The study examines the differential recurrence pattern between patients treated with lumpectomy and those treated with mastectomy at a single institution and reviews relevant literature-based data. Modification of the current breast cancer surveillance strategy for patients treated with breast-conserving surgery is recommended.
Keywords: Breast cancer, Lumpectomy, Mastectomy, Annual recurrence rate
Abstract
Purpose.
To investigate the recurrence pattern and annual recurrence risk after breast-conserving surgery and compare them with those after mastectomy.
Methods.
This retrospective analysis included 6,135 consecutive unilateral breast cancer patients undergoing surgery in 1998–2008, with 847 lumpectomy patients and 5,288 mastectomy patients. Recurrence patterns were scrutinized and annual recurrence rates were calculated. Furthermore, a literature-based review including seven relevant studies was subsequently performed to confirm our single-institution data-based observations.
Results.
After lumpectomy, 50.9% of recurrences occurred within 3 years and 30.2% of recurrences were detected at 3–5 years; after mastectomy, 64.9% of recurrences occurred within 3 years and 20.4% occurred at 3–5 years. The major locoregional recurrence pattern after lumpectomy was ipsilateral breast tumor recurrence, which mainly (81.3%) occurred ≤5 years postsurgery but with a low incidence of 37.5% ≤3 years postsurgery. Annual recurrence curves indicated that the relapse peak after mastectomy emerged in the first 2 years; however, recurrence after lumpectomy increased annually with the highest peak near 5 years. By reviewing relevant studies, we confirmed our finding of different annual recurrence patterns for lumpectomy and mastectomy patients. The hazard ratio of dying for those recurring ≤5 years postlumpectomy relative to patients relapsing >5 years postlumpectomy was 4.62 (95% confidence interval, 1.05–20.28; p = .042).
Conclusions.
Different recurrence patterns between mastectomy and lumpectomy patients imply that scheduling of surveillance visits should be more frequent during the 4–6 years after lumpectomy. Further prospective trials addressing the necessity of frequent and longer surveillance after lumpectomy are warranted.
Introduction
Breast cancer-related death is usually a result of disease recurrence, which occurs even in early breast cancer patients with small tumors and a negative lymph node status after long-term follow-up [1, 2]. Previous studies indicated that 70% of recurrences occur ≤3 years postsurgery, and the recurrence risk reaches a peak at 1–2 years after surgery [3, 4]. In 1997, the American Society of Clinical Oncology (ASCO) published breast cancer surveillance guidelines for the first time [5], in which the expert panel recommended that all women have a careful history and physical examination every 3–6 months for the first 3 years after primary therapy, then every 6–12 months for the next 2 years, then annually. The rationale for such an approach is that, because 60%–80% of all breast cancer recurrences are detected ≤3 years postsurgery, scheduling of surveillance visits should be more frequent during that time period [5]. Of note, the recurrence data that the guidelines were based on were derived from early clinical studies in which most patients underwent mastectomy rather than well-performed breast-conserving surgery (BCS). ASCO subsequently updated these guidelines several times, but the above-mentioned schedule of surveillance visits never changed despite the fact that the target population could have received either mastectomy or lumpectomy [6, 7].
BCS has been established as a local treatment for early-stage breast cancer. Several randomized controlled trials have shown no difference in overall survival (OS) rates after BCS or mastectomy [1, 2, 8], although BCS is associated with a higher risk for local recurrence than mastectomy [2]. Of note, ipsilateral breast tumor recurrence (IBTR) after lumpectomy might be a metastasis-related or survival-threat event rather than only a cosmetic failure [9–12]. The interval from lumpectomy to first recurrence is an important prognostic indicator. Of those patients presenting with IBTR ≤4 years postsurgery, 50% developed distant metastasis, versus only 17% of those presenting >4 years postsurgery (p < .01) [13]. Kurtz et al. [14] indicated that, whereas recurrence in the breast ≤5 years postlumpectomy profoundly affected survival, patients with late failure had a 15-year survival rate identical to that of other 5-year survivors who never failed locally. It is likely that early identification of recurrence in the breast ≤5 years postsurgery is important because these early recurrences may be more fatal than late recurrences. On the other hand, we also believe, to a large extent, that if the in-breast local recurrence could have been prevented, the breast cancer–specific survival rate would have been higher, because an overview of the “effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival” by the Early Breast Cancer Trialists' Collaborative Group [15] indicated that differences in local treatment that substantially affect local recurrence rates would, in the hypothetical absence of any other causes of death, avoid about one breast cancer death over the next 15 years for every four local recurrences avoided, and should reduce the 15-year overall mortality rate.
Thus far, there are few prospective trials investigating whether or not early detection of breast cancer recurrence results in a better outcome. It seems that surveillance aimed at early detection of “distant metastases” does not improve survival [16]; however, the effect on survival of early detection of “breast recurrences” is debatable [17–19]. Greater monitoring of the breast after BCS would identify more signs of early recurrence, whereas early detection of second breast cancers (either IBTR or contralateral breast cancer [CBC]) in the asymptomatic phase could improve relative survival by 27%–47%, as Houssami et al. [18] suggested. Perrone et al. [20] also reported that early detection of local recurrence might have a favorable impact on the prognosis of patients followed after primary treatment for breast cancer because a difference in survival was recorded in favor of cases detected in the asymptomatic state (p < .001). Another meta-analysis [17] found that recurrences assessed in patients without symptoms were related to a higher probability of survival than when symptoms were present (hazard ratio [HR], 1.56; 95% confidence interval [CI], 1.36–1.79). The authors thus concluded that detection of isolated locoregional or CBC recurrences in patients without symptoms has a beneficial impact on the survival of breast cancer patients, when compared with late symptomatic detection. Besides the potential benefits in terms of survival, a few patients who refuse salvage mastectomy at locoregional relapse after BCS could be treated with a second lumpectomy, albeit the treatment choice for locoregional relapse historically has been salvage mastectomy [3]. We believe that early detection of breast cancer allows for a higher proportion of reconservation. Of course, the safety and effectiveness of reconservation should be further evaluated.
In most studies, the risk for recurrence is generally described by survival curves rather than annual hazard rates. In recent years, some investigators have scrutinized patterns of recurrence and the annual recurrence rate (ARR) is used to assess dynamic changes in recurrence risk by year. Thus far, we have little information on the difference in the recurrence pattern between lumpectomy and mastectomy patients. The aim of the present study was to show the differential recurrence pattern between these two surgical modalities using our single-institution data, as well as to review relevant literature-based data to confirm our observations. A better understanding of the recurrence pattern with each type of surgery would be helpful in clinical surveillance and monitoring.
Materials and Methods
Patients
The study patients were from the Breast Malignancy Database established by the Department of Surgery, Shanghai Cancer Center of Fudan University. The information in this database has been reported previously [21, 22]. All patients provided informed consent for their information to be stored in the hospital database and be used for research. The present study was approved by the Ethical Committee of Shanghai Cancer Center. In this study, we selected 6,135 consecutive unilateral breast cancer patients undergoing surgery during the period 1998–2008, including 847 patients treated with BCS and 5,288 patients treated with modified radical or radical mastectomy. In our hospital, patients eligible for lumpectomy should have a tumor <4 cm in largest diameter, be without palpable axillary lymph nodes on clinical examination, have no absolute contraindication, and be willing to preserve their breast. The preoperative examination and performance of the surgical technique have been described previously [23, 24]. All patients having invasive carcinoma or carcinoma in situ with a microinvasion/invasion component undergo a level I and level II axillary lymph node dissection or a sentinel lymph node biopsy. Lumpectomy patients who were further pathologically proven to have a close (≤2 mm) or positive margin undergo a secondary mastectomy. For all BCS patients with invasive carcinoma, whole-breast irradiation was given as previously described [23]. Radiotherapy for lumpectomy patients with carcinoma in situ was mainly according to the Van Nuys Prognostic Index [25].
The postoperative recurrence risk category and adjuvant therapy choice were mainly determined by the St. Gallen consensus guidelines [26]. Patients having carcinoma in situ with an invasive component also received adjuvant chemotherapy. All patients with a positive hormone receptor status took tamoxifen or aromatase inhibitors (only for postmenopausal women) for 5 years. In this cohort, no one used adjuvant trastuzumab treatment. Systemic treatments were renewed according to updated St. Gallen consensus guidelines [27].
Recurrence Definition and Follow-Up
Recurrence was defined as the occurrence of locoregional relapse (LRR), distant metastasis, or CBC. In the present study, LRR after lumpectomy includes IBTR and other LRR (oLRR), whereas LRR after mastectomy includes only oLRR. In our definition, IBTR denotes tumor recurrence “in” the ipsilateral breast (either in the same quadrant or not), whereas oLRR indicates a first recurrence of a tumor in the ipsilateral chest wall, in the locoregional skin, or in regional lymph nodes (including the ipsilateral internal mammary, supraclavicular, infraclavicular, and axillary nodes). We specify that recurrence in the ipsilateral chest wall and breast skin after lumpectomy would not be considered an IBTR event because women who undergo total mastectomy are also at risk for such an event. Instead, only the occurrence of a tumor “in” the ipsilateral breast was classified as IBTR after lumpectomy, because women who undergo total mastectomy are not at risk for such an event. We made this classification in order to increase the comparability of oLRR between lumpectomy and mastectomy. Another notable issue is that CBC is usually considered as a new primary breast cancer rather than recurrent disease. In this paper, we defined “recurrence” in a broad sense rather than in a narrow sense. “Recurrence-free survival” in this paper is somewhat similar to “event-free survival.” Because we compare our ARR curve with those from other studies, most of which [28–30] presented their ARR curves derived from event-free survival or disease-free survival curves, we included CBC within the recurrence events to increase comparability, although this is flawed mechanistically. Recurrence time was defined as the interval between primary surgical treatment and the occurrence of the earliest relapse. In the absence of relapse, the observation time was censored at the date on which follow-up ended (date of death or last follow-up). The analysis of OS included all deaths. Follow-up was every 6 months during the first 2 years after surgery and every year since the third year. After surgery, patients had mammography, whole-body ultrasonic detection (including ultrasonography examination of the breast, axillary fossa, cervical parts, abdomen, and pelvis), and chest x-ray examination annually, as previously described. Study patients were followed up until June 2009, with median follow-up times of 61 months (range, 3–123 months) for patients receiving mastectomy and 53 months (range, 3–120 months) for patients receiving lumpectomy.
Literature Search and Literature-Based Data Extraction
We searched the MEDLINE, PubMed, and Web of Science databases (updated to September 1, 2010) using the following search terms: “annual” and “recurrence” and “breast cancer.” Eligible studies were retrieved and examined carefully. References were checked as well for other relevant publications. The literature inclusion criteria were as follows: (a) evaluation of ARR after surgery for primary breast cancer, (b) availability of information for ARR (numerical data or graphic data), and (c) full text published in English. Relevant information was carefully extracted from all eligible publications.
Statistical Analysis
Categorical variables were compared using Pearson's χ2 test or Fisher's exact test as appropriate. Student's t-test was used to compare continuous variables between two groups. The log-rank test was used for comparison of differences between survival curves that were derived by the Kaplan–Meier method. Cox proportional hazards regression was used to model the relationship between OS and recurrence time after lumpectomy. The corresponding HR was also calculated by the Cox model. All p-values were from two-sided tests and a p-value ≤.05 was considered statistically significant. Statistical analysis was performed using Stata/SE, version 10.0 (StataCorp LP, College Station, TX) and SPSS, version 12.0 (SPSS Inc., Chicago, IL) software. To graphically present our single-institution ARR data, annual hazard rates were estimated using the “smoothed hazard estimate” function in Stata. To collect ARR data from different publications, each annual hazard curve was read and measured using the Measure Tool of Adobe Acrobat 7.0 Professional (Adobe Systems Inc., San Jose, CA). A hazard rate of a given time point (every half year) was read and the corresponding ARR data were obtained; then the new annual recurrence curve was rebuilt by a nonparametric smoothing (LOWESS) method. The curves were made using GraphPad Prism (GraphPad Software, San Diego, CA).
Results
Recurrence Patterns of Breast Cancer After Mastectomy and Lumpectomy
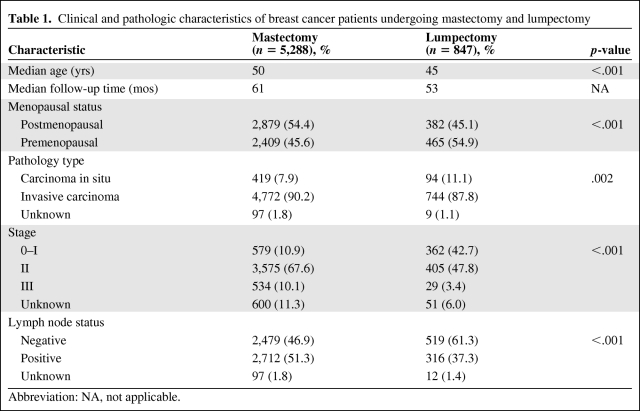
The basic characteristics of study patients are displayed in Table 1. Older age, postmenopausal status, invasive carcinoma, positive lymph node status, and later stage were correlated with mastectomy. Correspondingly, the recurrence-free survival interval of lumpectomy patients was longer than that of those undergoing mastectomy (log-rank p = .048) (Fig. 1A).
Table 1.
Clinical and pathologic characteristics of breast cancer patients undergoing mastectomy and lumpectomy
Abbreviation: NA, not applicable.
Figure 1.
Survival curves and annual recurrence rate curves after mastectomy and lumpectomy. (A): Kaplan–Meier curves for recurrence-free survival in breast cancer patients by surgical modality. (B): Kaplan–Meier curves for overall survival in relapsed breast cancer patients undergoing lumpectomy by recurrence time. (C): Annual recurrence hazard rate for breast cancer patients by surgical modality according to our single-institution data. (D): Annual recurrence hazard rate for breast cancer patients who undergo lumpectomy by menopausal status according to our single-institution data.
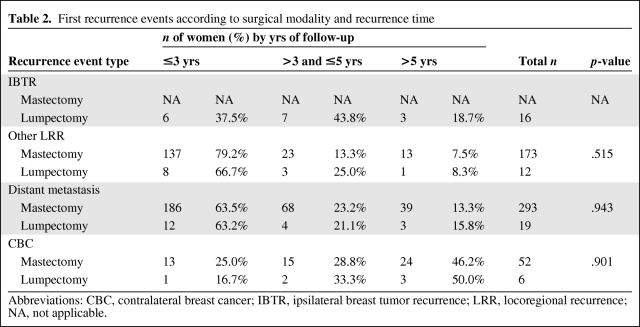
Table 2 displays the detailed recurrence type and recurrence time. During follow-up, 53 (6.3%) patients relapsed after lumpectomy and 518 (9.8%) patients relapsed after mastectomy. There were 28 patients (52.8%) with LRR after lumpectomy, 16 of whom (30.2%) developed IBTR; in contrast, 173 patients (33.4%) recurred with LRR after mastectomy. It seemed that the distribution of the recurrence types after lumpectomy were different from those after mastectomy (all LRR versus distant metastasis versus CBC; p = .011). More interestingly, of the 53 first recurrences after lumpectomy, 50.9% were detected ≤3 years postsurgery, 30.2% were detected at 3–5 years, and 18.9% were detected after 5 years, whereas of the 518 recurrences after mastectomy, 64.9% were detected ≤3 years postsurgery, 20.4% were detected at 3–5 years, and 14.7% were detected after 5 years. The time distribution of all recurrence events varied between lumpectomy and mastectomy patients, although with a borderline significant p-value (≤3 years versus >3 years and ≤5 years versus >5 years; p = .125). Notably, the distribution of recurrences ≤3 years versus >3 years postsurgery was significantly different between the two surgery types (p = .045).
Table 2.
First recurrence events according to surgical modality and recurrence time
Abbreviations: CBC, contralateral breast cancer; IBTR, ipsilateral breast tumor recurrence; LRR, locoregional recurrence; NA, not applicable.
Considering the recurrence type and recurrence time together, we found that 37.5% of IBTR, 66.7% of oLRR (all LRRs except for IBTR), 63.2% of distant relapse, and 16.7% of CBC events occurred ≤3 years postlumpectomy, whereas 79.2% of LRR, 63.5% of distant metastasis, and 25.0% of CBC events happened during this same period postmastectomy. Most IBTRs (81.3%) after lumpectomy occurred ≤5 years postsurgery, with a relatively low proportion of 37.5% in the first 3 years postsurgery. The time distributions of oLRR (p = .515), distant metastasis (p = .943), and CBC (p = .901) showed no significant difference between lumpectomy and mastectomy patients.
More interestingly, an evident association between early recurrence after lumpectomy and a lower OS rate was observed. Figure 1B shows that patients suffering early recurrences, either ≤3 years or at 3–5 years, had a lower OS rate than women not recurring until 5 years postsurgery (overall log-rank p = .025; ≤3 years versus 3–5 years, p = .140; ≤3 years versus >5 years, p = .034; 3–5 years versus >5 years, p = .056). Similarly, patients with recurrences ≤5 years postsurgery (≤3 years plus 3–5 years groups) had a significantly shorter OS time than patients without relapse until 5 years after lumpectomy (log-rank p = .027), with an HR of 4.62 (95% CI, 1.05–20.28; p = .042).
Annual Recurrence Pattern According to Our Single-Institution Experience and Literature-Based Data
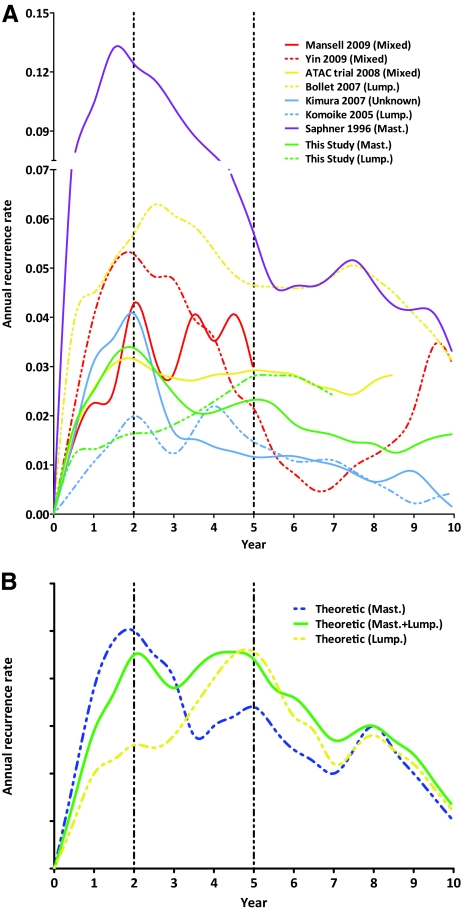
Figure 1C shows the smoothed ARR curves of breast cancer after mastectomy and lumpectomy. The ARR curve after mastectomy displayed a double-peaked pattern, with the major high peak at 2 years and a low flat peak at 5 years (3.4% and 2.3% per annum, respectively). In contrast, the ARR curve after lumpectomy exhibited one peak near 5–6 years (2.8% per annum). The first recurrence surge observed after mastectomy was not obvious after lumpectomy. In order to confirm the influence of menopausal status on recurrence timing and pattern, we performed an additional subgroup analysis according to menopausal status in women who underwent lumpectomy. The result (Fig. 1D) shows that there was a difference in recurrence timing between premenopausal and postmenopausal women who underwent lumpectomy. However, the timing difference is only about 1 year, which indicates that our observation of an obvious difference in recurrence timing (about 3–4 years) between lumpectomy and mastectomy is not a result of the higher proportion of premenopausal women in the lumpectomy group.
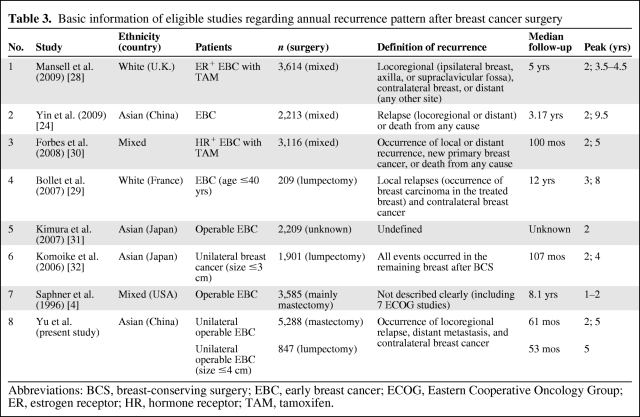
To further confirm the difference in the recurrence pattern between lumpectomy and mastectomy, we searched relevant literature for evidence. Seven publications that reported ARRs after surgery for primary breast cancer were identified as eligible [4, 24, 28–32]; another paper reporting the relapse pattern following LRR of breast cancer was excluded [33]. The basic characteristics of the seven studies are displayed in Table 3. Among them, three studies reported ARRs from mixed surgery populations (lumpectomy and mastectomy) [24, 28, 32], two studies were restricted to a lumpectomy population [29, 32], one study was performed mainly in a mastectomy population [4], and the remaining study did not specify the surgery type [31]. Figure 2A illustrates the ARR curves of all seven studies as well as our study by extracting ARR data from the original curve plots. We observed that lumpectomy populations had a recurrence peak at 4–6 years; in contrast, mastectomy patients had an earlier peak at 1–2 years. Interestingly, the recurrence pattern of the mixed surgery population had two comparable peaks at 1–2 years and near 5 years (refer to the curves from the Arimidex®, Tamoxifen, Alone or in Combination [ATAC] trial [30] and the study of Mansell et al. [28]). Some studies with a long follow-up time also exhibited a less prominent peak at 8–9 years.
Table 3.
Basic information of eligible studies regarding annual recurrence pattern after breast cancer surgery
Abbreviations: BCS, breast-conserving surgery; EBC, early breast cancer; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HR, hormone receptor; TAM, tamoxifen.
Figure 2.
Annual recurrence rate curves derived from our data and relevant studies. (A): Annual recurrence rate plots for seven eligible studies and the current study. (B): Theoretical annual recurrence rate plots for lumpectomy, mastectomy, and mixed surgical modalities.
Discussion
The present study is, to the best of our knowledge, the first retrospective analysis of the annual recurrence pattern for breast cancer patients after mastectomy or lumpectomy (BCS in this study). We observed a double-peaked time distribution (a major peak at 2 years and a moderate peak at 5 years) of recurrence risk in women undergoing mastectomy; however, in patients receiving lumpectomy, there was no first peak at 2 years and the recurrence risk kept rising with a peak at 5 years. We confirmed the double-peak recurrence surge after mastectomy and the single-peak relapse surge at 4–6 years after lumpectomy by reviewing relevant literature. Moreover, different from mastectomy, a large proportion of recurrence events after lumpectomy occurred ≤5 years postsurgery, rather than ≤3 years postsurgery. Early recurrence (≤5 years) after lumpectomy resulted in worse survival than later relapse after lumpectomy. Our findings suggest that the recurrence risk after primary tumor removal appears to have a different pattern between mastectomy and lumpectomy patients; therefore, the postoperative recurrence monitoring and follow-up strategy should be determined individually according to the patient's surgery type. We propose that a greater breast surveillance frequency after BCS at the recurrence peak would identify more early recurrences, whereas early detection of either IBTR or CBC in the asymptomatic phase might lead to a longer relative survival duration, as previous studies have implied [17, 18]. As well, we believe that early detection of breast cancer recurrence allows for a higher chance of reconservation for a few patients who refuse salvage mastectomy at LRR after lumpectomy.
For mastectomy, it has been established that 60%–80% of recurrences occur ≤3 years postsurgery; the recurrence risk reaches a peak at 1–2 years after surgery [3]. The present data reconfirmed the first peak at 2 years. The second peak at 5 years was absent in early reports [4] but observed in some recent studies [28, 30], indicating that the peak was not specific to the Chinese population. For lumpectomy, however, there was no first peak at 2 years, with the major peak emerging at 5 years. Our observations are consistent with a previous study [32] that showed a peak in IBTR at 3–5 years after primary operation and then a gradually decreasing ARR thereafter. Another report of lumpectomy showed an early peak in recurrence at 3 years [29]. There are some explanations. First, that study was restricted to young women treated with BCS, who tend to recur at an earlier time after surgery [34]. Second, the recurrence events used for ARR curve plotting were local relapse and CBC, but did not include distant recurrence that occurred at a later time after surgery [1].
The absence of a recurrence peak after lumpectomy at 1–2 years (during which time most relapses were local recurrence) in our study might be a result of the fact that we performed secondary mastectomy in patients with a close or positive margin after lumpectomy, treated with standard radiotherapy for patients undergoing lumpectomy, and had a relatively restrictive indication for BCS (i.e., tumor size ≤4 cm, distance between tumor and nipple–areola complex >3 cm). It has been well established that a close or positive margin, large tumor size, and involvement of the nipple–areola complex are associated with early local recurrence [3, 35, 36]. It has also been established that, compared with patients with estrogen receptor (ER)+ tumors, those with ER− breast cancers are more likely to recur earlier and the risk for the first peak is higher [24]. In our study, women who underwent lumpectomy were younger and more likely to be premenopausal, and premenopausal patients tend to have ER− tumors. We further performed an additional subgroup analysis according to menopausal status in women who underwent lumpectomy, and the result indicated that our observation of an obvious difference in the recurrence timing (about 3–4 years) between lumpectomy and mastectomy patients was not a result of the relatively higher proportion of premenopausal women in the lumpectomy group. Furthermore, there are other pieces of evidence in our previous study [24] showing that, in patients who undergo mastectomy, although premenopausal patients showed an earlier recurrence peak after surgery than their postmenopausal counterparts, the difference in timing was only about 1 year (peaks at 1.5 years and 2.5 years, respectively). Similarly, compared with patients with ER+ tumors, those with ER− breast cancers are more likely to recur early, but the difference in timing is still ∼1 year (peaks at 1.5 years and 2.5 years, respectively). Taken together, we suggest that the different recurrence patterns in mastectomy and lumpectomy patients are not a result of the different menopausal status between the two groups.
Interestingly, the mixed surgery population displayed an ARR curve with a merged shape of the recurrence curves from the lumpectomy and mastectomy populations. ARR curves from the ATAC trial (mastectomy, 47.7%; lumpectomy, 52.3%) [30] and the study of Mansell et al. [28] (mastectomy, 47.5%; lumpectomy, 52.5%) showed an equal or similar height of first peak at 2 years and a second peak at 5 years. Accordingly, we propose theoretical recurrence patterns for breast cancer after lumpectomy, mastectomy, and mixed modalities (Fig. 2B). The recurrence surge for mastectomy quickly increases to its highest peak within the first 2 years then decreases gradually, and then increases up to a low and flat peak at 5 years. In contrast, the recurrence surge after lumpectomy increases gradually and reaches a peak at 5 years.
Differences in the recurrence pattern after mastectomy and lumpectomy might have some implications for breast cancer follow-up and surveillance. The currently newest edition of the ASCO breast cancer surveillance guidelines suggests the same history and physical examination schedule for all breast cancer patients regardless of surgery type [6]. Examinations should be performed every 3–6 months for the first 3 years, every 6–12 months for years 4 and 5, and annually thereafter. As we propose, different from mastectomy, the recurrence peak after lumpectomy emerges near 5 years, rather than 2 years. According to our data, 80% of LRRs occur ≤3 years postmastectomy, whereas only 50% of LRRs and 37.5% of IBTRs occur postlumpectomy in this period. The current examination schedule is unfit for the features of relapse after lumpectomy, and incautious surveillance at 4–6 years might lead to failure in identification of recurrences. Recurrences within 5–6 years might be associated with shorter survival. Brooks et al. [37] observed a worse survival rate for patients with an IBTR <5.3 years after lumpectomy than for those who recurred >5.3 years postlumpectomy (HR, 2.49). We consistently observed an HR of 4.62 for patients recurring <5 years compared with >5 years postlumpectomy. Accordingly, we propose a modification of the current breast cancer surveillance guidelines: for patients treated with BCS, frequent examinations should be performed during years 4–6. Some investigators have suggested that in-breast recurrence is not a cause of systemic recurrence but rather is a marker of aggressive cancer biology [13]. Because early recurrence in the breast is a predictor of aggressive disease, we need to capture it through intensive monitoring, especially during the time of the recurrence peak. By identifying patients with aggressive disease, we could treat them with a positive strategy such as individualized chemotherapy, endocrine therapy, and targeted therapy. Currently, given the lack of prospective data, it seems reasonable to use the best available adjuvant systemic therapy for patients with aggressive early recurrences, because their metastatic potential approaches or exceeds that of newly diagnosed patients with node-positive disease [13]. However, consideration should be given to establishing guidelines for entering these patients into prospective protocols to evaluate systemic therapy [13].
Some limitations of our study should be acknowledged. First, as a retrospective study, recurrence events were probably underreported or misreported. Second, in the literature-based analysis, different studies have distinct definitions of “recurrence.” Our study is not an original data-based pooling analysis, and we could have overlooked the heterogeneity among different reports and curves. Third, we did not further scrutinize whether or not other clinicopathological factors and treatments would affect the ARR. Fourth, the current analysis is based on only a short follow-up and a small sample size of BCS patients; a more accurate annual recurrence pattern might be seen by analyzing more patients with a longer follow-up. Ideally, our observations should be validated in well-designed phase III clinical trials.
In conclusion, the recurrence pattern was different between mastectomy and lumpectomy patients. The relapse peak with mastectomy emerges in the first 2 years postsurgery, whereas the recurrence surge for lumpectomy increases annually with a peak near 5 years. The present breast cancer surveillance strategy for BCS patients should be modified because early detection of breast cancer recurrence in the asymptomatic phase might have a favorable impact on prognosis. Further large, prospective trials are needed to replicate our findings.
Acknowledgments
This research is supported by grants from the National Basic Research Program of China (2006CB910501), the 2009 Youth Foundation of Shanghai Public Health Bureau, the 2009 Youth Foundation of Shanghai Medical College, the Shanghai United Developing Technology Project of Municipal Hospitals (SHDC12010116), and the National Natural Science Foundation of China (30971143, 30972936, 81001169). The funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Zhi-Ming Shao had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We thank the women studied for their willingness to cooperate with our study, and we are also grateful to the anonymous reviewers for their very helpful suggestions and valuable comments.
Author Contributions
Conception/Design: Zhi-Ming Shao, Ke-Da Yu
Provision of study material or patients: Zhi-Ming Shao
Collection and/or assembly of data: Zhi-Ming Shao, Ke-Da Yu
Data analysis and interpretation: Zhi-Ming Shao, Ke-Da Yu, Shuang Li
Manuscript writing: Zhi-Ming Shao, Ke-Da Yu, Shuang Li
Final approval of manuscript: Zhi-Ming Shao, Ke-Da Yu, Shuang Li
References
- 1.Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 3.Huston TL, Simmons RM. Locally recurrent breast cancer after conservation therapy. Am J Surg. 2005;189:229–235. doi: 10.1016/j.amjsurg.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 4.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 5.Recommended breast cancer surveillance guidelines. American Society of Clinical Oncology. J Clin Oncol. 1997;15:2149–2156. doi: 10.1200/JCO.1997.15.5.2149. [DOI] [PubMed] [Google Scholar]
- 6.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 7.Smith TJ, Davidson NE, Schapira DV, et al. American Society of Clinical Oncology 1998 update of recommended breast cancer surveillance guidelines. J Clin Oncol. 1999;17:1080–1082. doi: 10.1200/JCO.1999.17.3.1080. [DOI] [PubMed] [Google Scholar]
- 8.Lichter AS, Lippman ME, Danforth DN, Jr, et al. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: A randomized trial at the National Cancer Institute. J Clin Oncol. 1992;10:976–983. doi: 10.1200/JCO.1992.10.6.976. [DOI] [PubMed] [Google Scholar]
- 9.Monteiro Grillo I, Jorge M, Marques Vidal P, et al. The effect of locoregional recurrence on survival and distant metastasis after conservative treatment for invasive breast carcinoma. Clin Oncol (R Coll Radiol) 2005;17:111–117. doi: 10.1016/j.clon.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 12.Whelan T, Clark R, Roberts R, et al. Ipsilateral breast tumor recurrence postlumpectomy is predictive of subsequent mortality: Results from a randomized trial. Investigators of the Ontario Clinical Oncology Group. Int J Radiat Oncol Biol Phys. 1994;30:11–16. doi: 10.1016/0360-3016(94)90513-4. [DOI] [PubMed] [Google Scholar]
- 13.Haffty BG, Reiss M, Beinfield M, et al. Ipsilateral breast tumor recurrence as a predictor of distant disease: Implications for systemic therapy at the time of local relapse. J Clin Oncol. 1996;14:52–57. doi: 10.1200/JCO.1996.14.1.52. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz JM, Spitalier JM, Amalric R, et al. The prognostic significance of late local recurrence after breast-conserving therapy. Int J Radiat Oncol Biol Phys. 1990;18:87–93. doi: 10.1016/0360-3016(90)90271-k. [DOI] [PubMed] [Google Scholar]
- 15.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 16.Rosselli Del Turco M, Palli D, Cariddi A, et al. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA. 1994;271:1593–1597. doi: 10.1001/jama.271.20.1593. [DOI] [PubMed] [Google Scholar]
- 17.Lu WL, Jansen L, Post WJ, et al. Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: A meta-analysis. Breast Cancer Res Treat. 2009;114:403–412. doi: 10.1007/s10549-008-0023-4. [DOI] [PubMed] [Google Scholar]
- 18.Houssami N, Ciatto S, Martinelli F, et al. Early detection of second breast cancers improves prognosis in breast cancer survivors. Ann Oncol. 2009;20:1505–1510. doi: 10.1093/annonc/mdp037. [DOI] [PubMed] [Google Scholar]
- 19.de Bock GH, Bonnema J, van der Hage J, et al. Effectiveness of routine visits and routine tests in detecting isolated locoregional recurrences after treatment for early-stage invasive breast cancer: A meta-analysis and systematic review. J Clin Oncol. 2004;22:4010–4018. doi: 10.1200/JCO.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 20.Perrone MA, Musolino A, Michiara M, et al. Early detection of recurrences in the follow-up of primary breast cancer in an asymptomatic or symptomatic phase. Tumori. 2004;90:276–279. doi: 10.1177/030089160409000302. [DOI] [PubMed] [Google Scholar]
- 21.Yu KD, Di GH, Wu J, et al. Development and trends of surgical modalities for breast cancer in China: A review of 16-year data. Ann Surg Oncol. 2007;14:2502–2509. doi: 10.1245/s10434-007-9436-2. [DOI] [PubMed] [Google Scholar]
- 22.Yu KD, Li JJ, Di GH, et al. A straightforward but not piecewise relationship between age and lymph node status in Chinese breast cancer patients. PLoS One. 2010;5:e11035. doi: 10.1371/journal.pone.0011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan J, Wang L, Wang XJ, et al. Breast conservative therapy in east part of China: A retrospective cohort study. J Cancer Res Clin Oncol. 2006;132:573–578. doi: 10.1007/s00432-006-0104-x. [DOI] [PubMed] [Google Scholar]
- 24.Yin W, Di G, Zhou L, et al. Time-varying pattern of recurrence risk for Chinese breast cancer patients. Breast Cancer Res Treat. 2009;114:527–535. doi: 10.1007/s10549-008-0022-5. [DOI] [PubMed] [Google Scholar]
- 25.Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77:2267–2274. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2267::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 26.Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. J Natl Cancer Inst. 1998;90:1601–1608. doi: 10.1093/jnci/90.21.1601. [DOI] [PubMed] [Google Scholar]
- 27.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansell J, Monypenny IJ, Skene AI, et al. Patterns and predictors of early recurrence in postmenopausal women with estrogen receptor-positive early breast cancer. Breast Cancer Res Treat. 2009;117:91–98. doi: 10.1007/s10549-008-0291-z. [DOI] [PubMed] [Google Scholar]
- 29.Bollet MA, Sigal-Zafrani B, Mazeau V, et al. Age remains the first prognostic factor for loco-regional breast cancer recurrence in young (<40 years) women treated with breast conserving surgery first. Radiother Oncol. 2007;82:272–280. doi: 10.1016/j.radonc.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 31.Kimura M, Yanagita Y, Fujisawa T, et al. Study of time-course changes in annual recurrence rates for breast cancer: Data analysis of 2,209 patients for 10 years post-surgery. Breast Cancer Res Treat. 2007;106:407–411. doi: 10.1007/s10549-007-9510-2. [DOI] [PubMed] [Google Scholar]
- 32.Komoike Y, Akiyama F, Iino Y, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: Risk factors and impact on distant metastases. Cancer. 2006;106:35–41. doi: 10.1002/cncr.21551. [DOI] [PubMed] [Google Scholar]
- 33.Kamby C, Sengeløv L. Survival and pattern of failure following locoregional recurrence of breast cancer. Clin Oncol (R Coll Radiol) 1999;11:156–163. doi: 10.1053/clon.1999.9033. [DOI] [PubMed] [Google Scholar]
- 34.Elkhuizen PH, van de Vijver MJ, Hermans J, et al. Local recurrence after breast-conserving therapy for invasive breast cancer: High incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys. 1998;40:859–867. doi: 10.1016/s0360-3016(97)00917-6. [DOI] [PubMed] [Google Scholar]
- 35.Voogd AC, Nielsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two large European randomized trials. J Clin Oncol. 2001;19:1688–1697. doi: 10.1200/JCO.2001.19.6.1688. [DOI] [PubMed] [Google Scholar]
- 36.Cense HA, Rutgers EJ, Lopes Cardozo M, et al. Nipple-sparing mastectomy in breast cancer: A viable option? Eur J Surg Oncol. 2001;27:521–526. doi: 10.1053/ejso.2001.1130. [DOI] [PubMed] [Google Scholar]
- 37.Brooks JP, Danforth DN, Albert P, et al. Early ipsilateral breast tumor recurrences after breast conservation affect survival: An analysis of the National Cancer Institute randomized trial. Int J Radiat Oncol Biol Phys. 2005;62:785–789. doi: 10.1016/j.ijrobp.2004.12.001. [DOI] [PubMed] [Google Scholar]