Whether or not the process of data disclosure regarding KRAS status and treatment of advanced colorectal cancer patients was effective in permitting timely decisions regarding ongoing publicly funded clinical trials and whether or not such decisions were rational and ethical are discussed with the overall goals of highlighting lessons learned regarding early disclosure of clinical trial results, as well as vetting and adoption of new scientific data, and proposing modifications for handling similar situations in the future.
Keywords: Ethics, KRAS, Clinical trials, Colorectal neoplasms
Abstract
Systemic therapy has led to a median survival time for patients with advanced colorectal cancer (CRC) almost fourfold longer than that expected with best supportive care, an outcome achieved through combining chemotherapeutic and targeted biologic agents. Although the latter can include anti–epidermal growth factor receptor antibodies, such as cetuximab and panitumumab, we now have strong evidence that patients whose tumors harbor mutated KRAS will not benefit from this class of agent. Acceptance of the reliability and importance of the KRAS data took several years to evolve, however, for a variety of reasons. The timeline from the presentation and publication of small, retrospective phase II studies to widespread acceptance of the KRAS predictive value and changes in behavior—specifically, modifications of ongoing national trials in advanced/metastatic CRC, changes in national guidelines and practice patterns, and adjustments to the labeled indications for the monoclonal antibodies—was lengthy. In this commentary, we discuss whether or not the process of data disclosure regarding KRAS status and treatment of advanced CRC patients was effective in permitting timely decisions regarding ongoing publicly funded clinical trials and whether or not such decisions were rational and ethical. The overall goals are to highlight lessons learned regarding early disclosure of clinical trial results, as well as vetting and adoption of new scientific data, and to propose modifications for handling similar situations in the future.
Introduction
Systemic therapy has led to a median survival time for patients with advanced colorectal cancer (CRC) almost fourfold longer than with best supportive care, an outcome achieved through combining chemotherapeutic and targeted biologic agents [1, 2]. Although the latter can include anti–epidermal growth factor receptor (EGFR) antibodies, such as cetuximab and panitumumab, we now have strong evidence that patients whose tumors harbor mutated KRAS will not benefit from this class of agent. Acceptance of the reliability and importance of the KRAS data took several years to evolve, however, because the data were initially all retrospectively derived. The timeline from the presentation and publication of small, retrospective phase II studies to widespread acceptance of the KRAS predictive value and changes in behavior—specifically, modifications of ongoing national trials in advanced/metastatic CRC, changes in national guidelines and practice patterns, and adjustments to the labeled indications for the monoclonal antibodies—was lengthy.
In this commentary, we discuss whether or not the process of data disclosure regarding KRAS status and treatment of advanced CRC patients was effective in permitting timely decisions regarding ongoing publicly funded clinical trials and whether or not such decisions were rational and ethical. The overall goals are to highlight lessons learned regarding early disclosure of clinical trial results, as well as vetting and adoption of new scientific data, and to propose modifications for handling similar situations in the future.
Background
The U.S. publicly funded clinical trials system consists of an interconnected network of cooperative groups, community oncology sites, cancer centers, universities, government contractors, and individual researchers, with coordination and oversight provided by the U.S. National Cancer Institute (NCI). The cooperative group component represents the largest publicly funded oncology clinical trial organization in the world, receiving about 145 million dollars per year in NCI support and involving >500,000 patients in research projects [3, 4].
In 2005, based on recommendations from the NCI Clinical Trials Working Group, disease-specific steering committees composed of experts from cooperative groups, specialized programs of research excellence, patient advocates, and other sources were established to prioritize scientific efforts, promote collaboration in performing clinical trials, and provide peer review for specific trial concepts [5]. Individual steering committees created disease-focused task forces that advise the steering committee on subtopics (e.g., the Colon Cancer Task Force of the GI Steering Committee). The Colon Cancer Task Force conducts monthly teleconferences to discuss accrual and other issues pertaining to ongoing trials, proposed studies, and research strategy and vision. In the conduct of its mission, the Colon Cancer Task Force struggled with the question of when accumulating data regarding KRAS mutation status as a predictor of cetuximab resistance warranted modification of ongoing national studies involving cetuximab. These discussions also raised issues regarding the early release of clinical trials data, and the current structure of embargo policies.
KRAS: Background and Timeline
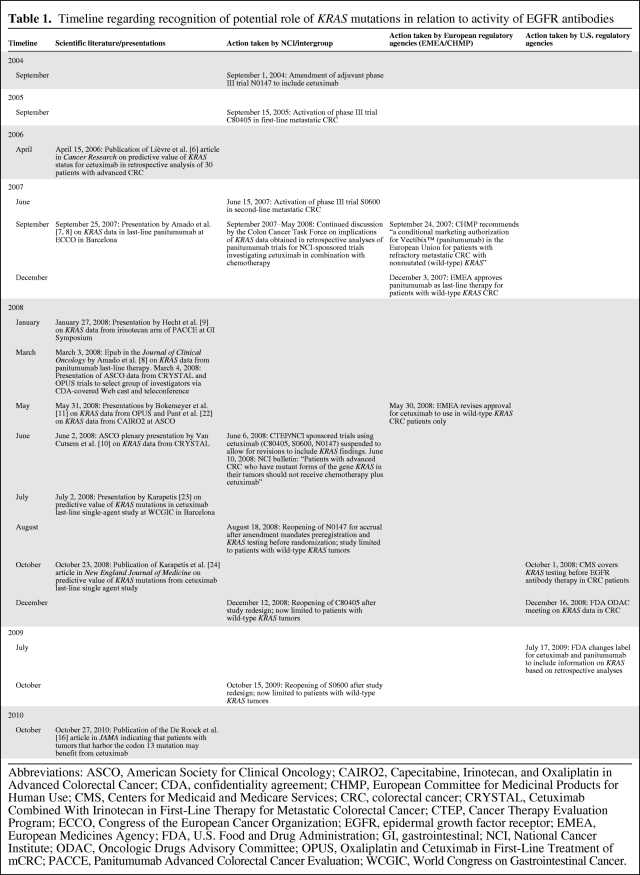
Although the prognostic value of KRAS mutational status remains unclear, KRAS mutations predict inactivity of anti-EGFR antibodies, such as cetuximab and panitumumab, in advanced CRC patients [6–9]. Some of the first data on a potential link between KRAS mutation and outcome were presented as early as 2006, starting with a retrospective analysis in 30 patients treated with cetuximab [6]. Over the next 2 years, several additional pieces of information (all from retrospective analyses of trials with cetuximab or panitumumab in advanced CRC patients) became available, culminating in several presentations at the American Society of Clinical Oncology (ASCO) 2008 Annual Meeting and leading to the June 2008 temporary suspension of all NCI-funded trials testing EGFR antibodies in CRC patients, to allow for amendments addressing the KRAS issue [7–11]. A specific timeline (Table 1), with some of the debate engendered, was as follows:
Table 1.
Timeline regarding recognition of potential role of KRAS mutations in relation to activity of EGFR antibodies
Abbreviations: ASCO, American Society for Clinical Oncology; CAIRO2, Capecitabine, Irinotecan, and Oxaliplatin in Advanced Colorectal Cancer; CDA, confidentiality agreement; CHMP, European Committee for Medicinal Products for Human Use; CMS, Centers for Medicaid and Medicare Services; CRC, colorectal cancer; CRYSTAL, Cetuximab Combined With Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer; CTEP, Cancer Therapy Evaluation Program; ECCO, Congress of the European Cancer Organization; EGFR, epidermal growth factor receptor; EMEA, European Medicines Agency; FDA, U.S. Food and Drug Administration; GI, gastrointestinal; NCI, National Cancer Institute; ODAC, Oncologic Drugs Advisory Committee; OPUS, Oxaliplatin and Cetuximab in First-Line Treatment of mCRC; PACCE, Panitumumab Advanced Colorectal Cancer Evaluation; WCGIC, World Congress on Gastrointestinal Cancer.
In September 2007, Amado et al. [7, 8] presented KRAS data involving a chemorefractory population administered panitumumab. In the same month, the European Committee for Medicinal Products for Human Use conditionally authorized panitumumab in the European Union for refractory CRC patients whose tumor had nonmutated KRAS. About 2 months later, the European Medicines Agency approved the same drug as late-line therapy, with the same restriction. In January 2008, Hecht et al. [9] presented an analysis of KRAS as a predictive factor in the Panitumumab Advanced Colorectal Cancer Evaluation (PACCE) trial, suggesting that frontline patients with KRAS-mutated tumors did not benefit from the addition of panitumumab to an irinotecan and bevacizumab–based regimen. The treatment regimens used in the PACCE trial did not mirror any arms of the ongoing NCI-sponsored clinical trials, and no changes were made to the design and conduct of the ongoing intergroup studies.
The phase III Cetuximab Combined With Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer (CRYSTAL) [12, 13] (5-fluorouracil, leucovorin, and irinotecan [FOLFIRI] with and without cetuximab) and phase II Oxaliplatin and Cetuximab in First-Line Treatment of mCRC (OPUS) trial [14, 15] (5-fluorouracil, leucovorin, and oxaliplatin with and without cetuximab) first-line trials had already been presented at the ASCO 2007 Annual Meeting, without a KRAS subgroup analysis. In 2008, various sources involved in these two industry-sponsored trials relayed the information that the KRAS subanalyses of both studies were to be presented at the ASCO 2008 Annual Meeting, as late-breaking abstracts, and that the presentations would make it clear that patients whose tumor harbored KRAS mutations would clearly not benefit from anti-EGFR antibody therapy. Some individuals from the Colon Cancer Task Force were also members of the ASCO Program Committee, and they had an opportunity to review the abstracts in January of 2008. The specific data in the abstracts were not yet widely revealed, but the Colon Cancer Task Force formally approached the industry sponsors of the CRYSTAL and OPUS trials to share their results. Under the cover of individual confidentiality agreements (CDAs), a webcast was held in March 2008, during which select investigators involved in the conduct and planning of cetuximab-based clinical trials in CRC patients were given access to the KRAS subanalyses. Indeed, the information presented during the webcast in March 2008 was identical to the data eventually presented 2 months later at the ASCO 2008 Annual Meeting. Unfortunately, not all members of the Colon Cancer Task Force were able to join the webcast, and no subsequent open discussions on the data were held during the next two monthly conference calls, in view of missing individual CDAs as well as concerns about the ASCO embargo policy. Specifically, it was felt that the presentation of one or both trials could be compromised if information regarding the trial results was leaked to the public. In addition, amending the cetuximab-based intergroup trials at that timepoint to allow only patients with wild-type KRAS tumors to be enrolled would have disclosed the pertinent information of the presentations planned for the ASCO 2008 Annual Meeting. Investigators also felt that the presentation of the results, the expert commentary from the podium, and the dialogue that would follow the meeting would inform the decision-making process regarding the reliability of the data. Absolute consensus regarding the strength of the data and the decisions that should follow acceptance were not reached within the Colon Cancer Task Force before the ASCO meeting.
Prompt action was implemented after those two late May/early June ASCO presentations. Temporary suspension of all NCI Cancer Therapy Evaluation Program–sponsored trials involving EGFR antibodies in CRC patients occurred on June 6, 2008. On June 10, an NCI bulletin was issued, stating “patients with advanced CRC who have mutant forms of the gene KRAS in their tumors should not receive chemotherapy plus cetuximab.” Subsequently, the three suspended phase III intergroup trials (C80405, S0600, and N0147) were redesigned to allow randomization only of patients with wild-type KRAS CRC, reopening to accrual in August 2008 (N1047), December 2008 (C80405), and October 2009 (S0600). In July 2009, the U.S. Food and Drug Administration (FDA) changed the labels for both cetuximab and panitumumab, incorporating information on KRAS. Most recently, a pooled data analysis suggesting that patients whose tumors harbor a codon 13, as opposed to codon 12 or 60 KRAS, mutation might have at least some potential to benefit from cetuximab treatment was published, indicating that further prospective trials are needed to assess whether or not the exclusion criteria of codon 13 mutations need to be reconsidered [16].
Problems and Solutions
The timing of the disclosure of these data engendered controversy. Critical issues surrounded the following questions: (a) Can data from retrospective biomarker analyses provide sufficient evidence to change the design of ongoing clinical trials, with additional potential impact on existing standard of care treatments? (b) What level of evidence would be necessary to accept such analyses?
To consider these issues raised by the process, and guide decision making around future analogous circumstances, the authors looked to principles of biomedical ethics while specifically considering the stakeholders involved. Ethical concepts underpinning clinical research include nonmaleficence (not doing harm), beneficence (doing good), autonomy (respect for individual rights), and justice (fair treatment). Failure to warn persons of newly discovered harm could certainly breach the principle of nonmaleficence. KRAS mutations predict a lack of efficacy of anti-EGFR antibodies in patients with metastatic CRC, so individuals whose tumors harbor such mutations are exposed to harm (adverse effects, shorter survival because of time spent taking ineffective therapy, cost) when taking the drugs without any realistic chance of benefit. If the release of early data would have definitively demonstrated the patient subgroups that could not benefit from EGFR inhibitors, then harm occurring during the time that the results were known to the investigators and the ASCO Program Committee prior to the ASCO meeting could have been potentially preventable. Similar considerations apply to the principle of beneficence. It could be argued that patients treated with these drugs, on or off trial, should have known if new evidence predicted “more” benefit for those whose tumors were wild-type KRAS.
On the other hand, considerations regarding not doing harm apply equally to the potentially damaging consequences of prematurely drawing conclusions or making recommendations based on data that ultimately are not correct. This possibility remained foremost in the minds of some Colon Cancer Task Force members, at least until the ASCO presentations highlighted above. For example, high-dose chemotherapy with bone marrow transplant was the standard of care for some women with breast cancer, based mostly on data derived from nonrandomized phase II trials. Phase III results, in general, did not replicate the early data [17]. Another potential example pertains to CRC BRAF mutational status. Two modestly sized studies identified a total of 20 patients with wild-type KRAS tumors who had V600E BRAF mutations; none responded to anti-EGFR monoclonal antibodies [18, 19]. On the basis of those trials, some clinicians began routinely assaying for BRAF mutations, withholding anti-EGFR therapies in patients with such mutations on the assumption that efficacy was not realistically possible. More recently, a pooled analysis of the CRYSTAL and OPUS first-line CRC studies contradicted the original finding, suggesting that adding cetuximab to chemotherapy in patients with BRAF mutations may lead to longer progression-free and overall survival times [20]. Were those patients with mutated BRAF, from whom cetuximab was withheld following presentation of the initial two studies, harmed?
Patients entering clinical trials make the decision to enroll expecting that the investigators assume equipoise. The principle of autonomy suggests that, if the investigators' views regarding the potential benefit of all the respective arms of a trial significantly change based on new evidence, then patients should be informed of this and have the ability to reconsider their treatment. Additionally, the principle of justice requires that the decision about whom to share early results with should fairly consider all patients, on or off trial, who are potentially affected by this information. Potential stakeholders in the KRAS decision included patients, physicians, the NCI, industry representatives, the FDA, the Securities and Exchange Commission (SEC), company shareholders, and professional societies (here, ASCO). While individual data and safety monitoring boards (DSMBs) are also charged with oversight for clinical trials, the DSMBs in this case would not have had access to the information from multiple unpublished clinical trials available to the Colon Cancer Task Force.
From an ethical standpoint, the rights and protection of patients are of primary importance, overriding concerns of nonpatient stakeholders. Nonetheless, the others have roles in patient protection and care advancement. Physicians obviously have to care for current and future patients; the FDA is charged with protecting the health of the general public; and the NCI and ASCO are invested in promoting the oncologic research agenda, ultimately leading to better cancer outcomes. Industry, the SEC, and shareholders obviously have a more financially based interest in drugs being proven effective. All groups are potentially affected, albeit for different reasons, by the decision to modify a trial or make a definitive statement about who should or should not be treated with an approved drug. Failure to disclose “convincing” data should invite scrutiny regardless of the group advocating waiting; however, the definition of “convincing” remains ambiguous. Certainly, a disclosure of data later found to be misleading equally invokes criticism.
Should these data have changed eligibility for ongoing clinical trials? The question must first be raised as to how much agreement was required that the data were reliable. Should one single believer and voice have the power to inform the public? Should unanimity among experts be required? Perhaps a simple majority is sufficient? The situation is clouded when the “experts” have signed legally binding confidentiality agreements with industry sponsors restricting public discussion and disclosure.
In answer to the question about whether or not ongoing trials should have been amended with the emergence of new KRAS data, the answer is clearly “yes,” if the assumptions underlying the arms were no longer valid. The determination of equipoise rested on a standard of evidence that could be determined only by the principal investigators conducting the trials. If this group believed that uncertainty no longer existed regarding the predictive ability of KRAS mutations, then the trial eligibility criteria should have been changed, as they ultimately were in this case (after the 2008 ASCO Annual Meeting presentations). However, earlier disclosure would have required that the investigators conducting these trials be informed of these results before the ASCO meeting, violating a well-established embargo policy. Currently, the ASCO confidentiality policy states that abstracts submitted to ASCO meetings are considered confidential from the time of submission. Information may not be made available to the public or the news media, nor may it be published or presented elsewhere, nor may it be used for securities trading purposes, prior to public release in conjunction with the meeting. In circumstances in which a high standard of evidence has been achieved and results are sufficiently compelling to warrant early release, then specific exceptions need to be created in order to comply with the policy. Whose responsibility is it to approach ASCO to ask for an exception?
Should these data have been released early to the public? This consideration required a higher level of evidence, in order to avoid inadvertent harm to patients. For reasons discussed earlier, ethical considerations that apply to patients suggest that this information should have been made available earlier if the data were felt to be definitive. However, early data often do not meet this standard of evidence, and misleading, premature conclusions may be drawn after early disclosure. “Formal” vetting by expert commentaries at national meetings like the ASCO meetings and “informal” vetting by discussions among stakeholders at these meetings can help to place these results in proper context for appropriate interpretation and subsequent decision making. However, even these methods of data interpretation are imperfect, and it may be most appropriate to use data to change clinical practice only after they have been presented in manuscript form and confirmed. Thus, it may be that, if there are circumstances in which results are so compelling that they change practice following a national meeting, perhaps even earlier disclosure of these results could and should have had the same effect.
Does the type of predictive classifier affect the level of evidence needed to change trial or treatment behavior? New efficacy markers may be used to include or exclude patients from specific systemic treatments. An example of an inclusionary marker would be the use of trastuzumab in human epidermal growth factor receptor 2–expressing gastric cancer [21]. KRAS mutations, in regard to anti-EGFR therapies, represent an exclusionary marker. How compelling did the evidence need to be to adopt KRAS mutational status as a hard stop exclusion from our using these agents in a previously approved setting? More specifically, how definitive do the data need to be that a marker does, in fact, exclude activity to the degree claimed, and to what degree does the marker need to fully exclude activity versus merely decrease the likelihood of activity? Illustrating the difficulties inherent in proposing solutions to these questions, substantial differences of opinion existed even among the national thought leaders in CRC as to when the burden of proof was sufficient to justify routine use of KRAS testing to make clinical decisions regarding anti-EGFR therapies. Some were comfortable deciding based on modest-sized retrospective analyses of treatment with cetuximab in the setting of refractory CRC. Some became persuaded by the retrospective analysis of the small, randomized, panitumumab versus best supportive care trials. However, others were not comfortable with the use of KRAS testing until demonstration of its utility in the CRYSTAL trial, the large randomized first-line trial of FOLFIRI with and without cetuximab [10, 13]. As a result, some experts were routinely testing all metastatic CRC patients for KRAS mutations as early as mid-2007, whereas sufficient consensus as per the National Comprehensive Cancer Network CRC guidelines committee was not reached until the autumn of 2008. Others chose not to change their practice until the FDA changed the label of cetuximab and panitumumab to exclude use in KRAS-mutated tumors, which occurred in the autumn of 2009. Clearly, many concerned, thoughtful, individuals, all deeply committed to optimizing care of patients with incurable CRC, had different interpretations of the reliability of the same evidence and reached their individual comfort levels at markedly different times.
The matter is further complicated when considering the other stakeholders involved. One could anticipate that clinicians, patient advocacy groups, and pharmaceutical manufacturers and their shareholders might all have relatively low thresholds for accepting a new inclusionary marker, and might have a higher threshold for embracing an exclusionary one, whereas third-party payers and those charged with containing national health care expenditures could possibly display the reverse, with a higher degree of evidence needed for acceptance of an inclusionary marker and a more rapid acceptance of an exclusionary one. Any solution to further ongoing questions of this nature will likely need to consider the needs, rights, and responsibilities of all these entities.
Conclusions
This case study highlights two important questions: (a) When is the level of evidence regarding a specific therapeutic agent or class of treatment sufficient to warrant change, both in practice and in ongoing studies? (b) When and by what mechanism should data be released from embargo restraints without penalty in terms of meeting presentation or manuscript publication guidelines? We believe that earlier dissemination is necessary either when trial results offer substantial potential benefit to patients or when data emerge that an ongoing therapy may substantially harm those treated. We feel the latter situation is much more likely to present a pressing need for early release of data. In general, trials are set up to demand some element of maturity and peer review, in order to validate positive findings. Indeed, it would be relatively rare for an ASCO abstract submission suggesting benefit to be so definitive and of such magnitude that waiting for formal public vetting or peer-review would deprive a significant number of patients from achieving a meaningful outcome from their current cancer treatment. On the other hand, an early signal of potential toxicity from an investigational treatment (in the context of no associated benefit) could emerge from a solitary trial and is very likely to be real. Beyond the practical considerations involved in data interpretation, there is also an ethical distinction between adding a potentially beneficial treatment that inevitably has some associated risks and subtracting a treatment that potentially has more toxicity (harm) than previously realized. The primacy of primum non nocere in medicine favors a lower threshold for making the latter decision than the former. Allowing patients to be exposed to potential harm while awaiting standard public vetting thus appears particularly challenging from an ethical standpoint.
We propose that an enhanced mechanism be developed to permit early sharing of otherwise “embargoed” preliminary research results that might bear on clinical care. This need is urgent. Currently, professional organizations that sponsor national meetings allow early disclosure of results for reasons of public health. However, as noted above and exemplified in the KRAS story, multiple threads of evidence rather than a single definitive trial may be present. In an effort to build consensus with a goal of developing a new mechanism for early results disclosure, we propose that a group of stakeholders, including clinical experts, biostatisticians, NCI members, those involved in major meeting embargo policies, representatives of the SEC and FDA, holders of research protocol intellectual property (including sponsoring pharmaceutical companies), and patient advocates, be convened to develop a policy with clear and transparent criteria for immediate disclosure and early discussion of significant data (most likely signifying that a treatment is harmful). The threshold for the “significance” of such data might be higher when the data contradict an existing standard of care, because presumably the standard of care has been supported by a larger body of data than the one or two trial(s) under consideration for early disclosure. The goals of the stakeholder group we propose would be to further discuss rules defining “significant data” and underlying identification of appropriate trials, as well as to discuss when, with whom, and how such data could be disclosed with a goal of rapidly determining whether widespread dissemination is appropriate.
Acknowledgments
This commentary was developed by the authors on behalf of the NCI GI Steering Committee Colon Cancer Task Force. Members of the Colon Cancer Task Force include:
Neal J. Meropol, M.D., Chair, Axel Grothey, M.D., Vice-Chair, Steven R. Alberts, M.D., Carmen Allegra, M.D., Jacqueline Benedetti, Ph.D., Al B. Benson III, M.D., Charles D. Blanke, M.D., George Chang, M.D., Fergus Coakley, M.D., S. Gail Eckhardt, M.D., Lee M. Ellis, M.D., Cathy Eng, M.D., Patrick J. Flynn, M.D., Sharlene Gill, M.D., Philip J. Gold, M.D., Richard M. Goldberg, M.D., Daniel G. Haller, M.D., Deborah Jaffe, Ph.D., Derek Jonker, M.D., Lisa A. Kachnic, M.D., Najjia Mahmoud, M.D., Robert Mattrey, M.D., Pam McAllister, Wells Messersmith, M.D., Margaret Mooney, M.D., Heidi Nelson, M.D., Peter J. O'Dwyer, M.D., Nicholas J. Petrelli, M.D., Mitchell C. Posner, M.D., Nancy Roach, David Ryan, M.D., Daniel J. Sargent, Ph.D., Anthony F. Shields, M.D., Elin R. Sigurdson, M.D., Ph.D., Joel E. Tepper, M.D., John J. Welch, M.D., Ph.D., Alan P. Venook, M.D.
The authors wish to thank Michael Anderson for expert editorial assistance and Deborah Jaffe for superlative administrative support. Drs. Meropol and Grothey receive financial support from the NCI for their roles on the NCI Colon Cancer Task Force. Drs. Alberts, Benedetti, Benson, Blanke, Ellis, Flynn, Haller, Jonker, Messersmith, Petrelli, Posner, Sargent, Tepper, and Venook and Ms. Roach receive financial support from the NCI for their roles on the NCI GI Steering Committee.
All authors contributed equally to this work.
Author Contributions
Conception/Design: Charles D. Blanke, Margaret Mooney, Axel Grothey, Richard M. Goldberg, Nancy Roach, Leonard B. Saltz, John J. Welch, William A. Wood, Neal Meropol
Data analysis and interpretation: Charles D. Blanke, Margaret Mooney, Axel Grothey, Richard M. Goldberg, Nancy Roach, Leonard B. Saltz, John J. Welch, William A. Wood, Neal Meropol
Manuscript writing: Charles D. Blanke, Margaret Mooney, Axel Grothey, Richard M. Goldberg, Nancy Roach, Leonard B. Saltz, John J. Welch, William A. Wood, Neal Meropol
Final approval of manuscript: Charles D. Blanke, Margaret Mooney, Axel Grothey, Richard M. Goldberg, Nancy Roach, Leonard B. Saltz, John J. Welch, William A. Wood, Neal Meropol
References
- 1.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 2.Scheithauer W, Rosen H, Kornek GV, et al. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306:752–755. doi: 10.1136/bmj.306.6880.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Committee on Cancer Clinical Trials and the NCI Cooperative Group Program. Institute of Medicine. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. In: Nass SJ, Moses HL, Mendelsohn J, editors. Washington, DC: The National Academies Press; 2010. pp. 1–316. [PubMed] [Google Scholar]
- 4.Mauer AM, Rich ES, Schilsky RL. Cancer Clinical Trials: Proactive Strategies. New York: Springer; 2007. The role of cooperative groups in cancer clinical trials. [DOI] [PubMed] [Google Scholar]
- 5.Coordinating Center for Clinical Trials, National Cancer Institute, National Institutes of Health. Transforming the NCI Clinical Trials Enterprise: Overview. [accessed May 5, 2011]. Available at http://transformingtrials.cancer.gov/initiatives/overview.
- 6.Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 7.Amado RG, Wolf M, Freeman D, et al. Analysis of KRAS mutations in patients with metastatic colorectal cancer receiving panitumumab monotherapy [abstract 7LB] Proc Eur Ca Conf. 2007;5:8. [Google Scholar]
- 8.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 9.Hecht JR, Mitchell EP, Chidiac T, et al. Interim results from PACCE: Irinotecan/bevacizumab +/- panitumumab as first-line treatment for metastatic colorectal cancer [abstract 279] Proc GI Symp. 2008;5:208. [Google Scholar]
- 10.Van Cutsem E, Lang I, D'haens G, et al. KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer treated with FOLFIRI with or without cetuximab: The CRYSTAL experience [abstract 2] Proc Am Soc Clin Oncol. 2008;26:5s. [Google Scholar]
- 11.Bokemeyer C, Bondarenko I, Hartmann JT, et al. KRAS status and efficacy of first-line treatment of patients with metastatic colorectal cancer with FOLFOX with or without cetuximab: The OPUS experience [abstract 4000] Proc Am Soc Clin Oncol. 2008;26:178s. [Google Scholar]
- 12.Van Cutsem E, Nowacki M, Lang I, et al. Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer: The CRYSTAL trial [abstract 4000] Proc Am Soc Clin Oncol. 2007;25:164s. [Google Scholar]
- 13.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 14.Bokemeyer C, Bondarenko I, Makhson A, et al. Cetuximab plus 5-FU/FA/oxaliplatin (FOLFOX-4) versus FOLFOX-4 in the first-line treatment of metastatic colorectal cancer: OPUS, a randomized phase II study [abstract 4035] Proc Am Soc Clin Oncol. 2007;25:172s. [Google Scholar]
- 15.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 16.De Roock W, Jonker DJ, Di Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–1820. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]
- 17.Peters WP, Rosner GL, Vredenburgh JJ, et al. Prospective, randomized comparison of high-dose chemotherapy with stem-cell support versus intermediate-dose chemotherapy after surgery and adjuvant chemotherapy in women with high-risk primary breast cancer: A report of CALGB 9082, SWOG 9114, and NCIC MA-13. J Clin Oncol. 2005;23:2191–2220. doi: 10.1200/JCO.2005.10.202. [DOI] [PubMed] [Google Scholar]
- 18.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 19.Ruzzo A, Cremolini C, Loupakis F, et al. Association of BRAF mutations and EGFR intron-1 L/L genotype with resistance to cetuximab plus irinotecan treatment in KRAS wild-type metastatic colorectal cancer patients [abstract 4058] Proc Am Soc Clin Oncol. 2009;27:15s. [Google Scholar]
- 20.Bokemeyer C, Kohne C, Rougier P, et al. Cetuximab with chemotherapy as first-line treatment for metastatic colorectal cancer: Analysis of the CRYSTAL and OPUS studies according to KRAS and BRAF mutation status [abstract 3506] Proc Am Soc Clin Oncol. 2010;28:15s. [Google Scholar]
- 21.Van Cutsem E, Kang Y, Chung H, et al. Efficacy results from the ToGA trial: A phase III study of trastuzumab added to standard chemotherapy in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer [abstract LBA4509] Proc Am Soc Clin Oncol. 2009;27:18s. [Google Scholar]
- 22.Punt CJ, Tol J, Rodenburg J, et al. Randomized phase III study of capecitabine, oxaliplatin, and bevacizumab with or without cetuximab in advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group [abstract LBA4011] Proc Am Soc Clin Oncol. 2008;26:1008s. doi: 10.1093/annonc/mdm607. [DOI] [PubMed] [Google Scholar]
- 23.Presented in part at the World Congress on Gastrointestinal Cancer; June 25–28, 2008; Barcelona. [Google Scholar]
- 24.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]