The most recent and interesting developments on diagnostic and therapeutic approaches in the treatment of neoplastic meningitis from solid tumors are reviewed.
Keywords: Neoplastic meningitis, Leptomeningeal disease, Leptomeningeal carcinomatosis, Solid tumors, Targeted therapy, Brain tumors
Learning Objectives
After completing this course, the reader will be able to:
Compare the use of i.t. therapy and systemic therapies for patients with neoplastic meningitis.
Describe new drugs showing promise for neoplastic meningitis.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Neoplastic meningitis is a result of the spread of malignant cells to the leptomeninges and subarachnoid space and their dissemination within the cerebrospinal fluid. This event occurs in 4%–15% of all patients with solid tumors and represents an important prognostic factor for poor survival. Neoplastic meningitis should be diagnosed in the early stages of disease to prevent important neurological deficits and to provide the most appropriate treatment. Despite new diagnostic approaches developed in recent years, such as positron emission tomography–computed tomography and new biological markers, the combination of magnetic resonance imaging without and with gadolinium enhancement and cytology still has the greatest diagnostic sensitivity.
Recently, no new randomized studies comparing intrathecal (i.t.) with systemic treatment have been performed, yet there have been a few small phase II studies and case reports about new molecularly targeted substances whose successful i.t. or systemic application has been reported. Trastuzumab, gefitinib, and sorafenib are examples of possible future treatments for neoplastic meningitis, in order to better individualize therapy thus allowing better outcomes.
In this review, we analyze the most recent and interesting developments on diagnostic and therapeutic approaches.
Introduction
Neoplastic meningitis (NM) results from the spread of malignant cells to the leptomeninges and subarachnoid space and their dissemination within the cerebrospinal fluid (CSF) compartment. NM is a frequent complication of systemic cancer and occurs in 4%–15% of all patients with solid tumors. Although NM has been described in nearly all types of solid tumors, the most common solid tumors causing NM are breast cancer (43%), lung cancer (31%), and melanoma (6%) [1]. NM secondary to breast cancer tends to be the most prevalent because of the overall number of women with the disease and because of better systemic control of the disease. Malignant cells may reach the subarachnoid space through the blood (venous or arterial), by growing along nerve and vascular sheaths, by migration from a tumor adjacent to CSF (30%–40% of patients with NM have coexistent parenchymal brain metastases), or by iatrogenic spread of tumor cells following resection of brain or cerebellar metastasis [2, 3].
In the last few years, it was discovered that the molecular factors implicated in NM development are numerous, including, in particular, metalloproteinases, activated integrin αvβ3, vascular endothelial growth factor (VEGF), and epidermal growth factor receptor (EGFR)-2 protein [4]. Moreover, NM has become an increasingly common diagnosis, most likely because of the prolonged survival of patients with metastatic disease and because of better diagnostic methods. Furthermore, NM resulting from tumors that were previously rarely associated with NM, such as prostate cancer, ovarian cancer, gastric cancer, cervical cancer, and endometrial cancer, appear to be increasing.
Treatment of NM aims to extend survival and stabilize or improve neurological symptoms, especially if it is diagnosed early. Therapy is not standardized, and includes surgery, radiation, local intrathecal (i.t.) or systemic chemotherapy, as well as available supportive measures. However, despite aggressive treatment, the median survival duration for patients with NM is in the range of 2–6 months and toxicity appears considerable [4]; hence, there is a need to achieve earlier diagnosis and to find new treatments.
Diagnostic Approaches
The diagnosis of NM is based on clinical symptoms and signs, imaging evidence, and CSF analysis.
Clinical Examination
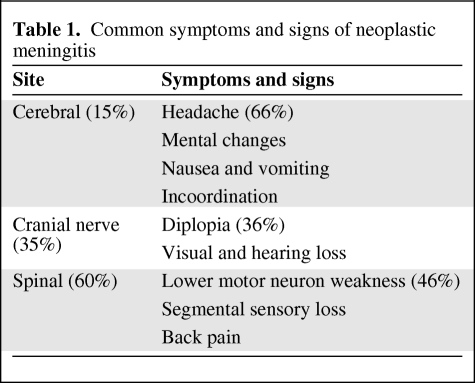
NM can involve the entire neuraxis, and therefore most patients present with pleomorphic and multifocal neurological symptoms according to the site involved (Table 1): cerebral (15%), cranial nerve (35%), or spinal (60%).
Table 1.
Common symptoms and signs of neoplastic meningitis
The most common manifestations of cerebral dysfunction are headache (66%), mental changes, nausea/vomiting, and incoordination; diplopia (36%), visual loss and hearing loss are the most common symptoms of cranial nerve dysfunction; spinal signs and symptoms include lower motor neuron weakness (46%), segmental sensory loss, and radicular and back/neck pain [5]. Of note, NM symptoms must be differentiated from meningitis resulting from other diseases such as sarcoidosis and tuberculosis; however, up to 25% of patients with NM can be diagnosed by clinical examination alone [2], although it is unthinkable to start therapy on the basis of clinical examination alone.
Diagnostic Imaging
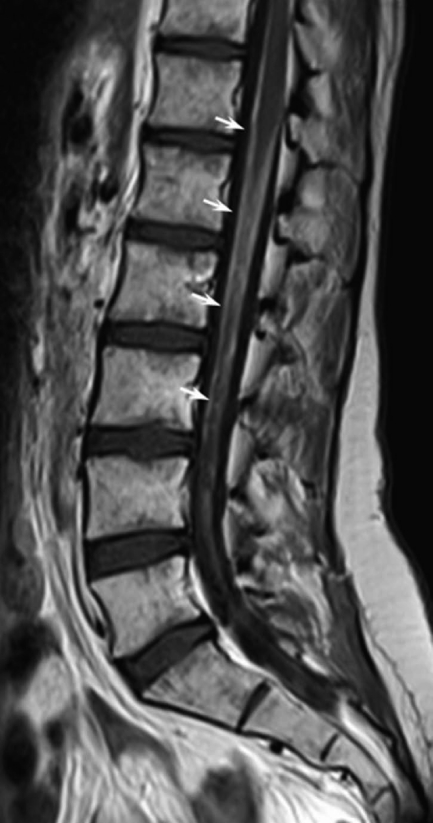
Magnetic resonance imaging (MRI) without and with gadolinium enhancement (Gd-MRI) of the entire central nervous system (CNS) is the radiological method of choice when NM is suspected, showing a higher sensitivity and specificity than contrast-enhanced computed tomography [6]. Combined T1-weighted and fat suppression T2-weighted sequences constitute the standard examination (Fig. 1). Important features for diagnosis of NM on Gd-MRI include focal or diffuse leptomeningeal enhancement, enhancement of intradural spinal nerves, and tumor nodules. These alterations are not pathognomonic because similar findings can be observed in several diseases; in fact, the differential diagnosis for meningeal enhancement must be made from granulomatous infiltration, inflammation (rheumatoid arthritis), infection, subarachnoid hemorrhage, intracranial hypotension, and chemical meningitis.
Figure 1.
A 27-year-old female patient with rhabdomyosarcoma and cytologically confirmed neoplastic meningitis. Sagittal T1-weighted image after contrast medium i.v. administration showing marked leptomeningeal enhancement of the conus and of cauda equina (white arrows).
In two studies [7, 8] analyzing 137 patients with NM from a primary solid tumor or hematalogic malignancy, Gd-MRI sensitivity and specificity were both about 75%; in particular, Freilich et al. [7] showed that Gd-MRI was abnormal in ∼90% of patients with both a solid tumor and positive CSF. In another study, Gd-MRI showed an ∼30% incidence of false-negative results [6], and so negative imaging does not exclude the diagnosis of NM in a patient with typical signs and symptoms. Positive Gd-MRI alone may be sufficient to establish an NM diagnosis and, in particular, Gd-MRI may be useful when the CSF is negative or if a lumbar puncture cannot be performed and the suspicion of NM remains high [8].
To our knowledge, there are no retrospective or prospective studies on the accuracy of 18F-fluorodeoxyglucose positron emission tomography–computed tomography (FDG-PET–CT) for NM diagnosis, probably because of resolution issues; only two papers describe one case of leptomeningeal carcinomatosis from lung cancer [9] and another case of meningeal metastases from neuroblastoma [10] detected using FDG-PET–CT. Likely, this imaging modality may play an important role in the detection of unexpected NM at an unusual site from certain cancers.
Results of radioisotope studies, using either 111indium-diethylenetriamine penta-acetic acid or 99Tc macroaggregated albumin, can be used to evaluate CSF flow dynamics and pathways before giving i.t. drugs [11, 12] in patients with a good performance status (PS), negative Gd-MRI, and positive CSF. In fact, this investigation, although not always readily available to practitioners, could be important because ∼60% of patients with NM have either normal neuroradiological examinations or CSF flow blocks [13]; moreover, involved-field radiotherapy (RT) to the site of CSF flow obstruction restores flow in 30%–50% of patients, and when followed by intra-CSF chemotherapy leads to a longer survival duration than in patients who have persistent CSF blocks [14].
CSF Analysis
CSF analysis could represent a valid method for diagnosis because the demonstration of tumor cells in the CSF is diagnostic for NM and anyone suspected of having NM should have a lumbar puncture (LP) performed if it is not otherwise contraindicated. CSF cytology is tumor positive on the first LP in ∼50% of cases [15], increasing to about 90% with a second LP, but little benefit is obtained from additional LPs [16]. However, CSF cytology can remain falsely negative in 14% of patients even after three samples. CSF should also be performed as closely to the site of clinically evident disease as possible. Chamberlain et al. [17], in a series of 60 patients with NM, showed that ventricular and lumbar puncture were discordant in 30% of cases; in fact, in the presence of spinal signs or symptoms, the lumbar CSF cytology was more likely to be positive than the ventricular, and conversely, in the presence of cranial signs or symptoms, the ventricular CSF cytology was more likely to be positive.
In a recent retrospective study, Clarke et al. [1] performed a comparison between CSF cytology and MRI in patients with NM from solid tumors. They showed that neuroimaging was positive for NM in 88% of cases and CSF was positive in 85% of those tested. In patients with both MRI of at least one segment and CSF analysis, both diagnostic tests were positive for NM in 55% of solid tumor patients, cytology alone was positive in 28% of patients, whereas neuroimaging alone was positive in 17% of patients. These results demonstrate that MRI and CSF cytology can be complementary for the NM diagnosis.
When cytology is not diagnostic, measurement of the opening pressure, cell count, and measurement of CSF protein and glucose can be helpful for the diagnosis, although these parameters are very nonspecific; a completely normal routine CSF examination almost excludes NM, and an opening pressure >15 cm H2O, elevated WBC, glucose <60 mg/dL, or protein >50 mg/dL can be suggestive of NM. In an effort to improve the sensitivity of CSF diagnostic immunocytochemistry, flow cytometry, polymerase chain reaction (PCR), and cytogenetic analysis were developed, although results have been less successful [18, 19]. In addition, numerous nonspecific markers, such as lactate dehydrogenase, or specific markers, such as α-fetoprotein, may be useful for monitoring the course of disease; in particular, VEGF was shown to be a sensitive and specific marker for diagnosis and prognosis [20, 21]. Recently, it was shown Dickkopf-3, a secreted tumor suppressor, may be a useful biologic marker for both the diagnosis and treatment response evaluation of NM, although its specificity is limited by similar CSF levels in patients with viral meningitis [22].
Response Evaluation
The low sensitivity of CSF cytology and the high incidence of false-negative results with Gd-MRI make it difficult to assess response to treatment [6, 7], leading to a lack of standardized evaluation criteria. In cases of nodular or bulky NM and when the diagnosis of NM is established by Gd-MRI, a subsequent Gd-MRI can be performed to evaluate the effectiveness of treatment using the Response Evaluation Criteria in Solid Tumors or World Health Organization (WHO) criteria [23, 24]. In cases with a negative Gd-MRI, patients may be scored as responders if their CSF cytology is converted from positive to negative at all sites (i.e., ventricle and/or lumbar sac) previously shown to be positive, and they remain neurologically stable or their neurological condition improves. In cases with negative CSF at the beginning of treatment, neurological improvement during therapy may be regarded as a sign of response [25]. Moreover, various studies have shown poor correlation between cytology and neurological examination during treatment, demonstrating that clinical response and survival are independent of cytologic improvement; also, many patients show neurological deterioration with negative cytology, thus neurological assessment may be a better predictor of treatment efficacy [26].
Recently, numerous biochemical markers have been evaluated and their use can be helpful to assess response to treatment when followed serially. In particular, α-fetoprotein and β-human chorionic gonadotropin from testicular cancer or prostate-specific antigen in prostate cancer when elevated in CSF can be used to evaluate the effectiveness of treatment. PCR and fluorescence in situ hybridization to detect genetic alterations may be additional diagnostic information but their sensitivity in NM from solid tumors is still low.
Treatment and Prognostic Factors
Treatment of NM is intended to improve or stabilize the neurological status as well as to extend survival. Most untreated patients with NM from solid tumors have a poor prognosis and die within 3–6 weeks, and progressive neurologic dysfunction is the most frequent cause of death. With treatment, the median survival time can be increased to 4–6 months [27], with a longer survival duration in a few cases.
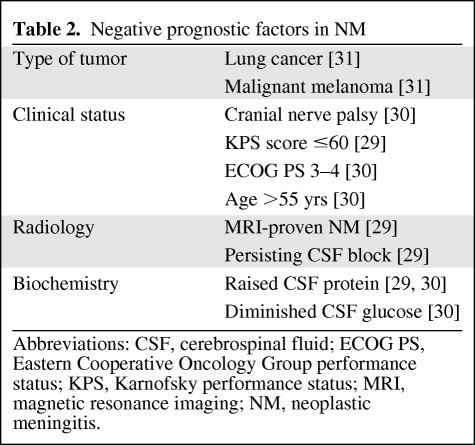
The evaluation of “poor” and “good” risk is important to identify patients who may benefit from aggressive treatment or supportive care alone (Table 2). Waki et al. [28] retrospectively analyzed data from 85 patients with cytologically proven NM from various solid tumors; a univariate analysis identified breast cancer, a good PS, time to development of NM, and treatment with i.t. chemotherapy as being associated with a good prognosis. A multivariate analysis identified poor PS (hazard ratio [HR], 1.72) and MRI-proven NM (HR, 1.82) as being associated with a poor prognosis. A prior study [29] showed that a Karnofsky KPS score ≤60 had a negative prognostic significance and independently predicted survival in patients with NM who were considered for i.t. chemotherapy.
Table 2.
Negative prognostic factors in NM
Abbreviations: CSF, cerebrospinal fluid; ECOG PS, Eastern Cooperative Oncology Group performance status; KPS, Karnofsky performance status; MRI, magnetic resonance imaging; NM, neoplastic meningitis.
In a recent study, Gauthier and colleagues [30] analyzed 91 patients with NM from breast cancer treated with i.t. methotrexate (MTX) and a multivariate analysis demonstrated a poor PS score (WHO–Eastern Cooperative Oncology Group PS score, 3–4), number of prior chemotherapy regimens (more than three), a negative hormone receptor status, age> 55 years, and high Cyfra 21–1 levels to be associated with poor survival.
Other negative prognostic factors in NM with respect to treatment outcome may include raised CSF protein, diminished CSF glucose, cranial nerve palsy, progressive systemic disease, and persistent CSF block [2]. Regarding the primary tumor, lung cancer and malignant melanoma seem to have a more negative prognosis [31].
Radiotherapy
Focal RT is performed in the treatment of bulky disease (intra-CSF chemotherapy is limited by diffusion to 2–3 mm penetration into tumor nodules) and in patients with CSF flow blocks. In addition, local RT should also be administered to symptomatic areas with a short palliative schedule such as 20 Gy in five fractions or 30 Gy in 10 fractions [25]. In patients with evidence of CSF flow obstruction, irradiation of the blockage before administration of i.t. drugs has been shown to result in better survival outcomes, with successful relief of CSF block by RT reported in ∼50% of cases [12, 13]. Another use of RT is in the treatment of cauda equina syndrome and cranial neuropathies from NM, whereas craniospinal irradiation is rarely used because of significant systemic toxicity and leukoencephalopathy.
Standard i.t. Chemotherapy
Intrathecal chemotherapy has been the mainstay treatment for patients with NM even though the extent of its benefit has not been proven in randomized controlled trials [25] and some retrospective studies showed discordant evidence [32, 33]. Intrathecal treatment offers local therapy with minimum systemic toxicity, and avoiding the blood–brain barrier, drugs are distributed throughout the entire subarachnoid space; although high drug concentrations can be achieved in the CSF, i.t. treatment is not effective for bulky disease in the meninges because intra-CSF agents penetrate only 2–3 mm into such lesions [34]. Chemotherapy administration can be undertaken either i.t. via a lumbar puncture or via an intraventricular device with a catheter into the lateral ventricle by Ommaya reservoir.
MTX and free and liposomal cytarabine are the i.t. drugs most commonly used against solid tumor NM. Unlike the other two drugs, liposomal cytarabine (DepoCyte®; Enzon Pharmaceuticals, Bridgewater, NJ) maintains a continuous and homogeneous cytotoxic drug concentration in the subarachnoid space over 2 weeks following the administration of a 50-mg dose. However, in the few randomized trials, the data were discordant when comparing treatments. In particular, Glantz et al. [35] compared i.t. lyposomal cytarabine with i.t. MTX; in the first phase (induction), patients received either liposomal cytarabine, 50 mg once every 2 weeks, or MTX, 10 mg twice a week. Patients who attained a response at the end of the induction phase were eligible to enter the consolidation phase; in that phase, liposomal cytarabine was administered every 2 weeks for 1 month and then every 4 weeks for 2 months, whereas MTX was given every week for 1 month and then every 2 weeks for 2 months. Sixty-one patients were randomized, and after the induction phase responses occurred in 26% of liposomal cytarabine–treated and 20% of MTX-treated patients (p = .76). Median survival times were 105 days in the liposomal cytarabine arm and 78 days in the MTX arm (p = .15). Interestingly, patients in the liposomal cytarabine group experienced a greater median time to neurological progression (58 days versus 30 days; p = .007). Chemical meningitis of any grade was common and occurred with slightly greater frequency in the liposomal cytarabine group than in the MTX group (23% versus 19% of cycles; p = .57). Only the frequencies of sensory/motor dysfunction and of visual impairment differed appreciably between treatment groups (p < .05).
In another randomized study [36], the authors compared i.t. liposomal cytarabine with MTX or nonliposomal cytarabine in the treatment of solid tumor NM, analyzing 103 patients. They demonstrated that liposomal cytarabine was noninferior to MTX (HR, 0.94, 95% confidence interval, 0.58–1.53), although the incidences of serious adverse events were 86% in the liposomal cytarabine group and 77% in the control group. Previously, Cole et al. [37], in a controlled trial, demonstrated an advantage with liposomal cytarabine over MTX in terms of time to neurological progression, survival time, and quality of life in the treatment of solid tumor NM. The median times to neurological progression were 30 days for the MTX group and 58 days for the liposomal cytarabine group (p = .007); the median overall survival (OS) periods were 78 days and 105 days for the MTX group and liposomal cytarabine group, respectively (p = .15). An adverse event of grade ≥3 occurred in 67% of the patients randomized to receive MTX and 77% of the patients randomized to received liposomal cytarabine, although the liposomal cytarabine regimen provided a significantly greater quality of life.
More recently, in a nonrandomized prospective trial [38], 25 patients with NM from breast cancer were treated with i.t. liposomal cytarabine. After five i.t. administrations, clinical stabilization or improvement was observed in 56.5% or patients, a CSF response was observed in 36% of patients, and the median OS duration was 7 months.
The safety and efficacy of i.t. liposomal cytarabine in 16 patients with NM from various solid tumors also were analyzed by Gil-Bazo et al. [39], demonstrating an improvement in all neurological symptoms in five patients and a median time to neurological progression of 14 days (range, 0–170 days). The most frequently reported adverse effect was headache (37.5%).
Boogerd et al. [25] randomized patients with NM from breast cancer to receive appropriate systemic therapy and, if necessary, RT with or without i.t. MTX. An intention-to-treat analysis showed neurological improvement or stabilization in 59% of the i.t. and in 67% of the non-i.t. group, with a median times to progression of 23 weeks in the i.t. arm and 24 weeks in the other arm. Interestingly, the median survival duration was 18.3 weeks for i.t. patients and 30.3 weeks for non-i.t. patients (p = 0.32). Neurological complications occurred in 47% and 6% (p = .0072) of patients in the i.t. group and non-i.t. group, respectively. The most frequent complications with i.t. MTX were aseptic and infectious meningitis (13%), early and late leukoencephalopathy (24%), and fatal encephalopathy (6%).
New Intrathecal Drugs
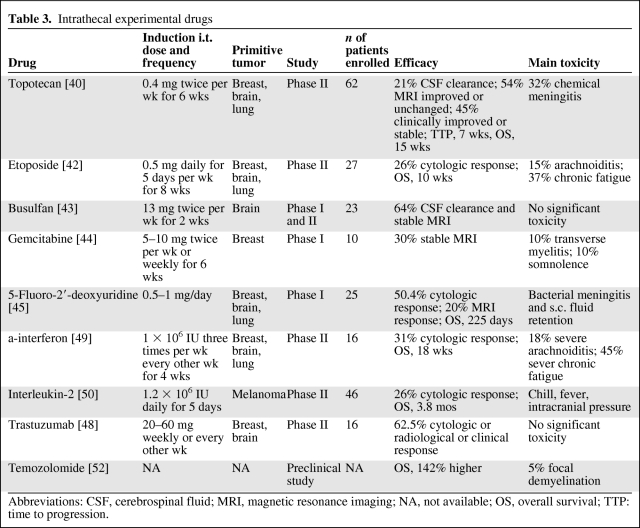
In the last few years, various experimental i.t. drugs have been reported upon from small clinical trials, a few case reports, and preclinical studies (Table 3).
Table 3.
Intrathecal experimental drugs
Abbreviations: CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; NA, not available; OS, overall survival; TTP: time to progression.
Topotecan
Topotecan is a topoisomerase-I inhibitor used for the systemic treatment of some types of solid tumors. In a phase 1 trial, 0.4 mg of i.t. topotecan was proven to be safe in 23 patients with NM, and six of those patients had evidence of benefit manifested as prolonged disease stabilization or response. In a subsequent nonrandomized and single-arm phase II trial, Groves et al. [40] tested i.t. topotecan in 62 patients with NM. Patients with breast cancer, primary brain tumors, and lung cancer made up 74% of the enrolled patients. During the induction phase, patients received 0.4 mg of i.t. topotecan twice weekly for a total of 6 weeks. If patients had no evidence of progressive NM, they received consolidation therapy with topotecan weekly for six doses; in patients who still showed no evidence of progression, maintenance therapy was administered (twice monthly for 4 months and then monthly thereafter). Forty patients (65%) completed the 6-week induction period. The CSF was cleared of malignant cells in 13 of 62 patients (21%) at 6 weeks. Gd-MRI improved in 10% of patients and remained unchanged in 44%. Clinically, 16% of patients improved and 29% remained stable. The median time to progression (TTP) was 7 weeks and the median OS time was 15 weeks. The most common side effect was chemical meningitis in 32% of patients (grade 3, 5%), whereas another 32% of patients experienced no drug side effects.
Walker and colleagues [41] treated four patients with NM originating from a variety of primary solid tumors. Five months and 10 months were the minimum and maximum lengths of treatment; nausea and fatigue were the most important adverse events.
In our experience, we treated one patient with NM from rhabdomyosarcoma with i.t. topotecan, and progression of the neurological symptoms occurred 1 month after the treatment as a result of progressive disease.
Etoposide
Etoposide is a topoisomerase-II inhibitor, and in clinical practice is active against numerous types of solid cancers such as ovarian and lung cancers. Chamberlain et al. [42] treated 27 patients with NM (85% from solid tumors) with i.t. etoposide. They received 0.5 mg of etoposide by intraventricular injection every day for five consecutive days per week every other week for a total of 8 weeks. Patients who were clinically stable or improved received 0.5 mg of etoposide every day for a total of five consecutive days every 4 weeks until disease progression. A cytological response was attained by 26% of the patients, with a median time to neurologic disease progression of 20 weeks. Among all patients, the median OS duration was 10 weeks. The difference in efficacy in lymphoma compared with solid tumors was not found to be statistically significant. The most common toxicities were arachnoiditis (15%) and chronic fatigue (37%).
Busulfan
Busulfan is an alkylating agent that has been used in the treatment of chronic myelogenous leukemia and in high-dose chemotherapy schedules; it was shown to have efficacy in preclinical studies using medulloblastoma, ependymoma, and malignant glioma xenografts. Gururangan et al. [43] conducted a phase I study analyzing a water-soluble formulation of busulfan in 23 pediatric patients with solid tumor NM. The final i.t. dose recommended for further phase II studies was 13 mg twice weekly for 2 weeks. Patients with an objective response or stable disease were allowed to continue therapy until disease progression. All patients completed the first 2 weeks of therapy and were evaluable for response. Nine patients had stable disease and 14 patients had progressive disease after the first 2 weeks of treatment. The seven patients treated with 13 mg of i.t. busulfan did not experience any significant toxicity.
Gemcitabine
Gemcitabine is a deoxycytidine analog that inhibits DNA polymerase and ribonucleotide reductase. It has clinical activity against a wide variety of cancers. A phase I trial by Bernardi and colleagues [44] investigated 10 patients with acute lymphoblastic leukemia and solid tumor (60%) NM. Patients received i.t. gemcitabine, with a dose range of 5–10 mg weekly or twice weekly. Five patients completed the induction phase (6 weeks of therapy) without any objective responses; three patients had stable disease. Two patients experienced dose-limiting neurological adverse events: one treated at the 5-mg twice-weekly dose level developed transverse myelitis and another patient treated at the 10-mg twice-weekly dose level developed somnolence. Thus, i.t. gemcitabine was associated with significant neurotoxicity and its use is not recommended.
5-Fluoro-2′-deoxyuridine
The role of continuous i.t. 5-fluoro-2′-deoxyuridine (FdUrd) against solid tumor NM was investigated by Nakagawa et al. [45] in a phase I study. They treated 25 patients with 0.5–1 g per day of i.t. FdUrd. Four patients had a complete cytologic response and nine patients had a partial reduction in CSF cell count after 1 month of the infusion. Two months after the treatment, a Gd-MRI response was observed in only five patients. The survival time was 225 days. Bacterial meningitis and s.c. fluid retention were the most common toxicities.
Trastuzumab
Trastuzumab is a humanized monoclonal antibody that binds to human epidermal growth factor receptor (HER)-2, which is overexpressed in some breast cancers, gastric cancers, glioblastoma, and medulloblastoma. A few case reports demonstrated that i.t. trastuzumab alone or in combination with thiotepa or MTX and/or systemic chemotherapy was effective and safe against NM from HER-2+ breast cancers. They used i.t. trastuzumab at a dosage of 20–50 mg weekly or every 3 weeks [46, 47], obtaining a good response. Noteworthy is the fact that a patient was alive 21 months after the first injection of i.t. trastuzumab [47]. Allison et al. [48] analyzed i.t. trastuzumab in 16 patients with NM from breast cancer, glioblastoma, and medulloblastoma. The patients were treated with 20–60 mg per dose of i.t. trastuzumab either weekly or every other week for four treatments. Patients who were neurologically and radiographically stable or improved after four treatments continued on every-other-week therapy until neurologic progression. Two of the four patients with breast cancer and the patient with medulloblastoma responded clinically and cytologically 4, 14, and 6 weeks, respectively, after initiating treatment. Seven of the 11 patients with glioblastoma responded (two cytologically, two radiographically, and all seven clinically), with response durations in the range of 4–12 weeks. The treatment was well tolerated with no adverse events related to trastuzumab.
α-Interferon
α-interferon (α-IFN) is an attractive agent for use in NM because of its modest efficacy against a variety of cancers and its biologic properties, including immunomodulatory activity, antiproliferative activity, and inhibition of angiogenesis. Chamberlain et al. [49] performed a phase II trial to establish the toxicity of intra-CSF α-IFN and to determine its antitumoral activity in the treatment of NM. They treated 22 patients, and 16 (73%) of these had NM from solid tumors. Patients received 1 × 106 IU α-IFN by intravenous (i.v.) injection every other day for a total of three doses per week for 4 weeks (induction period). Patients who were clinically stable or improved or who had negative CSF cytology received 1 × 106 IU α-IFN with the same schedule for a total of 4 weeks (consolidation period). Patients who were clinically stable or improved with negative CSF cytology continued to receive 1 × 106 IU α-IFN every other day for a total of three doses per week once a month for 4 months (maintenance period). About one third of the patients with solid tumor NM treated with α-IFN attained a cytologic response at the conclusion of the induction phase. The duration of response was in the range of 8–40 weeks. Overall, the median survival duration was 18 weeks. Nine percent of the patients had grade >2 arachnoiditis, and grade >2 chronic fatigue was seen in 45% of patients. There was no evidence of hematologic toxicity.
Interleukin-2
Papadopoulos et al. [50] analyzed the efficacy and safety of interleukin (IL)-2 in the treatment of NM from melanoma. They treated 46 patients with 1.2 × 106 IU IL-2 every day for 5 days, then three times a week via an Ommaya reservoir until response. There were 12 responses, defined as negative CSF cytology of 4 weeks duration. The median survival time was 3.8 months and the median survival duration of patients with a response was 11.5 months. Significant toxicities were chills, fever, and signs of elevated intracranial pressure.
Intrathecal Gene Therapy
Intrathecal gene therapy may be an interesting treatment for patients with NM from solid tumors, although no prospective studies have been performed so far. Heiss et al. [51] reported a case of a patient with NM secondary to malignant melanoma treated with this method. That patient received intraventricular delivery of NIH3T3 producer cells expressing the thymidine kinase (HSV-Tk1) gene via a retroviral vector followed by i.v. ganciclovir. The patient survived 9 months after treatment; side effects included acute hyperpyrexia, which was medically manageable.
Temozolomide and Pemetrexed
Intrathecal microcrystalline temozolomide and pemetrexed for the treatment of NM were analyzed in preclinical studies in rats.
Temozolomide is an oral methylating agent with activity against malignant glioma and melanoma. The standard formulation of temozolomide prevents its use as an i.t. drug, and to overcome this problem Sampson and colleagues [52] developed a microcrystalline formulation with enhanced solubility. They demonstrated that i.t. microcrystalline temozolomide in rats with NM led to a >142% longer median survival time. The toxicity directly attributable to i.t. administration was limited to small patchy areas of focal demyelination involving <5% of the spinal cord long tracks. Notably, some of the rats had no evidence of residual tumor on histological examination after treatment.
Pemetrexed is an antifolate antimetabolite showing cytotoxicity in a variety of tumors, including mesothelioma, non-small cell lung cancer (NSCLC), and breast cancer. Unfortunately, its concentration in CSF is very low after systemic administration. Thus, Sun et al. [53] studied the safety and pharmacokinetics of i.t. administration of pemetrexed in rats. They showed that 1-mg/kg dose of i.t. pemetrexed administered twice a week for 2 weeks was safe and capable of delivering a therapeutically durable concentration in rats. Thus, calculating the corresponding value in humans, the optimal starting dose for human studies could be 5–10 mg. On the basis of the data presented in these studies, a phase I trial of i.t. pemetrexed or microcrystalline temozolomide should be undertaken.
Systemic Therapies
Although most chemotherapeutic agents when given systemically have poor CSF penetration, various studies show that the blood–brain barrier in NM is permeable for i.v. drugs [54, 55]. Furthermore, NM from solid tumors is thick and well vascularized, and thus could be better penetrated by systemically administered drugs than by intra-CSF agents, which penetrate only 2–3 mm into such lesions, and efficacy will not be affected by CSF flow obstruction. However, systemic chemotherapy can be limited by systemic toxicity and the difficulty of using an effective treatment for NM as well as for the underlying disease that caused the meningeal spread.
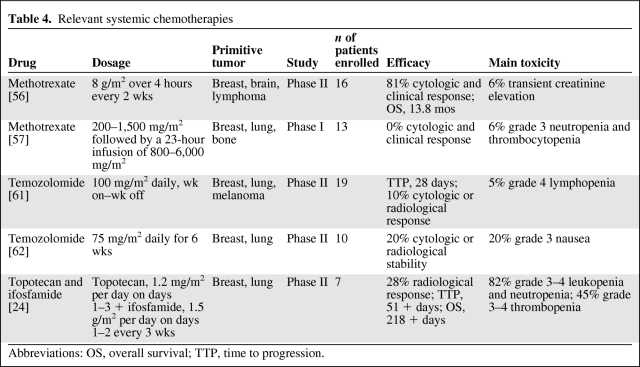
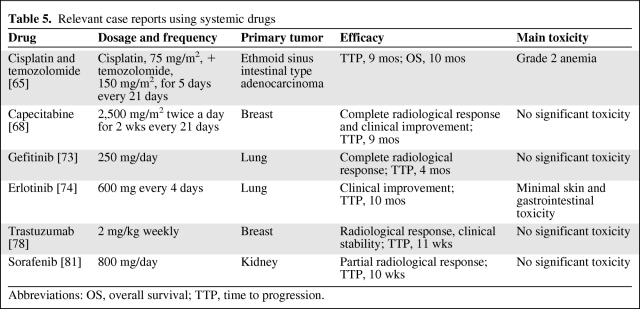
As with i.t. treatment, the few clinical studies and case reports also show discordant data regarding systemic therapies against solid tumor NM (Tables 4 and 5).
Table 4.
Relevant systemic chemotherapies
Abbreviations: OS, overall survival; TTP, time to progression.
Table 5.
Relevant case reports using systemic drugs
Abbreviations: OS, overall survival; TTP, time to progression.
MTX
High doses of MTX have favorable CSF penetration, obtaining cytotoxic concentrations, but the use of this agent can be limited by systemic toxicity. Moreover, MTX is not typically part of the standard regimens used to treat many of the underlying primary tumors, making its incorporation into systemic treatment difficult. On the other hand, in patients with MTX-sensitive cancers, such as breast cancer, its use may be effective.
Glantz et al. [56] treated 16 patients with solid tumor NM with high-dose i.v. MTX (8 g/m2 over 4 hours) and leucovorin rescue. Toxicity, response, and survival were retrospectively compared with a reference group of 15 patients treated with standard i.t. MTX (12 mg, two doses per week for 4 weeks). Cytologic and clinical responses were seen in 81% of patients treated with i.v. MTX. The rates of cytologic and clinical response seen in the control group were 60% and 47%, respectively. The median survival time in the i.v. MTX group was 13.8 months, versus 2.3 months in the i.t. MTX group (p = .003). Toxicities observed during i.v. MTX were minimal, and no important hematologic toxicities were seen. Among the patients in the control group, one developed reservoir-associated meningitis and another patient developed grade 2 stomatitis and grade 4 neutropenia with sepsis.
In contrast, in another study [57] of patients with NM from solid tumors, no patient had an objective response in the CSF following high-dose i.v. MTX despite cytotoxic CSF MTX levels—the mean plasma clearance of MTX was 84 ± 41 mL/min per m2 and the mean half-life of CSF MTX was 8.7 ± 3.4 hours. In that study, 13 patients with NM from breast cancer, lung cancer, and osteosarcoma were treated with MTX at loading doses of 200–1,500 mg/m2, followed by a 23-hour infusion of 800–6,000 mg/m2. All toxicities were grade ≤2, except grade 3 hematologic toxicity.
Temozolomide
Temozolomide has shown high penetration across the blood–brain barrier and a peak CSF concentration at 2.5 hours reaching 20% of the plasma concentration, demonstrating activity against primary brain tumors and brain metastases from breast cancer, melanoma, and NSCLC [58–60]. In a recent phase II study, 19 patients with NM from breast cancer, NSCLC, and melanoma were treated with temozolomide (100 mg/m2 daily) every other week, obtaining a response rate of 16% and a median TTP of 28 days, with a good safety profile [61]. In a pilot phase II trial, Davis et al. [62] treated 10 patients with NM from solid tumors with temozolomide at 75 mg/m2 daily for six continuous weeks, followed by a 4-week break. The treatment was extremely well tolerated and 20% of the patients obtained stable disease but progressed once treatment was discontinued. In another phase II study [63], eight patients with NM from breast cancer received irinotecan at 125 mg/m2 i.v. every 14 days with temozolomide at 100 mg/m2 orally on days 1–7 and days 15–21, obtaining a median TTP of 4.5 weeks (range, 4–10 weeks).
There are two case reports [64] of a beneficial contribution of i.t. liposomal cytarabine combined with dose-dense temozolomide in patients with NM from breast cancer. Intrathecal liposomal Ara-C every 2–4 weeks was combined with temozolomide, 100 mg/m2 on days 1–5 every week, improving neurological symptoms and with survival times of 10 months and 17 months after diagnosis of NM. Recently, we reported a case of a patient with NM from ethmoid sinus intestinal-type adenocarcinoma treated with cisplatin and temozolomide, in which prolonged reduction and stabilization of the lesion was obtained and the clinical condition of the patient improved [65]. Temozolomide was administered for five consecutive days at a single dose of 150 mg/m2 and cisplatin was given i.v. as a 60-minute infusion at a dose of 75 mg/m2 every 21 days. After two cycles, he had a partial reduction in the lesion on Gd-MRI, the TTP was 9 months, and OS time was 10 months. The patient showed no significant toxicity. Also, the same treatment was used in a patient with NM from melanoma, obtaining a survival duration >1 year [66].
Temozolomide was also effective against NM from oligodendroglioma with a 1p/19q deletion. That patient received temozolomide at 150 mg/m2 for 5 days every 4 weeks, showing marked clinical recovery and durable radiological remission [67].
Capecitabine
Capecitabine is an oral fluoropyrimidine enzymatically converted to 5-fluorouracil at the tumor site. It is used in the treatment of breast and colon cancer. Although a few studies demonstrated that capecitabine and its metabolites cross the blood–brain barrier in very limited quantities, various case reports showed good efficacy with capecitabine in treating NM from solid tumors. In particular, Giglio et al. [68] described four patients with NM from breast cancer treated with 2,000–2,500 mg/m2 twice a day for 2 weeks every 21 days, obtaining a prolonged response. Also, Tham et al. [69] reported a case of a long-term clinical response of 3.7 years in a patient with NM from breast cancer treated with capecitabine after whole-brain radiation. Another case report described a good response to capecitabine in a patient with NM from lung cancer [70].
Topotecan and Ifosfamide
Topotecan is a topoisomerase-I inhibitor and ifosfamide is an alkylating agent. They are both known to achieve high drug concentrations in the CSF and there is synergism between the two drugs. Moreover, both drugs are active against a variety of tumors. Kiewe et al. [24] analyzed this combination in patients with central nervous system involvement of solid tumors. In particular, they studied seven patients with NM from breast cancer and small-cell lung cancer treated with topotecan at a dose of 1.2 mg/m2 per day over 30 minutes i.v. on days 1–3 and ifosfamide at a dose of 1.5 g/m2 per day over 2 hours i.v. on days 1 and 2 every 3 weeks. NM response on Gd-MRI was found in two patients; three cases were not evaluated for response. The median TTP and OS time were 51+ and 218+ days, respectively. Elevated toxicity was observed: grade 3–4 leukopenia and neutropenia in at least one cycle was seen in 82% of patients, grade 3–4 thrombopenia and anemia were seen in 45% of patients.
Targeted Therapy
In the last few years, many new biological drugs have been developed against different types of solid neoplasms. To date, regarding the treatment of NM, no prospective or retrospective studies exist, but a few case reports have been described (Table 5).
Gefitinib is an oral tyrosine kinase inhibitor of EGFR with anticancer activity in patients with NSCLC with EGFR mutations. Several case reports have shown responses in patients with NM from NSCLC [71, 72]. Noteworthy, Sakai and colleagues [73] reported a good response in a patient with NM from NSCLC with an EGFR mutation in exon 19, administrating gefitinib at 250 mg/day. Cancer relapse was not observed 4 months after initiation of the drug.
Erlotinib is another tyrosine kinase inhibitor of EGFR used to treat NSCLC patients. It was reported [74] that erlotinib, at a dose of 600 mg orally every 4 days, improved the clinical condition of a patient with NM previously treated with erlotinib at 150 mg daily and subsequently with carboplatin, paclitaxel, and bevacizumab. The patient remained on treatment for a total duration of 10 months, with minimal skin and gastrointestinal toxicities. Likely, increasing the dose of orally administered erlotinib increased the CSF penetration and concentration.
Regarding NM from breast cancer, the efficacy of i.v. trastuzumab is in doubt. Trastuzumab is a large molecular and is easily blocked by the intact blood brain–barrier or partly impaired in meningeal carcinomatosis [75]. A few case reports demonstrated poor efficacy for trastuzumab against NM from HER-2/neu+ breast cancer [76], likely because of its low CSF concentration. In fact, the CSF level of trastuzumab was 300-fold lower than the serum level in another patient treated with i.v. trastuzumab [77]. On the other hand, Baculi et al. [78] reported good efficacy in a patient with NM from breast cancer treated with i.v. trastuzumab after whole-brain RT. Its penetration likely could be facilitated by RT. Stemmler et al. [79] measured trastuzumab levels in the serum and CSF of patients with brain metastases and concomitant NM before and after RT, showing that trastuzumab levels were higher after RT (226 ng/mL and 356 ng/mL before and after RT, respectively). In another patient, the combination of i.v. trastuzumab and oral capecitabine was used, leading to an improvement in the clinical condition and a remarkable reduction in the NM, although the response could be a result of capecitabine alone [80].
Sorafenib and sunitinib are two oral inhibitors of tyrosine kinase receptors showing efficacy against a few types of cancers, especially renal cancer. In a patient with NM from renal cancer [81], sorafenib treatment demonstrated modest activity. In fact, 10 days after the initiation of sorafenib therapy Gd-MRI showed partial remission that remained stable during the next 10 weeks of treatment. This case shows that sorafenib can achieve a rapid and prolonged tumor response. In contrast, sunitinib was tested in a patient with NM from renal cancer after RT without obtaining any signs of effectiveness against NM [82].
Conclusions
NM continues to be a devastating end-stage complication of solid tumors, and with improvements in treatment of systemic disease the incidence of NM is likely to increase.
Recently, new diagnostic approaches have been developed, allowing for earlier and more specific diagnoses of NM, although the goal is still far. Therefore, the combination of MRI and cytology to date still remains the approach with the greatest diagnostic sensitivity. In the last few years, the role of FDG-PET-CT is emerging and could be used in specific cases.
Specific or nonspecific markers in the CSF or in plasma could be developed and used either for better diagnosis or for monitoring the course of disease. For example, α-fetoprotein from testicular cancer and VEGF from various neoplasms when elevated in CSF can be used for diagnosis and to evaluate the effectiveness of treatment.
Regarding therapy for NM, no new randomized studies have been performed in recent years. Oral temozolomide and capecitabine have shown interesting results against NM from breast and lung cancer. The concept of combining i.t. and systemic drugs is growing. For instance, i.t. liposomal cytarabine is being employed in combination with capecitabine [83] and temozolomide [84]. In a few cases, new molecular drugs have been shown to be effective and safe against NM from solid tumors: the effectiveness of oral gefitinib against NM from NSCLC with mutated EGFR and i.t. trastuzumab for breast cancer with overexpressed HER-2 are examples. Thus, it is likely that, in future years, we will increasingly need to study the molecular characteristics of the tumor to deliver more individualized drug treatment.
In conclusion, because only 30% of patients with NM from solid tumors die solely as a result of NM, systemic treatment, especially new molecular drugs, against both NM and the primary tumor could be more effective and better tolerated than only i.t. treatment in patients with responsive systemic disease, especially, in cases with nodular NM. However, further randomized studies are needed to assess the best treatment.
Author Contributions
Conception/Design: Giuseppe Lombardi, Vittorina Zagonel, Patrizia Farina, Alessandro Della Puppa
Provision of study material or patients: Giuseppe Lombardi, Alessandro Cappetta, Antonella Brunello
Collection and/or assembly of data: Giuseppe Lombardi, Fable Zustovich, Alessandro Cappetta
Data analysis and interpretation: Vittorina Zagonel, Patrizia Farina, Diego Cecchin, Fable Zustovich, Renzo Manara
Manuscript writing: Vittorina Zagonel, Renzo Manara, Antonella Brunello
Final approval of manuscript: Giuseppe Lombardi
References
- 1.Clarke JL, Perez HR, Jacks LM, et al. Leptomeningeal metastases in the MRI era. Neurology. 2010;74:1449–1454. doi: 10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 2006;5:443–452. doi: 10.1016/S1474-4422(06)70443-4. [DOI] [PubMed] [Google Scholar]
- 3.van der Ree TC, Dippel DW, Avezaat CJ, et al. Leptomeningeal metastasis after surgical resection of brain metastases. J Neurol Neurosurg Psychiatry. 1999;66:225–227. doi: 10.1136/jnnp.66.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groves MD. New strategies in the management of leptomeningeal metastases. Arch Neurol. 2010;67:305–312. doi: 10.1001/archneurol.2010.18. [DOI] [PubMed] [Google Scholar]
- 5.Mammoser AG, Groves MD. Biology and therapy of neoplastic meningitis. Curr Oncol Rep. 2010;12:41–49. doi: 10.1007/s11912-009-0079-2. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain MC, Sandy AD, Press GA. Leptomeningeal metastasis: A comparison of gadolinium-enhanced MR and contrast-enhanced CT of the brain. Neurology. 1990;40:435–438. doi: 10.1212/wnl.40.3_part_1.435. [DOI] [PubMed] [Google Scholar]
- 7.Freilich RJ, Krol G, DeAngelis LM. Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol. 1995;38:51–57. doi: 10.1002/ana.410380111. [DOI] [PubMed] [Google Scholar]
- 8.Straathof CS, de Bruin HG, Dippel DW, et al. The diagnostic accuracy of magnetic resonance imaging and cerebrospinal fluid cytology in leptomeningeal metastasis. J Neurol. 1999;246:810–814. doi: 10.1007/s004150050459. [DOI] [PubMed] [Google Scholar]
- 9.Komori T, Delbeke D. Leptomeningeal carcinomatosis and intramedullary spinal cord metastases from lung cancer: Detection with FDG positron emission tomography. Clin Nucl Med. 2001;26:905–907. doi: 10.1097/00003072-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Chawla M, Reddy R, Kumar R, et al. PET-CT in detection of meningeal metastasis in neuroblastoma. Pediatr Surg Int. 2009;25:211–215. doi: 10.1007/s00383-008-2315-5. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain MC, Kormanik PA. Prognostic significance of 111indium-DTPA CSF flow studies in leptomeningeal metastases. Neurology. 1996;46:1674–1677. doi: 10.1212/wnl.46.6.1674. [DOI] [PubMed] [Google Scholar]
- 12.Mason WP, Yeh SD, DeAngelis LM. 111Indium-diethylenetriamine pentaacetic acid cerebrospinal fluid flow studies predict distribution of intrathecally administered chemotherapy and outcome in patients with leptomeningeal metastases. Neurology. 1998;50:438–444. doi: 10.1212/wnl.50.2.438. [DOI] [PubMed] [Google Scholar]
- 13.Glantz MJ, Hall WA, Cole BF, et al. Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer. 1995;75:2919–2931. doi: 10.1002/1097-0142(19950615)75:12<2919::aid-cncr2820751220>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain MC. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627–635. doi: 10.1097/CCO.0b013e32833de986. [DOI] [PubMed] [Google Scholar]
- 15.Glass JP, Melamed M, Chernik NL, et al. Malignant cells in cerebrospinal fluid (CSF): The meaning of a positive CSF cytology. Neurology. 1979;29:1369–1375. doi: 10.1212/wnl.29.10.1369. [DOI] [PubMed] [Google Scholar]
- 16.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: Experience with 90 patients. Cancer. 1982;49:759–772. doi: 10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Chamberlain MC, Kormanik PA, Glantz MJ. A comparison between ventricular and lumbar cerebrospinal fluid cytology in adult patients with leptomeningeal metastases. Neuro Oncol. 2001;3:42–45. doi: 10.1093/neuonc/3.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleinschmidt-DeMasters BK, Evans LC, Bitter MA, et al. Part II. Telomerase expression in cerebrospinal fluid specimens as an adjunct to cytologic diagnosis. J Neurol Sci. 1998;161:124–134. doi: 10.1016/s0022-510x(98)00254-8. [DOI] [PubMed] [Google Scholar]
- 19.van Oostenbrugge RJ, Hopman AH, Arends JW, et al. The value of interphase cytogenetics in cytology for the diagnosis of leptomeningeal metastases. Neurology. 1998;51:906–908. doi: 10.1212/wnl.51.3.906. [DOI] [PubMed] [Google Scholar]
- 20.Herrlinger U, Wiendl H, Renninger M, et al. Vascular endothelial growth factor (VEGF) in leptomeningeal metastasis: Diagnostic and prognostic value. Br J Cancer. 2004;91:219–224. doi: 10.1038/sj.bjc.6601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groves MD, Hess KR, Puduvalli VK, et al. Biomarkers of disease: Cerebrospinal fluid vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 levels in patients with neoplastic meningitis (NM) due to breast cancer, lung cancer and melanoma. J Neurooncol. 2009;94:229–234. doi: 10.1007/s11060-009-9819-2. [DOI] [PubMed] [Google Scholar]
- 22.Hutterer M, Medinger M, Untergasser G, et al. Dickkopf-3 (DKK-3) protein in cerebrospinal fluid (CSF): A biomarker for neoplastic meningitis? J Clin Oncol. 2010;28(suppl) Abstract e12517. [Google Scholar]
- 23.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Kiewe P, Thiel E, Reinwald M, et al. Topotecan and ifosfamide systemic chemotherapy for CNS involvement of solid tumors. J Neurooncol. 2011;103:629–634. doi: 10.1007/s11060-010-0434-z. [DOI] [PubMed] [Google Scholar]
- 25.Boogerd W, van den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: A randomised study. Eur J Cancer. 2004;40:2726–2733. doi: 10.1016/j.ejca.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert MR. Neoplastic meningitis: A unique disease process or a ‘test tube’ for evaluating cancer treatments? Curr Oncol Rep. 2003;5:11–14. doi: 10.1007/s11912-003-0081-z. [DOI] [PubMed] [Google Scholar]
- 27.Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25:103–119. doi: 10.1053/ctrv.1999.0119. [DOI] [PubMed] [Google Scholar]
- 28.Waki F, Ando M, Takashima A, et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neurooncol. 2009;93:205–212. doi: 10.1007/s11060-008-9758-3. [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain MC, Johnston SK, Glantz MJ. Neoplastic meningitis-related prognostic significance of the Karnofsky performance status. Arch Neurol. 2009;66:74–78. doi: 10.1001/archneurol.2008.506. [DOI] [PubMed] [Google Scholar]
- 30.Gauthier H, Guilhaume MN, Bidard FC, et al. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010;21:2183–2187. doi: 10.1093/annonc/mdq232. [DOI] [PubMed] [Google Scholar]
- 31.Oechsle K, Lange-Brock V, Kruell A, et al. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: A retrospective analysis. J Cancer Res Clin Oncol. 2010;136:1729–1735. doi: 10.1007/s00432-010-0831-x. [DOI] [PubMed] [Google Scholar]
- 32.Rudnicka H, Niwinska A, Murawska M. Breast cancer leptomeningeal metastasis—the role of multimodality treatment. J Neurooncol. 2007;84:57–62. doi: 10.1007/s11060-007-9340-4. [DOI] [PubMed] [Google Scholar]
- 33.Bokstein F, Lossos A, Siegal T. Leptomeningeal metastases from solid tumors: A comparison of two prospective series treated with and without intra-cerebrospinal fluid chemotherapy. Cancer. 1998;82:1756–1763. [PubMed] [Google Scholar]
- 34.Fleischhack G, Jaehde U, Bode U. Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. Clin Pharmacokinet. 2005;44:1–31. doi: 10.2165/00003088-200544010-00001. [DOI] [PubMed] [Google Scholar]
- 35.Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5:3394–3402. [PubMed] [Google Scholar]
- 36.Shapiro WR, Schmid M, Glantz M, et al. A randomized phase III/IV study to determine benefit and safety of cytarabine liposome injection for treatment of neoplastic meningitis. J Clin Oncol. 2006;24(18 suppl):1528. [Google Scholar]
- 37.Cole BF, Glantz MJ, Jaeckle KA, et al. Quality-of-life-adjusted survival comparison of sustained-release cytosine arabinoside versus intrathecal methotrexate for treatment of solid tumor neoplastic meningitis. Cancer. 2003;97:3053–3060. doi: 10.1002/cncr.11449. [DOI] [PubMed] [Google Scholar]
- 38.Le Rhun E, Zairi F, Baranzelli M, et al. Survival of a cohort of 25 breast cancer patients with neoplastic meningitis treated with intrathecal liposomal cytarabine. J Clin Oncol. 2009;27(15 suppl):1109. [Google Scholar]
- 39.Gil-Bazo I, Rodriguez J, Espinos J, et al. The safety and efficacy of intrathecal liposomal cytarabine in patients with carcinomatous meningitis from solid tumours. Eur J Cancer Suppl. 2009;7 Abstract 501. [Google Scholar]
- 40.Groves MD, Glantz MJ, Chamberlain MC, et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol. 2008;10:208–215. doi: 10.1215/15228517-2007-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker JG, Schultz DL, Grisdale KA, et al. Case studies of patients receiving intraventricular topotecan for treatment of neoplastic meningitis. J Clin Oncol. 2010;28(suppl) doi: 10.6004/jadpro.2012.3.4.4. Abstract e12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chamberlain MC, Tsao-Wei DD, Groshen S. Phase II trial of intracerebrospinal fluid etoposide in the treatment of neoplastic meningitis. Cancer. 2006;106:2021–2027. doi: 10.1002/cncr.21828. [DOI] [PubMed] [Google Scholar]
- 43.Gururangan S, Petros WP, Poussaint TY, et al. Phase I trial of intrathecal spartaject busulfan in children with neoplastic meningitis: A Pediatric Brain Tumor Consortium study (PBTC-004) Clin Cancer Res. 2006;12:1540–1546. doi: 10.1158/1078-0432.CCR-05-2094. [DOI] [PubMed] [Google Scholar]
- 44.Bernardi RJ, Bomgaars L, Fox E, et al. Phase I clinical trial of intrathecal gemcitabine in patients with neoplastic meningitis. Cancer Chemother Pharmacol. 2008;62:355–361. doi: 10.1007/s00280-007-0601-x. [DOI] [PubMed] [Google Scholar]
- 45.Nakagawa H, Miyahara E, Suzuki T, et al. Continuous intrathecal administration of 5-fluoro-2′-deoxyuridine for the treatment of neoplastic meningitis. Neurosurgery. 2005;57:266–280. doi: 10.1227/01.neu.0000166543.45294.f6. discussion 266–280. [DOI] [PubMed] [Google Scholar]
- 46.Ferrario C, Davidson A, Bouganim N, et al. Intrathecal trastuzumab and thiotepa for leptomeningeal spread of breast cancer. Ann Oncol. 2009;20:792–795. doi: 10.1093/annonc/mdp019. [DOI] [PubMed] [Google Scholar]
- 47.Platini C, Long J, Walter S. Meningeal carcinomatosis from breast cancer treated with intrathecal trastuzumab. Lancet Oncol. 2006;7:778–780. doi: 10.1016/S1470-2045(06)70864-6. [DOI] [PubMed] [Google Scholar]
- 48.Allison DL, Glantz M, Werner TL, et al. Intra-CSF trastuzumab in patients with neoplastic meningitis from breast cancer or primary brain tumors. J Clin Oncol. 2009;27(suppl) Abstract 2066. [Google Scholar]
- 49.Chamberlain MC. A phase II trial of intra-cerebrospinal fluid alpha interferon in the treatment of neoplastic meningitis. Cancer. 2002;94:2675–2680. doi: 10.1002/cncr.10547. [DOI] [PubMed] [Google Scholar]
- 50.Papadopoulos NE, Gerber DL, Eton O, et al. The role of intrathecal (IT) use of interleukin-2 (IL-2) in the treatment of leptomeningeal disease (LMD) in patients (pts) with melanoma. Proc Am Soc Clin Oncol. 2002;21 Abstract 1408. [Google Scholar]
- 51.Heiss JD, Taha S, Oldfield EH, et al. Intrathecal gene therapy for treatment of leptomeningeal carcinomatosis. J Neurooncol. 2010 Nov 26; doi: 10.1007/s11060-010-0458-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sampson JH, Archer GE, Villavicencio AT, et al. Treatment of neoplastic meningitis with intrathecal temozolomide. Clin Cancer Res. 1999;5:1183–1188. [PubMed] [Google Scholar]
- 53.Sun JM, Nam MH, Chung JY, et al. Safety and pharmacokinetics of intrathecal administration of pemetrexed in rats. Cancer Chemother Pharmacol. 2010 Nov 25; doi: 10.1007/s00280-010-1522-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Ushio Y, Shimizu K, Aragaki Y, et al. Alteration of blood-CSF barrier by tumor invasion into the meninges. J Neurosurg. 1981;55:445–449. doi: 10.3171/jns.1981.55.3.0445. [DOI] [PubMed] [Google Scholar]
- 55.Nakagawa H, Fujita T, Kubo S, et al. Difference in CDDP penetration into CSF between selective intraarterial chemotherapy in patients with malignant glioma and intravenous or intracarotid administration in patients with metastatic brain tumor. Cancer Chemother Pharmacol. 1996;37:317–326. doi: 10.1007/s002800050391. [DOI] [PubMed] [Google Scholar]
- 56.Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: Is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16:1561–1567. doi: 10.1200/JCO.1998.16.4.1561. [DOI] [PubMed] [Google Scholar]
- 57.Tetef ML, Margolin KA, Doroshow JH, et al. Pharmacokinetics and toxicity of high-dose intravenous methotrexate in the treatment of leptomeningeal carcinomatosis. Cancer Chemother Pharmacol. 2000;46:19–26. doi: 10.1007/s002800000118. [DOI] [PubMed] [Google Scholar]
- 58.Chua D, Krzakowski M, Chouaid C, et al. Whole-brain radiation therapy plus concomitant temozolomide for the treatment of brain metastases from non-small-cell lung cancer: A randomized, open-label phase II study. Clin Lung Cancer. 2010;11:176–181. doi: 10.3816/CLC.2010.n.022. [DOI] [PubMed] [Google Scholar]
- 59.Siena S, Crinoò L, Danova M, et al. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: A multicenter phase II study. Ann Oncol. 2010;21:655–661. doi: 10.1093/annonc/mdp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 61.Perez Segura P, Gil M, Balana C, et al. Phase II trial of temozolomide for leptomeningeal metastases: Safety and activity analysis. J Clin Oncol. 2010;28(suppl) Abstract e12528. [Google Scholar]
- 62.Davis TH, Fadul CE, Glantz MJ, et al. Pilot phase II trial of temozolomide for leptomeningeal metastases: Preliminary report. Proc Am Soc Clin Oncol. 2003;22 Abstract 460. [Google Scholar]
- 63.Melisko ME, Anderson M, Scott J, et al. Phase II study of irinotecan (IN) and temozolomide (TMZ) in breast cancer patients (pts) with brain metastases (BM) or leptomeningeal disease (LMD) that has progressed after stereotactic radiosurgery (SRS) or whole brain radiation (WBRT) J Clin Oncol. 2008;26(suppl) Abstract 237. [Google Scholar]
- 64.Hoffmann AL, Buhk JH, Strik H. Neoplastic meningitis from breast cancer: Feasibility and activity of long-term intrathecal liposomal Ara-C combined with dose-dense temozolomide. Anticancer Res. 2009;29:5191–5195. [PubMed] [Google Scholar]
- 65.Lombardi G, Zustovich F, Della Puppa A, et al. Cisplatin and temozolomide combination in the treatment of leptomeningeal carcinomatosis from ethmoid sinus intestinal-type adenocarcinoma. J Neurooncol. 2010 Dec 8; doi: 10.1007/s11060-010-0484-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Salmaggi A, Silvani A, Eoli M, et al. Temozolomide and cisplatin in the treatment of leptomeningeal metastatic involvement from melanoma: A case report. Neurol Sci. 2002;23:257–258. doi: 10.1007/s100720200053. [DOI] [PubMed] [Google Scholar]
- 67.Michotte A, Chaskis C, Sadones J, et al. Primary leptomeningeal anaplastic oligodendroglioma with a 1p36–19q13 deletion: Report of a unique case successfully treated with temozolomide. J Neurol Sci. 2009;287:267–270. doi: 10.1016/j.jns.2009.08.047. [DOI] [PubMed] [Google Scholar]
- 68.Giglio P, Tremont-Lukats IW, Groves MD. Response of neoplastic meningitis from solid tumors to oral capecitabine. J Neurooncol. 2003;65:167–172. doi: 10.1023/b:neon.0000003752.89814.ca. [DOI] [PubMed] [Google Scholar]
- 69.Tham YL, Hinckley L, Teh BS, et al. Long-term clinical response in leptomeningeal metastases from breast cancer treated with capecitabine monotherapy: A case report. Clin Breast Cancer. 2006;7:164–166. doi: 10.3816/CBC.2006.n.028. [DOI] [PubMed] [Google Scholar]
- 70.Paydas S, Bicakci K, Yavuz S. Dramatic response with capecitabine after cranial radiation to the brain parenchymal and leptomeningeal metastases from lung cancer. Eur J Intern Med. 2009;20:96–99. doi: 10.1016/j.ejim.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 71.Hashimoto N, Imaizumi K, Honda T, et al. Successful re-treatment with gefitinib for carcinomatous meningitis as disease recurrence of non-small-cell lung cancer. Lung Cancer. 2006;53:387–390. doi: 10.1016/j.lungcan.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 72.Kumamoto T, Suetsugu T, Iwakawa J, et al. [A case of meningeal carcinomatosis of lung adenocarcinoma well controlled by re-treatment with gefitinib] Gan to Kagaku Ryoho. 2009;36:2615–2617. In Japanese. [PubMed] [Google Scholar]
- 73.Sakai M, Ishikawa S, Ito H, et al. Carcinomatous meningitis from non-small-cell lung cancer responding to gefitinib. Int J Clin Oncol. 2006;11:243–245. doi: 10.1007/s10147-005-0558-x. [DOI] [PubMed] [Google Scholar]
- 74.Dhruva N, Socinski MA. Carcinomatous meningitis in non-small-cell lung cancer: Response to high-dose erlotinib. J Clin Oncol. 2009;27:e31–e32. doi: 10.1200/JCO.2008.21.0963. [DOI] [PubMed] [Google Scholar]
- 75.Platini C. [Trastuzumab and blood-brain barrier] Bull Cancer. 2007;94:857–859. In French. [PubMed] [Google Scholar]
- 76.Robins HI, Liu G, Hayes L, et al. Trastuzumab for breast cancer-related carcinomatous meningitis. Clin Breast Cancer. 2002;2:316. doi: 10.1016/s1526-8209(11)70432-3. [DOI] [PubMed] [Google Scholar]
- 77.Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000;18:2349–2351. doi: 10.1200/JCO.2000.18.11.2349. [DOI] [PubMed] [Google Scholar]
- 78.Baculi RH, Suki S, Nisbett J, et al. Meningeal carcinomatosis from breast carcinoma responsive to trastuzumab. J Clin Oncol. 2001;19:3297–3298. doi: 10.1200/JCO.2001.19.13.3297. [DOI] [PubMed] [Google Scholar]
- 79.Stemmler HJ, Schmitt M, Willems A, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18:23–28. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 80.Shigekawa T, Takeuchi H, Misumi M, et al. Successful treatment of leptomeningeal metastases from breast cancer using the combination of trastuzumab and capecitabine: A case report. Breast Cancer. 2009;16:88–92. doi: 10.1007/s12282-008-0056-x. [DOI] [PubMed] [Google Scholar]
- 81.Ranze O, Hofmann E, Distelrath A, et al. Renal cell cancer presented with leptomeningeal carcinomatosis effectively treated with sorafenib. Onkologie. 2007;30:450–451. doi: 10.1159/000105131. [DOI] [PubMed] [Google Scholar]
- 82.Dalhaug A, Haukland E, Nieder C. Leptomeningeal carcinomatosis from renal cell cancer: Treatment attempt with radiation and sunitinib (case report) World J Surg Oncol. 2010;8:36. doi: 10.1186/1477-7819-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soffietti R, Akerley W, Jensen RL, et al. The role of intra-cerebrospinal fluid treatment and prophylaxis in patients with solid tumors. Semin Oncol. 2009;36(suppl 2):S55–S68. doi: 10.1053/j.seminoncol.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 84.Passarin MG, Moretto G, Musso AM, et al. Intrathecal liposomal cytarabine in combination with temozolomide in low-grade oligoastrocytoma with leptomeningeal dissemination. J Neurooncol. 2010;97:439–444. doi: 10.1007/s11060-009-0040-0. [DOI] [PubMed] [Google Scholar]