Approximately one quarter of acute myeloid leukemia patients carry an internal tandem duplication mutation in the gene encoding FMS-like tyrosine kinase 3 (FLT3), which has a significantly deleterious impact on prognosis. The potential role of FLT3 as a target for therapy in these patients is examined and a survey of FLT3 inhibitors studied in clinical trials is provided.
Keywords: FLT3, Acute myeloid leukemia, Internal tandem duplication, Tyrosine kinase inhibitors, Targeted therapy
Learning Objectives
After completing this course, the reader will be able to:
Incorporate FLT3 mutational status into the initial diagnostic evaluation of AML to acquire prognostic information and guide the aggressiveness of consolidative therapy.
Select FLT3-mutant patients to participate in clinical trials of FLT3 inhibitors in order to help provide important insight into the future utility and promise of these compounds as adjuncts to therapy.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Acute myeloid leukemia (AML) is a hematologic malignancy with a poor prognosis. Approximately one quarter of the patients with AML also carry an internal tandem duplication (ITD) mutation in the gene encoding FMS-like tyrosine kinase 3 (FLT3), which has a significantly deleterious impact on prognosis. The ITD mutation renders FLT3 constitutively active and leads to uncontrolled proliferation of the leukemic blast. Over the course of the last decade, a variety of compounds have been developed in preclinical and clinical studies as potent inhibitors of FLT3. Many of the earlier agents under investigation, such as lestaurtinib, midostaurin, and sunitinib, were initially developed as inhibitors of other tyrosine kinases and as targeted therapies in a variety of malignancies. These compounds have been demonstrated to have some efficacy in clinical trials of AML, mainly manifesting as transient decreases in circulating blasts correlating with effective in vivo suppression of the FLT3 target. Nevertheless, the cumbersome pharmacokinetics of some compounds and the suboptimal specificity and potency of others have limited their therapeutic efficacy. In the last few years, newer, more potent and specific agents have been under investigation, with the leading example being AC220. This agent has shown significant promise in early phases of clinical investigation, and is currently in more advanced clinical trials. Hope remains that FLT3 inhibition will be become an effective therapeutic adjunct to our current treatment approach to AML.
Introduction
The internal tandem duplication (ITD) mutation in the gene encoding FMS-like tyrosine kinase 3 (FLT3) is found in approximately one quarter of the patients with acute myeloid leukemia (AML) and confers a markedly poor prognosis. Patients with this alteration often relapse following cytotoxic chemotherapy, and the majority die from their disease [1–4]. Recent studies have indicated 5-year overall survival (OS) and disease-free survival (DFS) rates as low as 15%–16% for patients with FLT3-ITD mutant disease, in contrast to the OS and DFS rates of ∼40% in those with wild-type (WT) FLT3 AML [1]. Inhibitors of the FLT3 tyrosine kinase have been extensively studied preclinically, and several have now been developed and investigated in clinical trials for the treatment of AML patients. Among these are a number of compounds initially developed to target other tyrosine kinases, but were later found to be potent inhibitors of FLT3. These include sorafenib, sunitinib (SU11428), lestaurtinib (CEP-701), semaxinib (SU5416), and midostaurin (PKC412) [5–8]. Midostaurin and lestaurtinib are currently the farthest along in clinical trials and have been associated with transient clinical responses. In recent years, attempts have been made to develop more specific and potent inhibitors of FLT3 for clinical investigation. One such agent, AC220, has shown great promise and dramatic responses in early-phase trials of patients with AML [9].
FLT3 as a Target
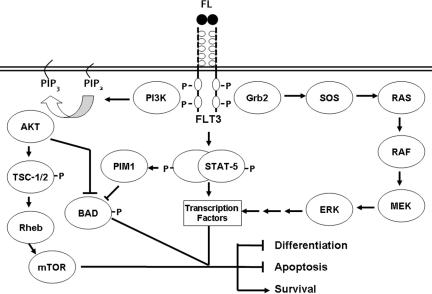
FLT3 was first cloned independently by two groups in the early 1990s [10, 11]. It resides on chromosome 13 and is comprised of 24 exons [12–14]. FLT3 is considered a type III receptor tyrosine kinase, a class that also includes KIT and platelet-derived growth factor receptor (PDGFR), proteins with very close homology to FLT3 [3, 15, 16]. FLT3 consists of an extracellular region with five immunoglobulin-like domains, a transmembrane region, a short intracellular juxtamembrane portion, followed by an intracellular tyrosine kinase domain (TKD). Upon binding of its ligand, FLT3 dimerizes, leading to eventual autophosphorylation on the inner leaflet of the membrane, with subsequent activation of the tyrosine kinase. phosphoinositide 3-kinase (PI3K), AKT, mitogen-activated protein kinase (MAPK), and signal transducer and activator of transcription (STAT)-5 are all significant mediators of downstream FLT3 signaling (Fig. 1) [17–25].
Figure 1.
Simplified diagram of signaling cascades downstream of FLT3 that are thought to promote leukemogenesis.
Abbreviations: BAD, Bcl-2-associated death promoter; ERK, extracellular signal–related kinase; FL, FLT3 ligand; FLT3, FMS-like tyrosine kinase 3; Grb2, growth factor receptor-bound protein 2; MEK, mitogen-activated protein kinase/ERK kinase; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase; PIM1, proto-oncogene serine/threonine-protein kinase 1; PIP2, phosphatidylinositol-bisphosphate; PIP3, phosphatidylinositol-trisphosphate; Rheb, Ras homolog enriched in brain; SOS, son of sevenless; STAT-5, signal transducer and activator of transcription 5; TSC, tuberous sclerosis protein.
Figure derived from one obtained courtesy of Dr. Mark Levis, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins, Baltimore, MD.
The cytokine that binds FLT3, the FLT3 ligand (FL), is ubiquitous to most tissues but appears functionally important only in hematopoietic and neural tissue [26, 27]. In the hematopoietic environment, FLT3 expression exists predominantly in CD34+ cells, although CD34− precursors of dendritic cells also express FLT3. FLT3 is a key mediator of early hematopoiesis and is involved with the reconstitution of early multilineage myeloid precursors [11, 28–30]. Disruption of FLT3 signaling in murine models is not lethal but does lead to a significant reduction in hematopoietic precursors. Specifically, when the FL gene was disrupted in mice, the numbers of myeloid and B lymphoid cells were markedly lower in the bone marrow. Interestingly, the numbers of dendritic and natural killer cells were also significantly lower in the spleen and thymus [31].
Overexpression of FLT3, or its constitutive activation, appears to play a major role in leukemias. Both FL and the FLT3 receptor have been demonstrated in the majority of human leukemia cell lines [32, 33]. FLT3 is expressed in higher amounts in AML blasts than in cells from normal bone marrow. Additionally, in this setting, its expression is no longer tightly associated with CD34 expression as it is in normal precursors. Indeed, the large majority of evaluated AML cell lines have amplified activity of FLT3 [34–36]. Some of these cells exhibited overexpression of WT FLT3, but others were found to have activating mutations that rendered FLT3 constitutively active [12, 37–40].
ITDs of nucleotide sequences in exon 14 were the first form of FLT3 mutation discovered in AML, and are found in approximately 23% of patients with AML. These mutations localize to the juxtamembrane domain of the receptor tyrosine kinase, where they presumably offset the negative regulatory functions of this domain [41–44]. Another category of FLT3 mutations consists of activating point mutations within the activation loop of the kinase domain, mostly localized at the aspartate 835 (D835) residue and found in an additional 7% of patients [12, 45]. FLT3 mutations result in constitutive activation, leading in turn to activation of STAT-5 as well as the MAPK and AKT signaling cascades. This results in suppression of apoptosis and dysregulated cell proliferation [38, 46, 47]. The ITD mutations, in particular, have been consistently found to have a negative prognostic impact. This was confirmed, in large part, by studies of banked AML samples from the European cooperative groups and multiple subsequent clinical studies, which have demonstrated that patients with FLT3 ITD mutations often present with leukocytosis, experience significantly higher rates of relapse, and have shorter DFS and OS times. According to recent studies, patients with newly diagnosed FLT3-ITD AML have DFS and OS rates as low as 15% at 5 years, in comparison with the ∼40% rates in those with WT FLT3. FLT3 point mutations, on the other hand, do not appear to carry with them the same degree of negative prognostic impact as FLT3 ITD mutations [1, 2, 4, 48–51]. These findings have brought forth a rationale and provided significant impetus to develop effective FLT3 inhibitors as therapy for AML patients.
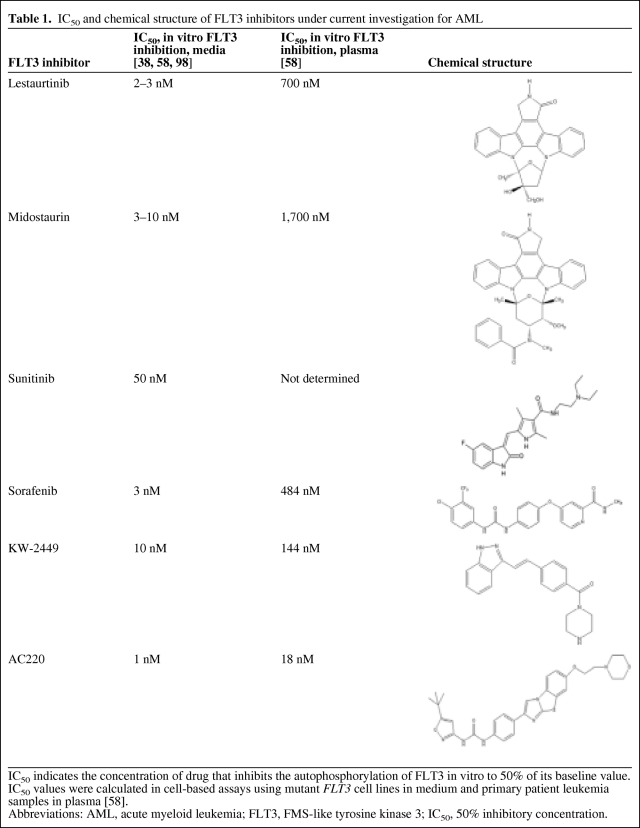
In recent years, multiple compounds that inhibit the kinase function of FLT3 have undergone preclinical investigation (Table 1). Most are structural mimics of the purine component of ATP, and in this manner occupy the ATP-binding pocket of the tyrosine kinase [52, 53]. A number of assays have been employed to assess the in vitro potency and selectivity of FLT3 inhibitors, including in vitro kinase assays, cell-based receptor autophosphorylation assays, and cytotoxicity assays. Results from these studies have indicated that, in general, a specific FLT3 inhibitor induces preferential cytotoxicity, as demonstrated by a significantly lower 50% inhibitory concentration (IC50), in FLT3-ITD AML cells. In addition, sustained and potent FLT3 inhibition (<10% FLT3 activity) is required to produce effective cell death of myeloblasts [5, 54].
Table 1.
IC50 and chemical structure of FLT3 inhibitors under current investigation for AML
IC50 indicates the concentration of drug that inhibits the autophosphorylation of FLT3 in vitro to 50% of its baseline value. IC50 values were calculated in cell-based assays using mutant FLT3 cell lines in medium and primary patient leukemia samples in plasma [58].
Abbreviations: AML, acute myeloid leukemia; FLT3, FMS-like tyrosine kinase 3; IC50, 50% inhibitory concentration.
The validation of FLT3 as a viable therapeutic target has also been supported by studies that have sought to suppress FLT3 by alternative mechanisms. One such manner of targeting FLT3 has been by RNA interference (RNAi) to induce downregulation of the tyrosine kinase. In those studies, downregulation of FLT3 led to a lower rate of phosphorylation of the FLT3 targets STAT-5, AKT, and MAPK. More importantly, the viability and growth of these cell lines were significantly lower following knockdown of FLT3. The investigators further demonstrated that the sensitivity of these cell lines to the FLT3 inhibitor tandutinib was enhanced by RNAi-induced downregulation of the FLT3 target [55]. Preclinical studies have therefore led to the conclusion that the activation of FLT3 results in activation of downstream signaling pathways and promotion of cell survival, and its downregulation can promote cytotoxicity, and therefore act as a potential therapeutic mechanism.
Sustained and effective inhibition of FLT3 appears necessary for clinical responses in trials, and therefore, presumably, for any FLT3 inhibitor to be successful clinically it must be able to accomplish this molecular feat in vivo. This is supported by results from correlative studies of clinical trials of FLT3 inhibitors, in which effective suppression of FLT3 phosphorylation strongly correlated with clinical response [54, 56]. The earlier, more multitargeted, generation of FLT3 inhibitors has been generally associated with transient, though often dramatic, decreases in peripheral blast counts. The suboptimal performance of these agents in clinical trials has at times been linked to their pharmacokinetic parameters, and in other cases to a lack of effective and sustained FLT3 inhibition. In the last few years, however, FLT3 inhibitors with more potent and constant suppression of their target have been associated with more impressive clinical outcomes, albeit in earlier phases of clinical investigation.
Inhibitors of FLT3
As mentioned above, the majority of the characterized FLT3 inhibitors are heterocyclic compounds with a component resembling a purine ring. They can thus act as competitors of ATP for the FLT3 binding site. The exact mechanism of binding for all FLT3 inhibitors is not completely clear, although some, such as the staurosporine derivatives (e.g., lestaurtinib), bind via an induced fit mechanism, whereas others interact in a lock and key manner [53, 57]. The clinical effectiveness of FLT3 inhibitors currently under study is quite variable, and in many ways dependent on the pharmacodynamic and pharmacokinetic properties of the compounds. Like other tyrosine kinase inhibitors (TKIs), the degree and duration of inhibition is determined by the potency of the inhibitor, its susceptibility to metabolism and protein binding, its pharmacokinetic properties such as half-life and rate of elimination, as well as the related factor of dosing frequency. Perhaps, most importantly, clinical efficacy depends on the dependence of leukemic cells on the FLT3 pathway. Very recent studies have indeed demonstrated that relapsed leukemia and other samples with a high mutant FLT3 allelic burden are more likely to be responsive to cytotoxicity from FLT3 inhibition [58].
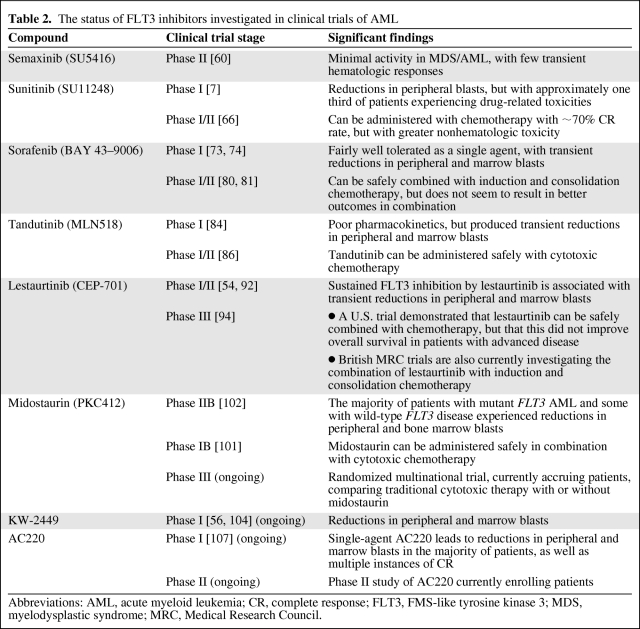
We henceforth review the results from clinical evaluation of FLT3 inhibitors. The structure and in vitro potency of a number of the evaluated FLT3 inhibitors are demonstrated in Table 1. The results of key clinical trials are summarized in Table 2.
Table 2.
The status of FLT3 inhibitors investigated in clinical trials of AML
Abbreviations: AML, acute myeloid leukemia; CR, complete response; FLT3, FMS-like tyrosine kinase 3; MDS, myelodysplastic syndrome; MRC, Medical Research Council.
Early FLT3 Inhibitors
Semaxinib
Semaxinib is an indolinone-derived TKI. This compound was not initially described as an inhibitor of FLT3 and entered clinical trials based on its suppression of other targets often upregulated in myeloblasts, such as c-KIT and vascular endothelial growth factor receptor (VEGFR). Semaxinib has been studied in clinical trials of refractory AML and myelodysplastic syndrome (MDS) patients. In one study of 55 patients, four instances of a transient hematologic response were noted, and in another phase II trial of 42 patients, one morphologic complete response (CR) and seven partial responses in the peripheral blood and marrow were found [59, 60]. Given the structural homology of c-KIT and FLT3, it was not surprising to find that semaxinib also effectively suppressed the latter target in leukemia cells [8]. Downstream effects of FLT3 inhibition by semaxinib included a lower rate of phosphorylation of the FLT3 targets STAT-5 and AKT. However, given the only modest clinical effects of this agent in patients with AML, no further advanced-phase studies have ensued.
Sunitinib
Like semaxinib, sunitinib can effectively inhibit multiple tyrosine kinases, including PDGFR, VEGFR, c-KIT, as well as FLT3 [61]. Sunitinib has been extensively studied in clinical trials of solid tumor malignancies [62–64] and is approved for use in metastatic renal cell carcinoma and gastrointestinal stromal tumors. O'Farrell et al. [61] demonstrated that sunitinib is a potent inhibitor of mutant FLT3 in AML cell lines, with an IC50 of 50 nM and single doses achieving potent inhibition for up to 16 hours in preclinical in vitro models. Sunitinib also inhibits the phosphorylation of WT FLT3 at an IC50 of 150 nM. A single-dose design, phase I trial of sunitinib followed in order to assess the in vivo effects of the compound in patients with AML. Clinical activity (decreases in peripheral blast counts) was noted in five of 29 patients, including subjects with both mutant FLT3 and WT FLT3 disease. Toxicity was mainly gastrointestinal and affected a third of patients [7]. A more traditional phase I trial of 15 patients with refractory AML again reported a small number of transient partial responses. These responses occurred in all four patients with mutant FLT3 AML and two patients with WT FLT3 disease. However, three patients experienced grade 3–4 toxicity, and six deaths were reported by the investigators (four cases of bleeding and two of cardiac dysfunction) [65].
Those studies demonstrated that sunitinib does lead to clinical responses in a fraction of treated patients with AML. Its associated significant toxicity, however, which has affected a number of patients studied, has limited the ability to maintain continuous dosing of the drug in patients with AML. A phase I/II trial of sunitinib administered concurrently with induction 7+3 chemotherapy is currently enrolling older patients with mutant FLT3 AML. Interim results were presented at the recent annual meeting of the American Society of Hematology (ASH). Twelve patients have been enrolled, eight of whom harbored FLT3 ITD mutations, and four of whom had FLT3 TKD mutations. Although seven of 10 evaluable patients achieved a CR or complete remission with insufficient blood count recovery, a high proportion of patients (>40%) experienced neutropenic fever, infection, and colitis [66], and long-term follow-up is not yet available.
Sorafenib
Sorafenib is now approved by the U.S. Food and Drug Administration for use in advanced renal cell and hepatocellular carcinoma patients [67, 68]. Sorafenib is a potent inhibitor of multiple receptor tyrosine kinases, including FLT3, c-KIT, NRAS, and RAF kinase, all of which can be upregulated in AML and appear to promote leukemogenesis and drive proliferation in myeloblasts [69, 70]. Indeed, sorafenib has been shown to suppress FLT3 phosphorylation and downstream signaling, leading to apoptosis of leukemia cells [71, 72]. In comparison with some other multitargeted FLT3 inhibitors, sorafenib is more effective in inducing sustained FLT3 inhibition in experimental models and in patients. This finding may be related to an N-oxide metabolite of sorafenib, which was incidentally found by Pratz et al. [73] to be 15-fold more potent than the parent compound in its inhibition of FLT3 autophosphorylation in treated patients.
One phase I study of 15 patients with diagnoses of relapsed or refractory leukemia, the majority of which were AML, reported that six patients experienced transient decreases in bone marrow blast percentage. Preliminary data from another phase I study, from the MD Anderson Cancer Center, found that 11 of 20 evaluable patients experienced a ≥50% reduction in bone marrow blasts. Nine of the responders had FLT3-ITD disease. Those studies also revealed that sorafenib is relatively well tolerated as a single agent in AML patients [73, 74]. There has been increasing use of sorafenib on an off-label and compassionate-use basis for patients with advanced mutant FLT3 AML, and indeed dramatic cases of CR to single-agent sorafenib have been reported [75, 76]. Sorafenib has also been studied in the setting of allogeneic stem cell transplantation of mutant FLT3 AML. In a recent abstract presentation, six of 11 patients with refractory disease underwent allogeneic stem cell transplantation after responding to treatment with sorafenib. The same group also retrospectively reported the experience of multiple centers with daily sorafenib in the peritransplant setting. In that abstract presentation, nine of 16 patients who had relapsed after transplant experienced reductions in peripheral blood or bone marrow myeloblasts, and four experienced a complete molecular remission [77, 78]. However, in another retrospective analysis, the use of sorafenib to treat 16 patients with relapsed FLT3-ITD AML following stem cell transplantation did not appear effective, because the drug produced only two transient partial responses [79].
A phase I/II trial of 51 newly diagnosed AML patients investigated sorafenib given concurrently with cytarabine- and idarubicin-based induction. Thirty-eight patients (75%) achieved a CR following induction, but remarkably the investigators reported very high response rates in particular for patients with FLT3-ITD AML, whereby 14 of 15 patients achieved a CR. Correlative studies reported effective suppression of FLT3 phosphorylation in FLT3 ITD mutant patients who achieved a CR, and there was a fivefold greater suppression of ITD FLT3 than WT FLT3 [80]. A European multicenter, randomized, placebo-controlled phase II trial in elderly patients is evaluating sorafenib combined with standard induction, consolidation, and maintenance chemotherapy. Data from that trial were presented at the recent ASH annual meeting. Although the combination was well tolerated in this group of 197 patients, no benefit in terms of event-free survival, OS, or the rate of CR has so far been noted, even in patients with mutant FLT3 AML [81]. Other ongoing clinical trials are further evaluating sorafenib in combination with targeted therapies and cytotoxic reg-imens (ClinicalTrials.gov identifiers, NCT00516828, NCT00908167, NCT 00373373, NCT00893373, NCT00875745, NCT00943943), but results are not yet available.
Tandutinib
Tandutinib, also known as MLN518, is somewhat unique among its predecessors because of its greater selectivity against FLT3, even though it does also display some inhibitory effects on c-KIT and PDGFR, which, as mentioned above, share structural homology with FLT3 [82]. Initial preclinical assays revealed that tandutinib was preferentially cytotoxic to FLT3-ITD cells lines. However, it was not found to be a particularly potent compound, inhibiting FLT3 phosphorylation at relatively high concentrations, with an IC50 of ∼30 nM in cell-based assays [82, 83].
A phase I trial in patients with relapsed/refractory AML and high-risk MDS revealed that a significant number of patients experienced nausea and vomiting, and that the dose-limiting toxicity was reversible muscle weakness. Correlative studies revealed that tandutinib was cleared slowly, leading to elevated plasma levels that may have exacerbated the therapy-related toxicity. Those experiencing the dose-limiting toxicity of profound muscle weakness all had persistently elevated plasma levels of tandutinib, which was felt to be responsible for the adverse therapeutic index of the agent. Nevertheless, two of eight evaluated patients with FLT3-ITD AML experienced transient decreases in blast percentage in the blood and bone marrow, both lasting <60 days. No antileukemic effects were noted in patients with WT FLT3 [84].
Tandutinib in combination with the antileukemic drugs cytarabine and daunorubicin displayed synergism when incubated with FLT3-ITD leukemia samples [85]. A phase I trial of tandutinib combined with induction therapy has been completed. Although the therapeutic efficacy results are not yet available, preliminary data reported at the 2006 ASH annual meeting suggested that the combination is well tolerated [86]. In summary, the suboptimal pharmacokinetics and the relatively low potency of tandutinib have limited its clinical utility and promise as an FLT3 inhibitor.
Lestaurtinib
Lestaurtinib has perhaps been the most extensively studied FLT3 inhibitor in clinical trials. Previously known as CEP-701, it is a polyaromatic indolocarbazole compound that effectively inhibits multiple tyrosine kinases, including RET, Janus kinase 2, tropomyosin related kinase (TRK), as well as FLT3 [87–89]. Lestaurtinib was initially evaluated as therapy in solid tumor malignancies, given its activity against TRK. In this setting, although the drug was well tolerated, no objective tumor responses were noted [90].
Preclinical studies of lestaurtinib suggested that it is a potent inhibitor of FLT3, inhibiting FLT3 autophosphorylation in ITD cell lines at an IC50 of 2 nM and exhibiting preferential cytotoxicity against FLT3-ITD cells [38]. In contrast to the negative results noted in clinical trials of solid tumors, a phase I/II trial of 17 patients with relapsed/refractory mutant FLT3 AML (with all but one having ITD mutations) reported four patients with decreases in peripheral myeloblasts and one with a dramatic decrease in bone marrow blasts to <5%. Lestaurtinib was also fairly well tolerated in these patients, with common toxicities of fatigue and nausea. It was further noted that sustained suppression of FLT3 phosphorylation (over the course of 4–5 weeks of therapy) correlated strongly with the observed clinical responses [54, 91]. A subsequent phase II trial of newly diagnosed elderly patients was not restricted on the basis of FLT3 mutational status. In that study, three of five patients with FLT3 mutations experienced transient hematologic responses, mainly manifested as decreases in peripheral blasts. Interestingly, an additional five patients with WT FLT3 experienced decreases in bone marrow blasts. These results were attributed to possible overexpression of FLT3 in these patients. These results may also be secondary to the multitargeted profile of lestaurtinib. In all eight patients who responded to lestaurtinib, the phosphorylation of FLT3 was continuously suppressed to <15% of baseline (as measured over time and on days 14, 28, and 56 of the study), again confirming that effective and sustained inhibition of FLT3 appears necessary for any clinical response [92].
In vitro studies demonstrated that lestaurtinib administered after cytotoxic chemotherapy led to synergistic leukemia cytotoxicity [93], which provided a rationale for this sequence. A multicenter trial of patients with relapsed AML randomized subjects to reinduction chemotherapy alone or chemotherapy followed by lestaurtinib. The results were presented at the 2009 ASH annual meeting. Unfortunately, the addition of lestaurtinib did not result in higher response rates or longer OS time in these patients with advanced disease. Correlative studies revealed that only a minority of patients achieved >85% FLT3 target inhibition by day 15 of therapy. The presenters speculated that this may, in turn, have been partly a result of elevations (as a response to cytotoxic chemotherapy) in plasma levels of FL [94]. The same investigators have indeed demonstrated a blunting of FLT3 inhibition by a variety of TKIs in the presence of increasing concentrations of FL [95].
Lestaurtinib was also incorporated into induction and consolidation chemotherapy regimens for mutant FLT3 patients in the British Medical Research Council 15 and 17 trials. The results of those trials have not been fully reported, but unlike the phase III trial above, the British studies have not been limited to relapsed patients and include patients receiving induction and consolidation regimens. Initial reports suggest effective inhibition (>85% inhibition) of FLT3 phosphorylation in samples from a majority of evaluated patients, and, to date, 77 of 83 (93%) evaluable patients have achieved a CR. The final results of these trials are eagerly anticipated [96].
Midostaurin
Midostaurin, also known as PKC412, is a staurosporine derivative, described initially as an inhibitor of protein kinase C. However, midostaurin was subsequently found to suppress the tyrosine kinases VEGFR, PDGFR, c-KIT, as well as FLT3. This multitargeted potential suggested promise in a variety of malignancies as an antiangiogenic and antiproliferative agent [97]. A phase I trial of midostaurin in solid tumors revealed minimal responses, that the primary toxicities were gastrointestinal, and that the drug was generally well tolerated [98].
Midostaurin was subsequently confirmed to be a potent inhibitor of FLT3 autophosphorylation, with an IC50 of 10 nM in FLT3-ITD cell lines [99]. A phase I trial of midostaurin was performed in patients with relapsed/refractory AML. Seven of 20 patients experienced transient decreases in peripheral blasts and five experienced decreases in bone marrow blasts, similar to results seen with other inhibitors of FLT3 [100].
Data from a phase Ib trial of midostaurin combined with induction chemotherapy in newly diagnosed patients was presented at the 2009 ASH annual meeting, revealing that mutant FLT3 patients had a rate of OS at 2 years similar to that of WT FLT3 AML patients [101]. Earlier this year, results of a phase IIb trial of midostaurin were published comparing two different dosages (50 mg and 100 mg daily) of the agent in patients with AML and MDS. Sixty-five of 92 patients (71%) with an FLT3 mutation experienced a significant decrease in marrow or peripheral blasts (≥50%) on therapy, as did 39 patients (42%) with WT FLT3 disease. Response rates did not differ according to dose. These results suggest that some patients with WT FLT3 disease may derive clinical benefit from this agent, perhaps explained by the multitargeted profile of midostaurin [102]. A randomized, multicenter, phase III study of midostaurin with induction and consolidation chemotherapy followed by midostaurin maintenance in newly diagnosed patients, is currently ongoing (ClinicalTrials.gov identifier, NCT00651261).
Inhibitors of FLT3: Newer Agents
Agents in the earlier generation of FLT3 inhibitors were initially developed against other tyrosine kinase targets for use in a variety of nonhematologic malignancies. Indeed, their relative nonselectivity could explain their efficacy in newly diagnosed mutant FLT3 AML patients, especially because multiple upregulated pathways, in addition to FLT3, may drive the proliferation of myeloblasts. This is especially true in the case of the broad TKIs sorafenib, lestaurtinib, and midostaurin. However, nonselectivity may also be associated with a broader range of toxicity, and in some cases a lesser degree of potency.
The newer generation of FLT3 inhibitors exhibit, in part, greater relative specificity for FLT3. This specificity may hold greater promise, especially in the setting of relapsed disease, wherein leukemic cells have been characterized as having a greater mutant FLT3 allele burden. These blasts appear addicted to and driven primarily by constitutively active FLT3 [58]. In such a setting, specific and potent FLT3 inhibitors, such as AC220 (currently in clinical trials), may hold greater promise.
KW-2449
KW-2449, a TKI from Kyowa Hakko Kirin Pharma Inc. (Princeton, NJ), effectively suppresses FLT3 phosphorylation, but also has activity against the Abl and aurora kinases. It has been studied in mutant FLT3 cell lines, where it was found to effectively suppress the phosphorylation of FLT3 and its downstream target STAT-5 at an IC50 of approximately 15 nM [103]. Given these promising preclinical findings, a clinical trial followed soon thereafter.
A phase I trial of KW-2449 reported that eight of 31 enrolled patients achieved a 50% reduction in peripheral or bone marrow blasts, with the majority (five) of responders harboring FLT3-activating mutations [104]. The responses consisted of transient decreases in blast percentage, and as with other FLT3 inhibitors, correlated with in vivo FLT3 inhibition. However, the plasma half-life of this agent is quite short (2.5–3.5 hours), leading to difficulty in maintaining plasma levels of the drug, and thus requiring frequent dosing for sustained FLT3 inhibition. This may be a limiting factor in the clinical use of this drug [56].
AC220
AC220, developed by Ambit Biosciences (San Diego, CA), is the most recent addition to the group of FLT3 inhibitors currently under clinical investigation. Preclinical studies of AC220 demonstrated significant selectivity in the inhibition of FLT3, but also higher potency, by one to two orders of magnitude, than other FLT3 inhibitors [105, 106]. AC220 also has a long plasma half-life of approximately 1.5 days, allowing sustained FLT3 inhibition and more practical dosing for patients. A recent survey of FLT3 inhibitors currently in clinical investigation (lestaurtinib, midostaurin, sorafenib, sunitinib, KW-2449, and AC220) found that all agents inhibited FLT3-ITD phosphorylation effectively in media-based cell lines, with an IC50 in the range of 1–10 nM, and with AC220 exhibiting the greatest potency (1 nM). However, the potency of these agents in plasma varied across two orders of magnitude (IC50 values in the range of 18–1,700 nM). AC220 again was the most potent, by a significant margin (Table 1). These results accentuate the importance of plasma protein binding and other pharmacokinetic factors affecting target inhibition in vivo, and suggest that AC220 most effectively maintains its potency in vivo, in comparison with other agents [58].
A phase I study of AC220 in patients with relapsed/refractory AML reported very promising preliminary results. Eleven of 45 evaluated patients (24%) experienced transient clinical responses, and four patients achieved a CR with single-agent AC220. Three of the responders harbored FLT3 mutations, but the other responders had WT FLT3 [9]. A phase II trial of AC220 in relapsed/refractory patients with mutant FLT3 AML is currently enrolling patients at multiple institutions (ClinicalTrials.gov identified, NCT00651261). The high specificity and potency against FLT3, along with a favorable pharmacokinetic profile, indicate significant promise for this new agent as effective therapy for patients with mutant FLT3 AML.
Conclusion
Patients with AML and an FLT3 ITD mutation have a particularly poor prognosis. Their disease is marked by an aggressive presentation and a high propensity for relapse. In fact, the large majority of patients relapse after induction therapy and most ultimately succumb to their disease. Since the cloning of FLT3 more than 15 years ago, we have learned much regarding the structure and function of this receptor tyrosine kinase and the mutations that affect its function. ITD alterations, the most common FLT3 mutations in AML, render the receptor constitutively active and lead to uncontrolled proliferation of blasts. Inhibition of FLT3 in model systems suppresses the autophosphorylation of FLT3, inhibits downstream signaling, and leads to apoptosis.
In the last decade, multiple inhibitors of FLT3 have been investigated in clinical trials. Some of the earlier agents were initially developed as inhibitors of other tyrosine kinases, and some, such as sorafenib and sunitinib, are effectively used today as therapy in solid tumor malignancies. These compounds were found to also be potent inhibitors of FLT3 and displayed clinical promise in mutant FLT3 AML, with a proportion of treated patients experiencing transient clinical responses in the peripheral blood and bone marrow. However, the relative nonselectivity of some FLT3 inhibitors and the suboptimal pharmacokinetics of others may have been responsible for the disappointing results in clinical trials. Despite the lower selectivity and modest potency of most available FLT3 inhibitors, compounds such as lestaurtinib and midostaurin are currently being studied in advanced phases of clinical investigation and may still hold promise as future adjuncts to the treatment of AML. AC220, a more selective and potent FLT3 inhibitor, has been demonstrated to have promising activity against AML in phase I studies. However, it is still in early phases of clinical investigation as single-agent therapy and in combination with traditional cytotoxic regimens. Therefore, despite encouraging preclinical results that appear to validate FLT3 as a target and the emergence of newer compounds with more therapeutic promise, it has yet to be established that FLT3 inhibitors will add clinical benefit to our current management of AML.
Author Contributions
Conception/Design: Amir T. Fathi
Manuscript writing: Amir T. Fathi, Bruce A. Chabner
Final approval of manuscript: Amir T. Fathi, Bruce A. Chabner
References
- 1.Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 2.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 3.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 4.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 5.Knapper S, Mills KI, Gilkes AF, et al. The effects of lestaurtinib (CEP701) and PKC412 on primary AML blasts: The induction of cytotoxicity varies with dependence on FLT3 signaling in both FLT3-mutated and wild-type cases. Blood. 2006;108:3494–3503. doi: 10.1182/blood-2006-04-015487. [DOI] [PubMed] [Google Scholar]
- 6.Levis M, Smith BD, Beran M, et al. A randomized, open-label study of lestaurtinib (CEP-701), an oral FLT3 inhibitor, administered in sequence with chemotherapy in patients with relapsed AML harboring FLT3 activating mutations: Clinical response correlates with successful FLT3 inhibition. Blood. 2005;106:121a. [Google Scholar]
- 7.O'Farrell AM, Foran JM, Fiedler W, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9:5465–5476. [PubMed] [Google Scholar]
- 8.Yee KW, O'Farrell AM, Smolich BD, et al. SU5416 and SU5614 inhibit kinase activity of wild-type and mutant FLT3 receptor tyrosine kinase. Blood. 2002;100:2941–2949. doi: 10.1182/blood-2002-02-0531. [DOI] [PubMed] [Google Scholar]
- 9.Cortes J, Foran J, Ghirdaladze D, et al. AC220, a potent, selective, second generation FLT3 receptor tyrosine kinase (RTK) inhibitor, in a first-in-human (FIH) phase 1 AML study. Blood. 2009;114 Abstract 636. [Google Scholar]
- 10.Rosnet O, Schiff C, Pébusque MJ, et al. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood. 1993;82:1110–1119. [PubMed] [Google Scholar]
- 11.Small D, Levenstein M, Kim E, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci U S A. 1994;91:459–463. doi: 10.1073/pnas.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Duhier FM, Goodeve AC, Wilson GA, et al. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113:983–988. doi: 10.1046/j.1365-2141.2001.02850.x. [DOI] [PubMed] [Google Scholar]
- 13.Agnès F, Shamoon B, Dina C, et al. Genomic structure of the downstream part of the human FLT3 gene: Exon/intron structure conservation among genes encoding receptor tyrosine kinases (RTK) of subclass III. Gene. 1994;145:283–288. doi: 10.1016/0378-1119(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 14.Carow CE, Kim E, Hawkins AL, et al. Localization of the human stem cell tyrosine kinase-1 gene (FLT3) to 13q12-->q13. Cytogenet Cell Genet. 1995;70:255–257. doi: 10.1159/000134046. [DOI] [PubMed] [Google Scholar]
- 15.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 16.van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- 17.Dosil M, Wang S, Lemischka IR. Mitogenic signalling and substrate specificity of the Flk2/Flt3 receptor tyrosine kinase in fibroblasts and interleukin 3-dependent hematopoietic cells. Mol Cell Biol. 1993;13:6572–6585. doi: 10.1128/mcb.13.10.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavagna-Sévenier C, Marchetto S, Birnbaum D, et al. FLT3 signaling in hematopoietic cells involves CBL, SHC and an unknown P115 as prominent tyrosine-phosphorylated substrates. Leukemia. 1998;12:301–310. doi: 10.1038/sj.leu.2400921. [DOI] [PubMed] [Google Scholar]
- 19.Lavagna-Sévenier C, Marchetto S, Birnbaum D, et al. The CBL-related protein CBLB participates in FLT3 and interleukin-7 receptor signal transduction in pro-B cells. J Biol Chem. 1998;273:14962–14967. doi: 10.1074/jbc.273.24.14962. [DOI] [PubMed] [Google Scholar]
- 20.Marchetto S, Fournier E, Beslu N, et al. SHC and SHIP phosphorylation and interaction in response to activation of the FLT3 receptor. Leukemia. 1999;13:1374–1382. doi: 10.1038/sj.leu.2401527. [DOI] [PubMed] [Google Scholar]
- 21.Mizuki M, Schwable J, Steur C, et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood. 2003;101:3164–3173. doi: 10.1182/blood-2002-06-1677. [DOI] [PubMed] [Google Scholar]
- 22.Scheijen B, Ngo HT, Kang H, et al. FLT3 receptors with internal tandem duplications promote cell viability and proliferation by signaling through Foxo proteins. Oncogene. 2004;23:3338–3349. doi: 10.1038/sj.onc.1207456. [DOI] [PubMed] [Google Scholar]
- 23.Levis M. Recent advances in the development of small-molecule inhibitors for the treatment of acute myeloid leukemia. Curr Opin Hematol. 2005;12:55–61. doi: 10.1097/01.moh.0000148761.23036.e6. [DOI] [PubMed] [Google Scholar]
- 24.Fathi AT, Grant S, Karp JE. Exploiting cellular pathways to develop new treatment strategies for AML. Cancer Treat Rev. 2010;36:142–150. doi: 10.1016/j.ctrv.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fathi AT, Swinnen I, Rajkhowa T, et al. PIM: An integral component of FLT3 signaling and a potential therapeutic target in acute myeloid leukemia. Blood. 2010;114 Abstract 1735. [Google Scholar]
- 26.Hannum C, Culpepper J, Campbell D, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 1994;368:643–648. doi: 10.1038/368643a0. [DOI] [PubMed] [Google Scholar]
- 27.Lyman SD, James L, Johnson L, et al. Cloning of the human homologue of the murine flt3 ligand: A growth factor for early hematopoietic progenitor cells. Blood. 1994;83:2795–2801. [PubMed] [Google Scholar]
- 28.Broxmeyer HE, Lu L, Cooper S, et al. Flt3 ligand stimulates/costimulates the growth of myeloid stem/progenitor cells. Exp Hematol. 1995;23:1121–1129. [PubMed] [Google Scholar]
- 29.Gotze KS, Ramirez M, Tabor K, et al. Flt3high and Flt3low CD34+ progenitor cells isolated from human bone marrow are functionally distinct. Blood. 1998;91:1947–1958. [PubMed] [Google Scholar]
- 30.Veiby OP, Jacobsen FW, Cui L, et al. The flt3 ligand promotes the survival of primitive hemopoietic progenitor cells with myeloid as well as B lymphoid potential. Suppression of apoptosis and counteraction by TNF-α and TGF-β. J Immunol. 1996;157:2953–2960. [PubMed] [Google Scholar]
- 31.McKenna HJ, Stocking KL, Miller RE, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 32.Drexler HG. Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells. Leukemia. 1996;10:588–599. [PubMed] [Google Scholar]
- 33.Meierhoff G, Dehmel U, Gruss HJ, et al. Expression of FLT3 receptor and FLT3-ligand in human leukemia-lymphoma cell lines. Leukemia. 1995;9:1368–1372. [PubMed] [Google Scholar]
- 34.Birg F, Courcoul M, Rosnet O, et al. Expression of the FMS/KIT-like gene FLT3 in human acute leukemias of the myeloid and lymphoid lineages. Blood. 1992;80:2584–2593. [PubMed] [Google Scholar]
- 35.Carow CE, Levenstein M, Kaufmann SH, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87:1089–1096. [PubMed] [Google Scholar]
- 36.Rosnet O, Bḧring HJ, Marchetto S, et al. Human FLT3/FLK2 receptor tyrosine kinase is expressed at the surface of normal and malignant hematopoietic cells. Leukemia. 1996;10:238–248. [PubMed] [Google Scholar]
- 37.Armstrong SA, Kung AL, Mabon ME, et al. Inhibition of FLT3 in MLL. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell. 2003;3:173–183. doi: 10.1016/s1535-6108(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 38.Levis M, Allebach J, Tse KF, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–3891. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 39.Quentmeier H, Reinhardt J, Zaborski M, et al. FLT3 mutations in acute myeloid leukemia cell lines. Leukemia. 2003;17:120–124. doi: 10.1038/sj.leu.2402740. [DOI] [PubMed] [Google Scholar]
- 40.Yokota S, Kiyoi H, Nakao M, et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia. 1997;11:1605–1609. doi: 10.1038/sj.leu.2400812. [DOI] [PubMed] [Google Scholar]
- 41.American Cancer Society Cancer Facts & Figures 2010. [Accessed November 2010]. Available at http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf.
- 42.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 43.Kiyoi H, Towatari M, Yokota S, et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12:1333–1337. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- 44.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 45.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 46.Hayakawa F, Towatari M, Kiyoi H, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19:624–631. doi: 10.1038/sj.onc.1203354. [DOI] [PubMed] [Google Scholar]
- 47.Mizuki M, Fenski R, Halfter H, et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96:3907–3914. [PubMed] [Google Scholar]
- 48.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: A study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 49.Rombouts WJ, Blokland I, Löwenberg B, et al. Biological characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplications in the Flt3 gene. Leukemia. 2000;14:675–683. doi: 10.1038/sj.leu.2401731. [DOI] [PubMed] [Google Scholar]
- 50.Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 51.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: Correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 52.Böhmer FD, Karagyozov L, Uecker A, et al. A single amino acid exchange inverts susceptibility of related receptor tyrosine kinases for the ATP site inhibitor STI-571. J Biol Chem. 2003;278:5148–5155. doi: 10.1074/jbc.M209861200. [DOI] [PubMed] [Google Scholar]
- 53.Lamers MB, Antson AA, Hubbard RE, et al. Structure of the protein tyrosine kinase domain of C-terminal Src kinase (CSK) in complex with staurosporine. J Mol Biol. 1999;285:713–725. doi: 10.1006/jmbi.1998.2369. [DOI] [PubMed] [Google Scholar]
- 54.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 55.Walters DK, Stoffregen EP, Heinrich MC, et al. RNAi-induced down-regulation of FLT3 expression in AML cell lines increases sensitivity to MLN518. Blood. 2005;105:2952–2954. doi: 10.1182/blood-2004-07-2758. [DOI] [PubMed] [Google Scholar]
- 56.Pratz KW, Cortes J, Roboz GJ, et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2009;113:3938–3946. doi: 10.1182/blood-2008-09-177030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levis M, Small D. Small molecule FLT3 tyrosine kinase inhibitors. Curr Pharm Des. 2004;10:1183–1193. doi: 10.2174/1381612043452604. [DOI] [PubMed] [Google Scholar]
- 58.Pratz KW, Sato T, Murphy KM, et al. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115:1425–1432. doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fiedler W, Mesters R, Tinnefeld H, et al. A phase 2 clinical study of SU5416 in patients with refractory acute myeloid leukemia. Blood. 2003;102:2763–2767. doi: 10.1182/blood-2002-10-2998. [DOI] [PubMed] [Google Scholar]
- 60.Giles FJ, Stopeck AT, Silverman LR, et al. SU5416, a small molecule tyrosine kinase receptor inhibitor, has biologic activity in patients with refractory acute myeloid leukemia or myelodysplastic syndromes. Blood. 2003;102:795–801. doi: 10.1182/blood-2002-10-3023. [DOI] [PubMed] [Google Scholar]
- 61.O'Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–3605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 62.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 63.Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–3410. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- 64.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 65.Fiedler W, Serve H, Döhner H, et al. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 66.Fiedler W, Krauter J, Götze K, et al. A phase I/II study combining sunitinib with standard ara-C/daunorubicin chemotherapy in patients 60 years or older with FLT3 mutated AML. Blood. 2010;116 Abstract 3285. [Google Scholar]
- 67.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 68.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 69.Christiansen DH, Andersen MK, Desta F, et al. Mutations of genes in the receptor tyrosine kinase (RTK)/RAS-BRAF signal transduction pathway in therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2005;19:2232–2240. doi: 10.1038/sj.leu.2404009. [DOI] [PubMed] [Google Scholar]
- 70.Clark JW, Eder JP, Ryan D, et al. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43–9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 71.Auclair D, Miller D, Yatsula V, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21:439–445. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- 72.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: A direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 73.Pratz KW, Cho E, Levis MJ, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. 2010;24:1437–1444. doi: 10.1038/leu.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delmonte J, Kantarjian HM, Andreeff M, et al. Update of a phase I study of sorafenib in patients with refractory/relapsed acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2007;110 Abstract 893. [Google Scholar]
- 75.Lee SH, Paietta E, Racevskis J, et al. Complete resolution of leukemia cutis with sorafenib in an acute myeloid leukemia patient with FLT3-ITD mutation. Am J Hematol. 2009;84:701–702. doi: 10.1002/ajh.21511. [DOI] [PubMed] [Google Scholar]
- 76.Safaian NN, Czibere A, Bruns I, et al. Sorafenib (Nexavar) induces molecular remission and regression of extramedullary disease in a patient with FLT3-ITD+ acute myeloid leukemia. Leuk Res. 2009;33:348–350. doi: 10.1016/j.leukres.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 77.Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: Sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113:6567–6571. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 78.Metzelder S, Finck A, Fey M, et al. Sorafenib monotherapy is effective in relapsed and refractory Flt3-ITD positive acute myeloid leukemia, particularly after allogenic stem cell transplantation. Blood. 2010;116 Abstract 3314. [Google Scholar]
- 79.Sharma MR, Ravandi F, Chiattone A, et al. Treatment of FLT3-ITD positive AML relapsing after allogeneic hematopoietic stem cell transplant (HSCT) with sorafenib. Blood. 2010;116 Abstract 3471. [Google Scholar]
- 80.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serve H, Wagner R, Sauerland C, et al. Sorafenib in combination with standard induction and consolidation therapy In elderly AML patients: Results from a randomized, placebo-controlled phase II trial. Blood. 2010;116 Abstract 333. [Google Scholar]
- 82.Kelly LM, Yu JC, Boulton CL, et al. CT53518, a novel selective FLT3 antagonist for the treatment of acute myelogenous leukemia (AML) Cancer Cell. 2002;1:421–432. doi: 10.1016/s1535-6108(02)00070-3. [DOI] [PubMed] [Google Scholar]
- 83.Griswold IJ, Shen LJ, La Rosée P, et al. Effects of MLN518, a dual FLT3 and KIT inhibitor, on normal and malignant hematopoiesis. Blood. 2004;104:2912–2918. doi: 10.1182/blood-2003-05-1669. [DOI] [PubMed] [Google Scholar]
- 84.DeAngelo DJ, Stone RM, Heaney ML, et al. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: Safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108:3674–3681. doi: 10.1182/blood-2006-02-005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schittenhelm MM, Kampa KM, Yee KW, et al. The FLT3 inhibitor tandutinib (formerly MLN518) has sequence-independent synergistic effects with cytarabine and daunorubicin. Cell Cycle. 2009;8:2621–2630. doi: 10.4161/cc.8.16.9355. [DOI] [PubMed] [Google Scholar]
- 86.DeAngelo DJ, Amrein PC, Kovacsovics TJ, et al. Phase 1/2 study of tandutinib (MLN518) plus standard induction chemotherapy in newly diagnosed acute myelogenous leukemia (AML) Blood. 2006;108 Abstract 158. [Google Scholar]
- 87.Camoratto AM, Jani JP, Angeles TS, et al. CEP-751 inhibits TRK receptor tyrosine kinase activity in vitro exhibits anti-tumor activity. Int J Cancer. 1997;72:673–679. doi: 10.1002/(sici)1097-0215(19970807)72:4<673::aid-ijc20>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 88.Hexner EO, Serdikoff C, Jan M, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008;111:5663–5671. doi: 10.1182/blood-2007-04-083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strock CJ, Park JI, Rosen M, et al. CEP-701 and CEP-751 inhibit constitutively activated RET tyrosine kinase activity and block medullary thyroid carcinoma cell growth. Cancer Res. 2003;63:5559–5563. [PubMed] [Google Scholar]
- 90.Marshall JL, Kindler H, Deeken J, et al. Phase I trial of orally administered CEP-701, a novel neurotrophin receptor-linked tyrosine kinase inhibitor. Invest New Drugs. 2005;23:31–37. doi: 10.1023/B:DRUG.0000047103.64335.b0. [DOI] [PubMed] [Google Scholar]
- 91.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): A pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108:3477–3483. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knapper S, Burnett AK, Littlewood T, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 93.Levis M, Pham R, Smith BD, et al. In vitro studies of a FLT3 inhibitor combined with chemotherapy: Sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 94.Levis M, Ravandi F, Wang ES, et al. Results from a randomized trial of salvage chemotherapy followed by lestaurtinib for FLT3 mutant AML patients in first relapse. Blood. 2009;114 doi: 10.1182/blood-2010-08-301796. Abstract 788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sato T, Yang X, Knapper S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117:3286–3293. doi: 10.1182/blood-2010-01-266742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knapper S, Burnett AK, Hills RK, et al. Lestaurtinib FLT3 inhibitory activity is modulated by concomitant azole therapy and may influence relapse risk. Blood. 2009;114 Abstract 789. [Google Scholar]
- 97.Fabbro D, Ruetz S, Bodis S, et al. PKC412—a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des. 2000;15:17–28. [PubMed] [Google Scholar]
- 98.Propper DJ, McDonald AC, Man A, et al. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J Clin Oncol. 2001;19:1485–1492. doi: 10.1200/JCO.2001.19.5.1485. [DOI] [PubMed] [Google Scholar]
- 99.Weisberg E, Boulton C, Kelly LM, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 100.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 101.Stone RM, Fischer T, Paquette R, et al. A phase 1b study of midostaurin (PKC412) in combination with daunorubicin and cytarabine induction and high-dose cytarabine consolidation in patients under age 61 with newly diagnosed de novo acute myeloid leukemia: Overall survival of patients whose blasts have FLT3 mutations is similar to those with wild-type FLT3. Blood. 2009;114 Abstract 634. [Google Scholar]
- 102.Fischer T, Stone RM, Deangelo DJ, et al. Phase IIB trial of oral midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28:4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shiotsu Y, Kiyoi H, Ishikawa Y, et al. KW-2449, a novel multikinase inhibitor, suppresses the growth of leukemia cells with FLT3 mutations or T315I-mutated BCR/ABL translocation. Blood. 2009;114:1607–1617. doi: 10.1182/blood-2009-01-199307. [DOI] [PubMed] [Google Scholar]
- 104.Cortes J, Roboz GJ, Kantarjian H, et al. A phase I dose escalation study of KW-2449, an oral multi-kinase inhibitor against FLT3, Abl, FGFR1 and Aurora in patients with relapsed/refractory AML, ALL and MDS or resistant/intolerant CML. Blood. 2008;112 Abstract 2967. [Google Scholar]
- 105.Chao Q, Sprankle KG, Grotzfeld RM, et al. Identification of N-(5-tert-butyl-isoxazol-3-yl)-N′-{4-[7-(2-morpholin-4-yl-ethoxy)imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl}urea dihydrochloride (AC220), a uniquely potent, selective, and efficacious FMS-like tyrosine kinase-3 (FLT3) inhibitor. J Med Chem. 2009;52:7808–7816. doi: 10.1021/jm9007533. [DOI] [PubMed] [Google Scholar]
- 106.Zarrinkar PP, Gunawardane RN, Cramer MD, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114:2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cortes J, Ghirdaladze D, Foran JM, et al. Phase 1 AML study of AC220, a potent and selective second generation FLT3 receptor tyrosine kinase inhibitor. Blood. 2008;112 Abstract 767. [Google Scholar]