010-9055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]