Using Medicaid enrollment as a proxy for poverty, potential disparities in survival after a diagnosis of acute myeloid leukemia or Hodgkin's lymphoma were assessed in a nonelderly population. Poverty did not affect survival for acute myeloid leukemia patients but did appear to be associated with survival for Hodgkin's lymphoma patients.
Keywords: Health care disparities, Medicaid, Socioeconomic factors, Neoplasms, Hodgkin's disease, Acute myeloid leukemia
Abstract
Background.
Because poverty is difficult to measure, its association with outcomes for serious illnesses such as hematologic cancers remains largely uncharacterized. Using Medicaid enrollment as a proxy for poverty, we aimed to assess potential disparities in survival after a diagnosis of acute myeloid leukemia (AML) or Hodgkin's lymphoma (HL) in a nonelderly population.
Methods.
We used records from the New York (NY) and California (CA) state cancer registries linked to Medicaid enrollment records for these states to identify Medicaid enrolled and nonenrolled patients aged 21–64 years with incident diagnoses of AML or HL in 2002–2006. We compared overall survival for the two groups using Kaplan–Meier curves and Cox proportional hazards analyses adjusted for sociodemographic and clinical factors.
Results.
For HL, the adjusted risk for death for Medicaid enrolled compared with nonenrolled patients was 1.98 (95% confidence interval [CI], 1.47–2.68) in NY and 1.89 (95% CI, 1.43–2.49) in CA. In contrast, for AML, Medicaid enrollment had no effect on survival (adjusted hazard ratio, 1.00; 95% CI, 0.84–1.19 in NY and hazard ratio, 1.02; 95% CI, 0.89–1.16 in CA). These results persisted despite adjusting for race/ethnicity and other factors.
Conclusions.
Poverty does not affect survival for AML patients but does appear to be associated with survival for HL patients, who, in contrast to AML patients, require complex outpatient treatment. Challenges for the poor in adhering to treatment regimens for HL could explain this disparity and merit further study.
Introduction
It is well established that cancer outcomes are influenced by socioeconomic factors and that poverty puts patients at risk for inferior health outcomes [1–4]. However, socioeconomic status (SES) is not routinely measured at the individual level and is not available from medical records or from large population-based data sources. Typically, SES is estimated using ecological surrogates, which provide a composite educational and/or income level based on an average for the area in which a person lives. This methodology is easy to apply to large population-based databases; however, it is likely to underestimate the effect of individual-level SES on medical outcomes [5]. Moreover, potentially better measures of individual SES, such as household income or education level, are most often available in studies that are small and thus lack the power to truly detect SES-associated disparities.
In contrast, ascertainment of Medicaid enrollment status provides an alternative strategy for measuring SES and represents a reasonable proxy for poverty at the individual level [6]. State Medicaid programs provide health coverage for persons who meet region-specific income criteria based on family structure and disability status. The majority of Medicaid recipients have income that is near the federal poverty level, and thus can reasonably be assumed to be poor based on individual income level. Moreover, comparison of health outcomes for patients insured by state Medicaid programs with outcomes for otherwise similar state residents insured by other health care payers permits benchmarking of health care delivery for the poor.
Much of the literature relating to socioeconomic inequalities in cancer outcomes has centered on the finding that patients with a lower SES have a more advanced cancer stage at diagnosis [6–9]. Other possible pathways that may contribute to unequal outcomes for the poor have been less thoroughly investigated. These include difficulty accessing appropriate cancer care providers and quality cancer care, a higher burden of comorbid conditions, and impediments to following through with treatment recommendations (e.g., competing employment or caregiving responsibilities).
Using Medicaid enrollment as a proxy for poverty, we sought to evaluate how poverty influences outcomes for acute myeloid leukemia (AML) and Hodgkin's lymphoma (HL) patients. We chose these hematological cancers because no routine screening exists for either, and thus the observed disparities cannot be attributed to lower rates of prevention or early detection among Medicaid enrollees. We also sought to avoid cancers with risk factors that are highly correlated with a lower SES, such as tobacco use, alcohol use, and human papillomavirus infection [9–12].
AML and HL differ in terms of prognosis and where treatment is delivered. AML is treated almost exclusively in the inpatient setting and has relative 5-year survival rates <40% [13]. In contrast, HL is highly curable provided patients can adhere to complex chemotherapy regimens that require multiple outpatient visits. By comparing survival for patients with these two malignancies who were insured by Medicaid at the time of diagnosis with survival for patients of a similar age who were not insured by Medicaid at the time of diagnosis, our aim was to measure the magnitude of a potential disparity in survival that may be attributed to poverty. Secondarily, our goal was to evaluate any observed disparities in the context of variation in patient, clinical, and health system factors in order to gain better insight into the pathways that cause disparities in cancer outcomes for the poor.
Methods
Data Sources
Data for this project were obtained from linkages created between two state Medicaid plans and their respective statewide tumor registries. In collaboration with leadership of the health departments of New York (NY) and California (CA), we merged individual patient records for patients diagnosed with incident cancer in 2002–2006 with Medicaid enrollment files for 2001–2008 in NY and 2000–2007 in CA. The first reason to merge these data sources was to facilitate measurement of SES using Medicaid enrollment at or before diagnosis as a proxy for poverty. An additional reason for linking these data sources was to supplement the detailed information about cancer that the registries provide with the longitudinal information about health care use that Medicaid claims and encounter data provide for its program enrollees. Whereas tumor registries characterize cancer stage, initial treatment, and vital status, Medicaid administrative records permit ascertainment of hospitalizations, outpatient visits, and health care use that extends beyond the scope of information collected by the registries [14].
Tumor Registries
We used information from NY and CA tumor registries. Analyses for the two states were carried out in parallel. The California Cancer Registry (CCR) participates in the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program and meets all their standards for data quality [15]. Although not part of the SEER program, the NY state cancer registry (NYSCR) is one of the largest programs in the country and has consistently achieved the highest level of certification from the North American Association of Central Cancer Registries with respect to accuracy, timeliness, and completeness [16].
Medicaid Data
Both the NY state Medicaid program and CA Medicaid (Medi-Cal) track monthly enrollment. We focused this analysis on those diagnosed with HL or AML in the age range of 21–64 years in each state. We chose to exclude patients diagnosed with these malignancies after age 65 both because the prognosis is very different for older persons and because the ≥65 year-old population is also covered by Medicare. In NY, 19% of the adult population (ages 19–64) lives in poverty (below 100% of the federal poverty level [FPL]) and Medicaid provides insurance for 16% of the state's adult population. In comparison, 20% of the adult population in CA lives in poverty and Medicaid provides insurance for 11% of the state's adult population [17].
Linkage
NY
A detailed description of the NY linkage was reported previously [7]. Briefly, the NY statewide linkage was established for patients with incident cancer diagnoses in 2002–2006 who were linked to Medicaid enrollment, eligibility, encounter, and claims files for 2001–2008 by a probabilistic matching algorithm using social security numbers; first, middle, and last names; date of birth; and gender. The NYSCR–Medicaid linkage was further linked to Medicare files as well as to statewide hospital discharge records (Statewide Planning and Research Cooperative System). Data were merged by the tumor registry and then deidentified and encrypted for use by researchers at the Dana Farber Cancer Institute (DFCI). Linkage and research were approved by institutional review boards (IRBs) at both the DFCI and the NY State Department of Health.
CA
Linkage of the CCR (cancer diagnoses in 2002–2006) and Medicaid enrollment files (2001–2007) was constructed by merging the unique identifiers on tumor registry records with the unique identifiers on the Medicaid files maintained by Centers for Medicare & Medicaid Services (CMS). The CCR submitted a finder file to the CMS that provided a merged record that indicated those patients diagnosed with an incident cancer who were also enrolled in Medicaid for ≥1 month in 2000–2007. The CMS created this file and returned it to the CCR. At the CCR, records were encrypted and released to the research team at the DFCI for analysis. The CCR required administrative review but not state IRB approval to use encrypted data files. IRBs at DFCI provided approval for analyses of both NY and CA data.
Cohort
Incident diagnoses of AML and HL in patients aged 21–64 reported to the NY and CA registries in 2002–2006 were identified. We excluded patients with a prior diagnosis of cancer except nonmelanoma skin cancers.
Outcome
Overall survival was the main outcome. Vital statistics were obtained from the tumor registries, which rely on state records that link to the National Death Index. Patients were followed from diagnosis until death or the censoring date of December 31, 2007.
Medicaid Enrollment
We categorized any cancer patient enrolled in Medicaid prior to a cancer diagnosis or up to 6 months subsequent to a diagnosis as having Medicaid, irrespective of Medicare enrollment. Patients who first enrolled in Medicaid >6 months after their cancer diagnosis were excluded from the analysis. Those who were never on Medicaid were categorized as not having Medicaid. With Medicaid enrollment records spanning the interval 2001–2008 in NY and 2000–2007 in CA and incident cancer diagnoses for 2002–2006, we had a minimum of 1 year of Medicaid enrollment records prior to diagnosis and 1 year subsequent to diagnosis. The majority of cancer patients aged 21–64 years not insured by Medicaid are insured by commercial plans. However, because state tumor registries do not report insurance type, we could not ascertain the specific health plan nor could we identify the subset of patients who lacked any insurance. Estimates are that 20% of the NY and 26% of the adult CA population lack health insurance and that 63% and 61%, respectively, have private medical insurance [17].
Covariates
For all patients, we included information on age at diagnosis (21–29, 30–39, 40–49, 50–59, or 60–64 years), sex (male, female), race/ethnicity (white non-Hispanic, Hispanic, black, Asian/Pacific Islander), marital status at cancer diagnosis (married, not married), and the year of cancer diagnosis. We also report the basis of Medicaid eligibility (cash assistance or not, disability or not). We evaluated clinical information collected by the tumor registries. For HL, we considered stage at diagnosis (I/II, early; III/IV, advanced; or unknown), “B” symptoms (yes, no, or unknown), and whether the tumor was reported to the registry as HIV related (yes, no/unknown). The latter two variables were available only from the cancer registries for 2004–2006. In order to estimate comorbidity in both the Medicaid insured and non-Medicaid enrolled populations, we relied on counts of the number of hospitalizations during a 12-month period prior to the month that the cancer diagnosis was reported to the tumor registry (hereafter, referred to as the year prior to diagnosis). A high number of hospital admissions is associated with poorer survival and is a crude metric for the burden of comorbid disease [18, 19]. For NY, this estimation was possible because of the linkage to the statewide hospital discharge system that permitted reliable counts of the number of hospital admissions. Comparable data for CA were not available for analysis.
Analysis
We ran parallel analyses for the databases of state cancer registries linked to State Medicaid files for NY and CA. For both AML and HL patients, we created Kaplan–Meier curves of survival for 48 months stratified by Medicaid enrollment status. We calculated Cox proportional hazards ratios (HRs) for the Medicaid and non-Medicaid cohorts considering all the covariates shown in Table 1 (except the basis of Medicaid eligibility, which is not applicable to those not on Medicaid, and the treatment variables, which was done as a sensitivity analysis). Variables were included in the initial multivariate model based on either their a priori clinical relevance or a p-value < .1. We also performed survival analyses for AML patients, stratifying by age (<50 years, >50 years), and for HL patients, stratifying by stage (I/II versus III/IV), to assess whether there were any differences between the Medicaid and non-Medicaid groups in the good versus poor prognosis patient subgroups. In a series of sensitivity analyses, we included treatment information recorded by the registries (no/unknown, chemotherapy, radiation, or combination chemotherapy plus radiation for HL and no/unknown or chemotherapy for AML). A p-value < .05 was used to determine significance. All analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC).
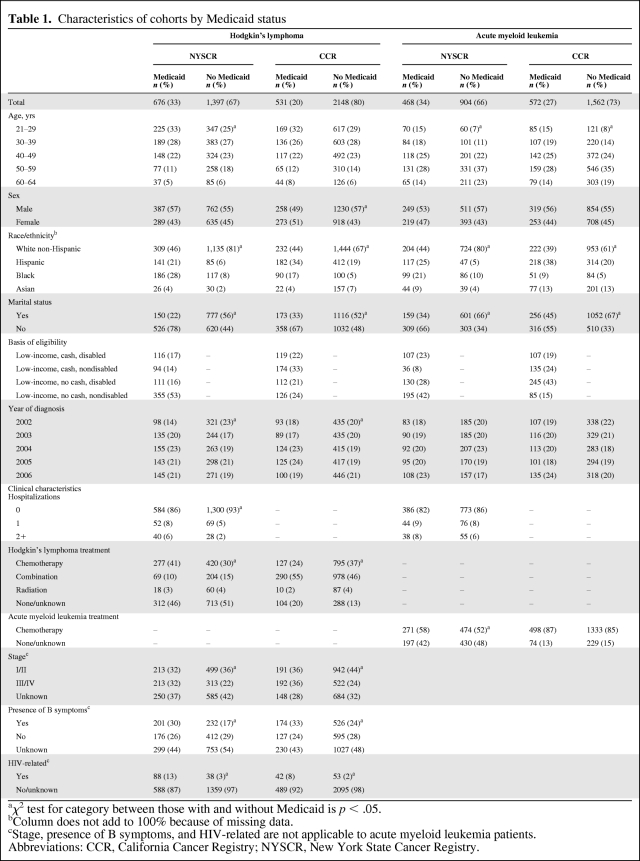
Table 1.
Characteristics of cohorts by Medicaid status
aχ2 test for category between those with and without Medicaid is p < .05.
bColumn does not add to 100% because of missing data.
cStage, presence of B symptoms, and HIV-related are not applicable to acute myeloid leukemia patients.
Abbreviations: CCR, California Cancer Registry; NYSCR, New York State Cancer Registry.
Results
Characteristics of the Cohort
We identified 2,073 and 2,679 patients diagnosed with HL at age 21–64 years in 2002–2006 in NY and CA, respectively. There were 1,372 and 2,134 patients diagnosed with AML at age 21–64 years in NY and CA, respectively. In NY, compared with CA, a higher percentage of the statewide population with these diagnoses was enrolled in Medicaid (33% in NY versus 20% in CA for HL and 34% in NY versus 27% in CA for AML). Table 1 shows the characteristics of patients with HL or AML by state and Medicaid status. Notable differences between the Medicaid enrolled and nonenrolled patients included the presence of greater racial and ethnic diversity and fewer married individuals among Medicaid enrollees. For HL, Medicaid enrollees had more advanced stage and a greater proportion of associated HIV diagnoses, and in NY, Medicaid enrollees had more hospitalizations in the year prior to diagnosis than those patients not enrolled in Medicaid.
Association of Medicaid Enrollment with Survival
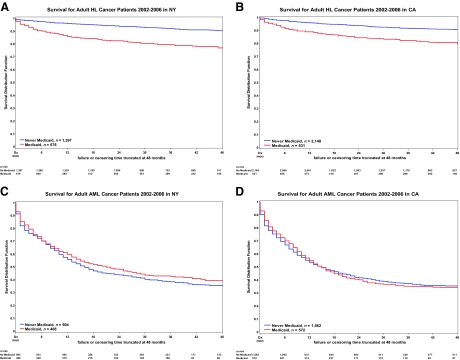
For HL patients, survival outcomes were inferior for Medicaid enrollees. In contrast, for AML patients, survival outcomes appear to be similar for Medicaid enrolled and nonenrolled patients. Survival outcomes for patients diagnosed in NY and CA were largely consistent. Kaplan–Meier survival curves for both HL and AML patients in NY and CA, stratified by Medicaid enrollment, are depicted in Figure 1.
Figure 1.
Kaplan–Meier curves by state (NY and CA) stratified for enrollment in Medicaid. (A, B): HL patients. (C, D): AML patients. For HL patients, survival is inferior for those with Medicaid insurance. This is in contrast to AML patients, for whom the survival lines overlap for those with Medicaid and those without Medicaid.
Abbreviations: AML, acute myeloid leukemia; CA, California; HL, Hodgkin's lymphoma; NY, New York.
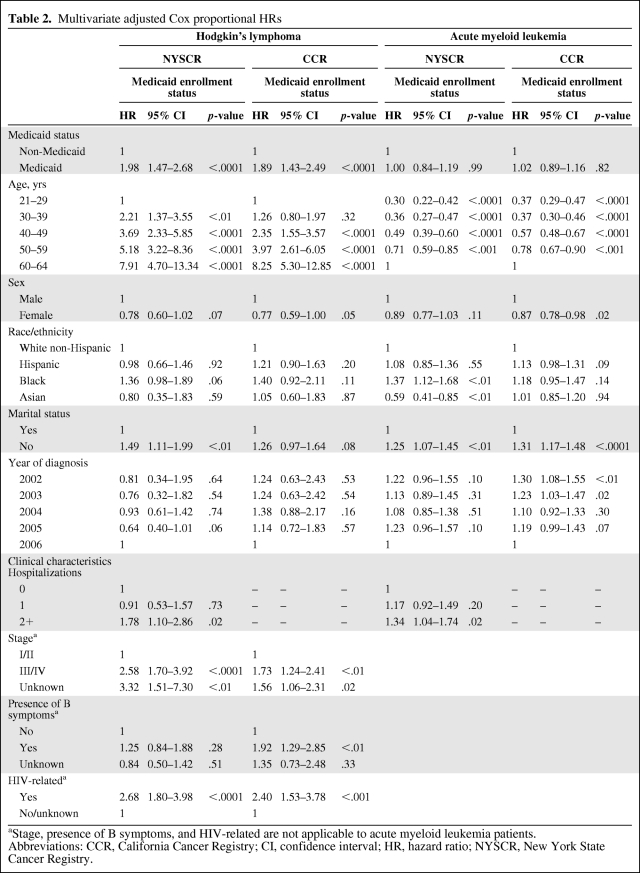
In the adjusted multivariate analysis for HL (Table 2), the adjusted Cox proportional HR for those on Medicaid compared with those not on Medicaid was 1.98 (95% confidence interval [CI], 1.47–2.68; p < .0001) in NY and 1.89 (95% CI, 1.43–2.49; p < .0001) in CA. This effect persisted despite correcting for the higher stage at diagnosis seen in the Medicaid group. Older age, male sex (in CA), not being married, advanced or unknown stage, B symptoms (in CA), HIV, and comorbidity (in NY) remained significant independent predictors of death. Older age, male sex, advanced stage, and B symptoms are all included in risk scores used clinically to predict worse outcome [20]. Although race/ethnicity was significant on univariate analysis, in the adjusted model it was no longer predictive of death; however, there was a trend toward worse survival for blacks in NY (HR, 1.36; 95% CI, 0.98–1.89; p = .06), and although not significant, CA had a similar effect measure (HR, 1.40; 95% CI, 0.92–2.11). Stratified analysis for early versus advanced stage (I/II versus III/IV) disease did not show a differential effect on survival by prognostic group (data not shown).
Table 2.
Multivariate adjusted Cox proportional HRs
aStage, presence of B symptoms, and HIV-related are not applicable to acute myeloid leukemia patients.
Abbreviations: CCR, California Cancer Registry; CI, confidence interval; HR, hazard ratio; NYSCR, New York State Cancer Registry.
For AML, the unadjusted and adjusted (Table 2) models showing Cox proportional HRs were very similar. In the final adjusted model for AML, Medicaid enrollment had no effect on survival (HR, 1.00; p = .99 in NY and HR, 1.02; p = .82 in CA). Among all patients in the cohort, we found that older age and higher comorbidity, the two most important and well-established clinical risk factors for worse survival [21], were also significant in our study population. Stratified analysis on lower risk patients versus higher risk patients (age, <50 years compared with >50 years) did not demonstrate that Medicaid status was a predictor of survival in either strata (data not shown).
Although Medicaid enrollment was not associated with survival for AML patients, other sociodemographic factors, including marital status, race, and sex, were associated with survival. Not being married was associated with worse survival in both NY and CA. In NY, blacks had worse survival (HR, 1.37; 95% CI, 1.12–1.68; p < .01) whereas Asians had better survival (HR, 0.59; 95% CI, 0.41–0.85; p < .01) than white non-Hispanics. Female sex was associated with better survival in CA (HR, 0.87; 95% CI, 0.78–0.98; p = .02), with a similar effect measure in NY, although not significant (HR, 0.89; 95% CI, 0.77–1.03).
Sensitivity Analysis of Treatment Effect
In adjusted models for both HL and AML, patients categorized as having no/unknown treatment had significantly worse survival than those treated with chemotherapy. Additionally, HL patients treated with radiation and chemotherapy had better survival than those treated with chemotherapy alone. The measures of treatment available, however, did not explain all the survival disparity for HL patients on Medicaid compared with those not on Medicaid, which was evident in both NY (HR, 1.97; 95% CI, 1.45–2.66; p < .0001) and CA (HR, 1.77; 95% CI, 1.34–2.33; p < .0001).
Discussion
Using data constructed from linkage of Medicaid and cancer registry data from the health departments of two states with large and diverse populations, we compared survival following a diagnosis of HL and AML for adults aged <65 years enrolled and not enrolled in Medicaid. For HL patients, a significant gap in survival outcomes based on Medicaid enrollment was observed, with a nearly twofold higher risk for death for Medicaid enrolled patients than for nonenrolled patients. In contrast, for AML patients, there was no significant disparity in survival based on Medicaid enrollment.
This study seeks to provide further insight into the causes of disparities in cancer outcomes for persons with a low SES. Whereas most of the disparities literature focuses on race because it is most easily measured, we used Medicaid data as a proxy for low SES. In addition, we focused on disparities in survival outcomes subsequent to diagnosis for two malignancies for which there is no screening or early detection. A plausible explanation for a gap in survival between Medicaid HL patients and their non-Medicaid counterparts but no such gap for AML relates to the predominant site of disease treatment. In the younger age groups we studied, AML is consistently treated in an inpatient hospital setting. In contrast, HL is treated with combination chemotherapy and/or radiation, typically in the outpatient setting.
We speculate that the need to access outpatient treatment centers is more challenging for Medicaid insured patients, perhaps contributing to the gap in survival. The complexity of HL outpatient treatment might pose barriers to those with limited resources or psychosocial support or other issues accessing the health care system—factors that might be more intensely experienced by the poor. In addition, although we did not find that the measures of initial treatment available from the cancer registries explained the differences in HL patient survival, it remains possible that important details of initial treatment or treatment for relapsed disease—such as receiving each cycle of chemotherapy in a timely manner—are the source of the survival disparities we observed. HIV infection can be associated with worse survival in HL patients, and the CCR and NYSCR did not have complete capture of HIV status during the years of our study. With contemporary antiretroviral treatment, HL outcomes for patients with HIV infection have improved significantly [22]. However, if a greater proportion of Medicaid enrollees than nonenrollees had HIV-related HL, this factor might contribute to our findings. Given the striking disparity in HL outcomes that we identified, the underlying reasons deserve further investigation, which will require detailed review of medical records.
The effects of poverty and race/ethnicity are highly correlated and difficult to tease apart, especially when poverty is measured by the ecological methods often used in population-based research. In contrast, using an individual-level measure of poverty (Medicaid enrollment), we found that poverty was not associated with survival for AML patients, but that race/ethnicity was still an independent predictor of survival. Although not significant, there were also race/ethnicity differences in survival for HL patients after adjusting for the effects of Medicaid status. Including measures of both poverty and race facilitates better specification of individuals at greatest risk for poor outcomes and identifies groups that should be prioritized for careful scrutiny and intervention studies.
Our finding of a twofold greater risk for death among those on Medicaid than among non-Medicaid enrollees with HL is mostly consistent with previous studies showing that lower income level detrimentally affects outcomes in HL [23, 24]. Additionally, previous work has suggested that patients of nonwhite [25] and black [26] races have shorter survival, but these studies have not controlled for SES. A CCR study evaluating both race and estimated income (by census block divided into quintiles) found that black and Hispanic race/ethnicity and lower average neighborhood income were independent predictors of both overall and HL-specific survival [24]. Those authors found a moderately higher risk for death for black patients (HR, 1.05–1.63 based on age) and for Hispanic patients (HR, 1.11–1.30 based on age) than for non-Hispanic whites. In the same adjusted models, they found a similar magnitude of risk for death for the lowest SES quintile compared with the highest SES quintile (HR, 1.44–1.81 based on age).
These results are generally consistent with our findings; however, using Medicaid enrollment instead of area-level measures, we found poverty to be an even stronger predictor of survival than race. Discrepancies in the magnitude of the effects of poverty and race possibly reflect Medicaid enrollment status as a more precise surrogate of income level. Another possible explanation for the disagreement between our study and the previous one is a residual confounding effect of race on income level when using ecological variables for income. Lastly, although our study uniquely includes HIV-related diagnoses to attempt to correct for confounding from comorbid illnesses, and specifically HIV, it is possible that there is residual confounding, specifically from incomplete recording of HIV-related tumors.
Although we did not find that Medicaid enrollment predicted worse survival for those with AML, we did find that a lack of social support (not being married) and black and Hispanic race/ethnicity were significant sociodemographic factors predicting worse survival. Prior studies of disparities in outcomes for AML patients have evaluated race; three found that being black predicted worse survival [27–30]. Interestingly, in one study, poorer survival was still evident after correcting for cytogenetics [30]. Three studies looked at ecological surrogates for income, and one of those also included insurance status [28]. The effect of income was consistently small (HR, 1.15–1.21) [27, 28] or nonsignificant [29]; however, in the largest study, Medicaid insurance was a significant and meaningful (HR, 1.23; p = .01) predictor of worse survival in a Florida population [28]. Discrepancies between these prior findings and our own could be a result of the variability and generosity of Medicaid programs in different states. For example, in Florida, low income parents must be below 59% of the FPL to be eligible for Medicaid, compared with 106% of the FPL in CA and 150% of the FPL in NY [17]. This suggests that Medicaid enrollees in Florida are relatively more impoverished than those in NY and CA.
The consistency of results for NY and CA suggests that our findings are generalizable. Our study covers approximately 15% of the U.S. population. Additionally NY and CA have significant racial and ethnic diversity, which allowed us to evaluate the role of race and poverty simultaneously. Finally, adjusted models found known clinical risk factors to be significant, lending further credibility to the study's findings.
Our study has limitations. First, we did not have information on insurance status for cancer patients not enrolled in Medicaid. Whereas most adults aged <65 years not enrolled in Medicaid are enrolled in commercial plans, a small percentage are uninsured. Inclusion of the uninsured would bias our study to finding no significant difference between the Medicaid and non-Medicaid groups. Had we been able to eliminate the uninsured from the non-Medicaid comparator group, the magnitude of the disparities we report might be greater. Second, we used overall survival rather than cancer-specific survival as our primary outcome, and therefore it is plausible that the disparities we observed are attributable to factors other than malignancy or its treatment. We chose overall survival as our primary outcome because cause-specific death coding on death certificates can be inaccurate. However, when we repeated our analyses using cause-specific mortality for CA, our results were unchanged. Third, several important prognostic clinical characteristics are not collected by state cancer registries or are incomplete (e.g., cytogenetics for AML, HIV status), limiting our ability to fully adjust for these factors. Similarly, because of the lack of detailed claims data for the non-Medicaid population, we were not able to further investigate many potentially interesting pathways that might lead to our findings. This included details of treatment, such as exact type and timing, or a more complete analysis of comorbidity, for example, examining the effect of mental illness. Lastly, all administrative data are subject to potential misclassification.
Conclusions/Summary
Medicaid enrolled patients with HL diagnosed at age 21–64 years have a nearly twofold greater risk for death than similarly aged patients not enrolled in Medicaid. This excess risk for death was manifest in two large states and across racial groups, and persisted after adjusting for other clinical and demographic variables. In contrast, there was no such variation in survival outcomes by insurance status for patients with AML. Whereas HL treatment requires adherence to a complex outpatient regimen, AML treatment requires intensive inpatient care—perhaps a clue to the difference. Given that HL is a curable cancer, the mechanisms that contribute to this difference warrant further investigation. More generally, because of the importance of state Medicaid programs as the primary source of insurance for the nation's indigent, consistent scrutiny of how outcomes for enrollees compare with those of patients with commercial insurance plans should be regularly evaluated to prioritize areas for intervention.
Acknowledgments
We thank the California Cancer Registry for willingness to permit construction of the merged dataset and Mark Allen for assistance constructing and encrypting the analytic files.
Work for this project was supported by Cooperative Agreement S3888 from the Association of Schools of Public Health/Centers for Disease Control and Prevention (awarded to Dr. Schymura) and National Cancer Institute R01-CA131847-01A1 (awarded to Dr. Schrag).
Author Contributions
Conception/Design: Deborah Schrag, Rachel L. Yung, Kun Chen
Provision of study material or patients: Deborah Schrag, Foster C. Gesten, Patrick J. Roohan, Francis Boscoe, Amber Sinclair, Maria J. Schymura
Collection and/or assembly of data: Deborah Schrag, Foster C. Gesten, Patrick J. Roohan, Francis Boscoe, Amber Sinclair, Kun Chen, Maria J. Schymura
Data analysis and interpretation: Rachel L. Yung, Gregory A. Abel, Kun Chen, Maria J. Schymura
Manuscript writing: Deborah Schrag, Rachel L. Yung, Francis Boscoe, Amber Sinclair, Gregory A. Abel, Maria J. Schymura
Final approval of manuscript: Deborah Schrag, Rachel L. Yung, Foster C. Gesten, Patrick J. Roohan, Francis Boscoe, Amber Sinclair, Gregory A. Abel, Kun Chen, Maria J. Schymura
Other: Principal investigator: Deborah Schrag
References
- 1.Ayanian JZ, Kohler BA, Abe T, et al. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329:326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 2.Boyd C, Zhang-Salomons JY, Groome PA, et al. Associations between community income and cancer survival in Ontario, Canada, and the United States. J Clin Oncol. 1999;17:2244–2255. doi: 10.1200/JCO.1999.17.7.2244. [DOI] [PubMed] [Google Scholar]
- 3.Lannin DR, Mathews HF, Mitchell J, et al. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA. 1998;279:1801–1807. doi: 10.1001/jama.279.22.1801. [DOI] [PubMed] [Google Scholar]
- 4.Singh GK, Miller BA, Hankey BF, et al. Bethesda, MD: National Cancer Institute; 2003. Area socioeconomic variations in U.S. cancer incidence, mortality, stage, treatment, and survival, 1975–1999. NCI Cancer Surveillance Monograph Series, Number 4. NIH Publication No. 03-5417. [Google Scholar]
- 5.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: A review. Ann Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 6.Bradley CJ, Given CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer. 2001;91:178–188. doi: 10.1002/1097-0142(20010101)91:1<178::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Boscoe FP, Schrag D, Chen K, et al. Building capacity to assess cancer care in the Medicaid population in New York state. Health Serv Res. 2011;46:805–820. doi: 10.1111/j.1475-6773.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley CJ, Gardiner J, Given CW, et al. Cancer, Medicaid enrollment, and survival disparities. Cancer. 2005;103:1712–1718. doi: 10.1002/cncr.20954. [DOI] [PubMed] [Google Scholar]
- 9.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menvielle G, Kunst AE, Stirbu I, et al. Socioeconomic inequalities in alcohol related cancer mortality among men: To what extent do they differ between Western European populations? Int J Cancer. 2007;121:649–655. doi: 10.1002/ijc.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benard VB, Johnson CJ, Thompson TD, et al. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer. 2008;113(10 suppl):2910–2918. doi: 10.1002/cncr.23742. [DOI] [PubMed] [Google Scholar]
- 12.Mouw T, Koster A, Wright ME, et al. Education and risk of cancer in a large cohort of men and women in the United States. PloS One. 2008;3:e3639. doi: 10.1371/journal.pone.0003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Surveillance Epidemiology and End Results. Cancer Statistics. [accessed March 15, 2011]. Available at http://seer.cancer.gov/statistics/
- 14.U.S. Department of Health & Human Services, Centers for Medicare & Medicaid Services. Overview. [accessed March 14, 2011]. Available at http://www.cms.gov/MedicaidGenInfo/
- 15.California Cancer Registry. California Department of Public Health. [accessed February 14, 2011]. Available at http://www.ccrcal.org/
- 16.New York State Department of Health. NYS Cancer Registry and Cancer Statistics. [accessed February 14, 2011]. Available at http://www.nyhealth.gov/statistics/cancer/registry/
- 17.The Kaiser Family Foundation. statehealthfacts.org. [accessed February 14, 2011]. Datasource http://pdf.kff.org/mfs/NYUS.pdf and http://pdf.kff.org/mfs/CAUS.pdf. Available at http://www.statehealthfacts.org/profileglance.jsp?rgn=6&rgn=34.
- 18.Schneeweiss S, Seeger JD, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154:854–864. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 19.Wang PS, Walker A, Tsuang M, et al. Strategies for improving comorbidity measures based on Medicare and Medicaid claims data. J Clin Epidemiol. 2000;53:571–578. doi: 10.1016/s0895-4356(00)00222-5. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Hodgkin Lymphoma Guidelines. [accessed March 15, 2011]. Available at http://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf.
- 21.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 22.Glaser SL, Clarke CA, Gulley ML, et al. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988–1998. Cancer. 2003;98:300–309. doi: 10.1002/cncr.11459. [DOI] [PubMed] [Google Scholar]
- 23.Cella DF, Orav EJ, Kornblith AB, et al. Socioeconomic status and cancer survival. J Clin Oncol. 1991;9:1500–1509. doi: 10.1200/JCO.1991.9.8.1500. [DOI] [PubMed] [Google Scholar]
- 24.Keegan TH, Clarke CA, Chang ET, et al. Disparities in survival after Hodgkin lymphoma: A population-based study. Cancer Causes Control. 2009;20:1881–1892. doi: 10.1007/s10552-009-9382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke CA, Glaser SL, Prehn AW. Age-specific survival after Hodgkin's disease in a population-based cohort (United States) Cancer Causes Control. 2001;12:803–812. doi: 10.1023/a:1012240222032. [DOI] [PubMed] [Google Scholar]
- 26.Zaki A, Natarajan N, Mettlin CJ. Early and late survival in Hodgkin disease among whites and blacks living in the United States. Cancer. 1993;72:602–606. doi: 10.1002/1097-0142(19930715)72:2<602::aid-cncr2820720244>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Baker KS, Davies SM, Majhail NS, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:1543–1554. doi: 10.1016/j.bbmt.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrne MM, Halman LJ, Koniaris LG, Cassileth PA, Rosenblatt JD, Cheung MC. Effects of Poverty and Race on Outcomes in Acute Myeloid Leukemia. Am J Clin Oncol. 2011;34:297–304. doi: 10.1097/COC.0b013e3181dea934. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez CP, Baz R, Jawde RA, et al. Impact of socioeconomic status and distance from treatment center on survival in patients receiving remission induction therapy for newly diagnosed acute myeloid leukemia. Leuk Res. 2008;32:413–420. doi: 10.1016/j.leukres.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Sekeres MA, Peterson B, Dodge RK, et al. Differences in prognostic factors and outcomes in African Americans and whites with acute myeloid leukemia. Blood. 2004;103:4036–4042. doi: 10.1182/blood-2003-09-3118. [DOI] [PubMed] [Google Scholar]