The most common symptoms in patients with breast cancer metastases are identified and an overview of their management is provided.
Keywords: Metastatic breast cancer, Symptom management, Palliative care, Treatment
Abstract
Approximately 40,000 women die as a result of breast cancer each year and many more live with advanced disease. When breast cancer recurs, the goals of treatment often shift from one of cure to controlling the disease for as long as possible while palliating symptoms interfering with the patient's functional status and quality of life. This requires ongoing discussions with the patient and family about the goals of care. Many symptoms depend on the site of metastasis, with bone being the most frequent, and commonly occur with fatigue, depression, insomnia, and pain. The purpose of this paper is to identify and provide an overview of the management of the most common symptoms in patients with breast cancer metastases.
Introduction
Breast cancer is the most common cancer in women, accounting globally for 23% of all cancers in women and 14% of cancer deaths [1]. Of the 1,529,560 estimated new cancer cases and 549,490 cancer deaths in the U.S. in 2010, 209,600 were estimated to be new cases of breast cancer and 40,230 were estimated to be deaths resulting from metastases [2]. For ∼5% of the patients with metastases (stage IV), these are found at the time of their initial diagnosis, whereas the other 95% are seen in those with potentially curable disease at diagnosis who have since relapsed. (Breast cancer primarily occurs in women, but 2,140 men were estimated to be diagnosed and 450 men were estimated to have died as a result of breast cancer in 2011.) Although the mortality rates for breast cancer patients have improved over the last decade, the loss of 40,000 lives each year as a result of metastasis has remained constant.
Breast cancer is a heterogeneous disease with a number of factors that influence the prognosis and chance for cure. Data from the Surveillance, Epidemiology, and End Results project of the National Cancer Institute for patients diagnosed in 1999–2005 indicate that ∼25% of all patients diagnosed with metastatic (stage IV) breast cancer survive for 5 years, including 25% of white and 16% of African-American patients [3]. These data further suggest that, of the >2.5 million breast cancer survivors in the U.S., a substantial number have metastatic breast cancer (MBC). All patients with metastases will likely have problems with symptoms related to organ involvement, treatment, or both that affect their quality of life.
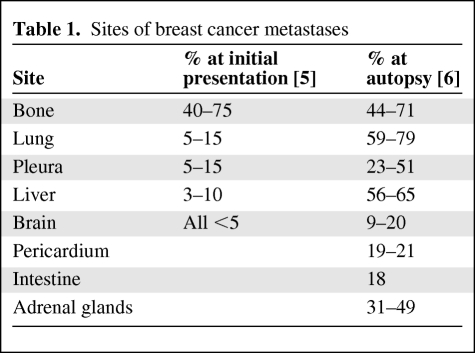
The fact that MBC can involve virtually any organ and the myriad of treatment options available frequently make management challenging. The goal of any treatment in this scenario is disease control, palliation of symptoms, and maintenance of the highest quality of life [4]. About one third of patients with MBC present with a local or regional recurrence involving lymph nodes and/or the chest wall. Over time, 75% of these patients develop metastases to other organs. The remainder initially present with distant metastasis, described in Table 1 [5, 6]. The optimal management of symptoms related to organ involvement and dysfunction as well as the psychosocial factors associated with these symptoms is the focus of this review.
Table 1.
Sites of breast cancer metastases
Common Problems
Seeking to live as normal a life as possible, including the ability to fulfill roles and maintain relationships, is a goal for many that is negatively affected by MBC and decreasing physical function [7]. Many patients with metastases experience major concerns, including fear of dying, declining quality of life, side effects of treatment, the ability to care for family, and care at the end of life [8]. Managing symptoms to maintain an optimum quality of life is the major goal of care in the metastatic setting because all therapy is palliative care.
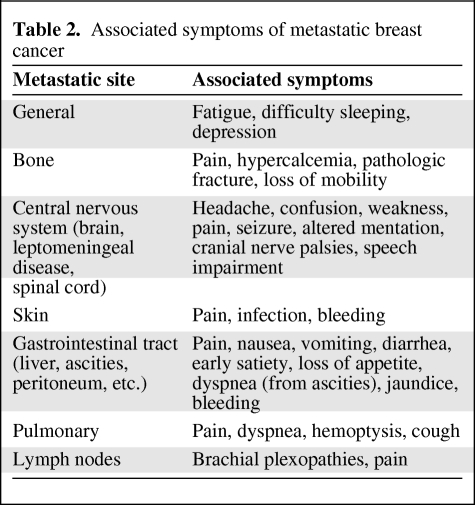
Many symptoms in the metastatic setting occur based on the site of metastasis or together with fatigue, depression, insomnia, and pain, among the most common (Table 2) [9, 10]. The most reported unmet supportive care needs in patients with MBC in one study were psychological support, counseling, and adequate information concerning: things they could do themselves to help feel better, which member of the staff was available for communication, the status of their cancer, an explanation of test results, the risks and benefits of treatment, and how to obtain access to counseling [11]. In another study, the average number of symptoms reported by 56 patients with MBC was 14. Whereas declining sexual function was the most frequently reported symptom, worry was the most severe symptom and pain was the most distressing symptom [10].
Table 2.
Associated symptoms of metastatic breast cancer
Pain
Pain is among the most common and most distressing symptoms in cancer, with 73% of patients reporting pain at hospital admission [12]. Pain may be categorized as “nociceptive” pain (caused by tissue damage) and “neuropathic” pain (caused by dysfunction of the nervous system) [13]. The first steps in approaching pain in the MBC patient are taking a careful history to assess location (have the patient point to location if possible to avoid misunderstanding) and trusting the patient's report concerning severity. Other assessments include describing the type of pain (sharp, dull, etc.), timing and duration, inciting factors, and interference with activities, and identifying what provides relief.
The prevalence of chronic pain in patients with advanced cancer is estimated at 70%–90% [14]. Bone metastases are a common cause of chronic pain, with pain resulting directly from expanding lesions, pathologic fracture, or damage to adjacent structures. Pain in cancer may also be a symptom of an oncologic emergency such as pathologic bone fracture, brain or epidural metastasis, leptomeningeal metastasis, infection, or an obstructed or perforated viscus. Spinal cord compression occurs in 5%–20% of cancer patients at autopsy and is a medical emergency requiring prompt evaluation and treatment [15]. Patients should be evaluated for impending cord compression if there are complaints of back pain because bone metastases are present in 95% of cases. Breast cancer occasionally (2%–6%) involves the leptomeninges, with the diagnosis being made by finding malignant cells on lumbar puncture [16, 17]. Leg weakness, multifocal abnormalities at more than one level of the neural system (spine, cranial nerves, cerebrum), headaches, and mentation changes are frequent symptoms associated with leptomeningeal disease [17].
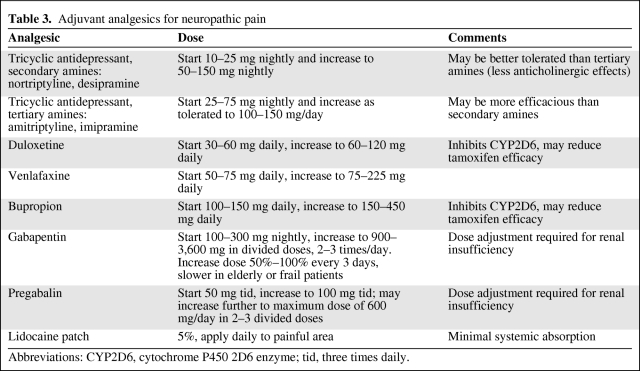
The treatment of pain in the cancer patient involves several interventions. For neuropathic pain related to metastases or treatment, adjuvant analgesics such as antidepressants (i.e., the tricyclics amitriptyline, imipramine, nortriptyline, desipramine, or duloxetine, venlafaxine, or bupropion) [18] and anticonvulsants (i.e., gabapentin or pregabalin) [19–21] are first-line therapies in conjunction with opioids, with the recommendation to start with a low dose and increase every 3–14 days as tolerated (Table 3) [22]. Topical anesthetics such as a lidocaine patch may also be helpful in treating this type of pain. Psychological support as well as patient and family education should be provided. For inflammatory pain or pain related to nerve compression, a trial of glucocorticoids should be considered. For bone pain not associated with spinal cord compression or fracture, nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen in combination with opioids are recommended by the National Comprehensive Cancer Network (NCCN) guidelines [22]. For diffuse bone pain resulting from metastases, bisphosphonates, glucocorticoids, or systemic administration of radioisotopes should be considered. For localized bone pain, external-beam radiation therapy or nerve block (e.g., for rib pain) may be helpful [23–27]. For treatment of refractory pain, interventional procedures such as regional infusions with the insertion of an infusion pump, percutaneous kyphoplasty or vertebroplasty, and radiofrequency ablation are potential treatment options [22].
Table 3.
Adjuvant analgesics for neuropathic pain
Abbreviations: CYP2D6, cytochrome P450 2D6 enzyme; tid, three times daily.
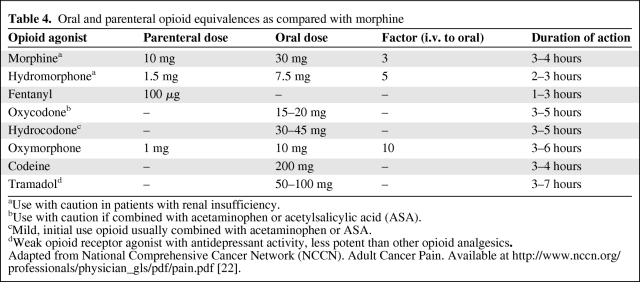
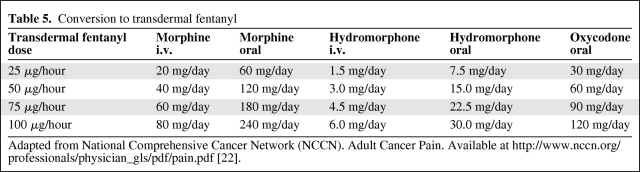
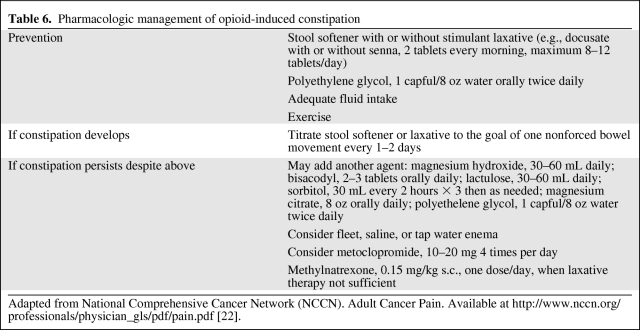
When using opioids for the treatment of cancer pain, the use of short-acting breakthrough opioids along with long-acting opioids is recommended as symptoms fluctuate or worsen with disease progression [15, 22]. Table 4 summarizes opioid equivalences and the relative potencies of drugs as compared with morphine, and Table 5 shows conversions to transdermal fentanyl. Of note, meperidine, pentazocine, nalbuphine, butorphanol, and dezocine are not recommended in the treatment of cancer pain because of a central nervous system toxic metabolite of meperidine (normeperidine) and the potential for withdrawal crises with the other medications [22]. Not listed in Table 4 is methadone, because the oral conversion ratio of methadone varies and practitioners are advised to consult with a pain specialist if not familiar with methadone prescribing. The duration of action for transdermal fentanyl is usually 72 hours, but some patients may require replacement after 48 hours. Pain should be well controlled on short-acting opioids prior to transdermal fentanyl use. Constipation is the most common adverse event associated with opioid use and occurs in 25%–50% of cancer patients. NCCN guidelines recommend instituting a bowel regimen to all patients on chronic opioids unless otherwise contraindicated [22, 28] (Table 6).
Table 4.
Oral and parenteral opioid equivalences as compared with morphine
aUse with caution in patients with renal insufficiency.
bUse with caution if combined with acetaminophen or acetylsalicylic acid (ASA).
cMild, initial use opioid usually combined with acetaminophen or ASA.
dWeak opioid receptor agonist with antidepressant activity, less potent than other opioid analgesics.
Adapted from National Comprehensive Cancer Network (NCCN). Adult Cancer Pain. Available at http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf [22].
Table 5.
Conversion to transdermal fentanyl
Adapted from National Comprehensive Cancer Network (NCCN). Adult Cancer Pain. Available at http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf [22].
Table 6.
Pharmacologic management of opioid-induced constipation
Adapted from National Comprehensive Cancer Network (NCCN). Adult Cancer Pain. Available at http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf [22].
Fatigue
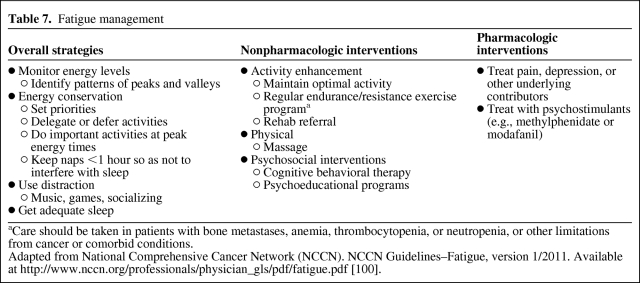
Fatigue is the most commonly reported symptom in cancer patients, with an estimated prevalence of 25%–99% during treatment and 20%–35% off treatment [9]. Potential contributing factors include the tumor burden, pain, difficulty sleeping, anemia, poor nutrition, inactivity, and other comorbid conditions. Some of these contributing factors are more amenable to interventions than others. Methylphenidate has been effective in treating fatigue in advanced cancer [29]. Nonpharmacologic management includes regular physical activity and psychosocial interventions [30, 31]. With advanced disease, strategies include energy conservation, exercise, cognitive behavioral therapy, and the use of psychostimulants (Table 7). Regular physical activity has been mostly studied in the adjuvant setting, but it may be helpful in this setting. However, a referral to physical rehabilitation medicine to make recommendations on metastatic disease sites, treatment, symptoms, and physical condition may be needed.
Table 7.
Fatigue management
aCare should be taken in patients with bone metastases, anemia, thrombocytopenia, or neutropenia, or other limitations from cancer or comorbid conditions.
Adapted from National Comprehensive Cancer Network (NCCN). NCCN Guidelines–Fatigue, version 1/2011. Available at http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf [100].
Depression and Anxiety
Sadness, disappointment, fear, and worry are common reactions when breast cancer recurs or progresses [8, 32], and social support is critical as patients try to cope with and adapt to this news. Psychological distress may be evident as depression and/or anxiety and is influenced by feelings of uncertainty, lack of control, and concerns about physical functioning [33]. Estimates of depression are in the range of 20%–50%, but the diagnosis of a major depressive disorder is much lower [9]. In one study assessing psychosocial morbidity that included 303 women with early-stage breast cancer and 200 women with MBC, there were no significant differences in the prevalence of psychiatric disorders [34]. For those with advanced cancer, 31% were found to have mood disorders, 6.5% had major depression, 24.5% had minor depression, and 6% had anxiety disorders [34]. Fatigue, a previous history of depression or another psychiatric illness, and feelings of helplessness, hopelessness, or resignation may increase the risk for depression. Common vegetative symptoms of depression (fatigue, loss of appetite, insomnia) may be difficult to differentiate from symptoms related to metastases. Antidepressants and anxiolytics can be effective in this situation, but monitoring for potential drug interactions is essential. However, if possible, women taking tamoxifen for breast cancer should avoid taking cytochrome P450 (CYP450) 2D6 strong inhibitors such as fluoxetine, paroxetine, and bupropion [35] because data show that the concurrent use of medications that inhibit CYP2D6 reduces the disease-free survival interval [36–39], although this is somewhat controversial [40]. Psychosocial support, whether one-to-one or group counseling, and cognitive behavioral therapy may also be useful.
Difficulty Sleeping
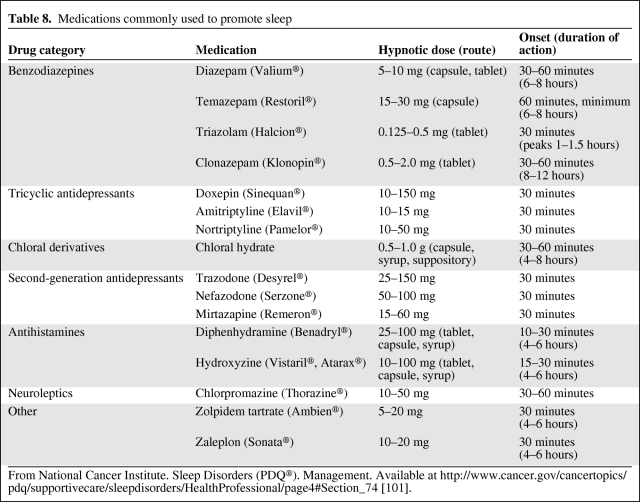
Many patients (up to 75%) experience problems falling asleep or maintaining sleep, whereas a smaller group meet the criteria for insomnia (19%) [41]. These difficulties may have existed prior to a cancer diagnosis but may be exacerbated after diagnosis, especially if hot flashes are experienced. Insomnia is associated with poorer satisfaction across all domains of quality of life and higher rates of depression [42]. Fatigue and depression are often associated with sleep problems, whereas pain and menopausal vasomotor symptoms may interfere with sleep quantity and quality. A number of pharmacologic and nonpharmacologic approaches have been studied and found to be helpful in addressing sleep issues; however, there are a number of methodological issues (e.g., small sample size, sleep not the primary outcome). Approaches that have been helpful include cognitive behavior therapy, benzodiazepines, benzodiazepine receptor agonists, mindfulness medication, and yoga [41, 43]. Although there has been a number of studies establishing the effectiveness of cognitive behavioral therapy in improving sleep, the effectiveness of complementary therapies (e.g., aromatherapy, expressive therapy, expressive writing, healing, autogenic training, massage, muscle relaxation, mindfulness-based stress reduction, and yoga), education or information, and exercise interventions is yet to be established [44]. Attention to sleep hygiene, including attending to the environment by decreasing noise, dimming or turning off lights, keeping the room temperature cool, and consolidating patient care tasks to reduce the number of interruptions, can increase the amount of uninterrupted sleep. Pharmacologic interventions may be useful short term if other measures are not working (Table 8). However, use for >1–2 weeks may impair natural sleep patterns, and these should be used with caution because tolerance and dependence may develop.
Table 8.
Medications commonly used to promote sleep
From National Cancer Institute. Sleep Disorders (PDQ®). Management. Available at http://www.cancer.gov/cancertopics/pdq/supportivecare/sleepdisorders/HealthProfessional/page4#Section_74 [101].
Lymphedema
Lymphedema may develop in the upper extremity and/or trunk of the primary tumor site and can be especially disabling if associated with infection or local recurrence [45]. Lymphedema may also be associated with other arm and shoulder problems (difficulty lifting, reaching, pain) [46]. Having had an axillary lymph node dissection or radiation to the axilla increases the risk, but any breast cancer patient may develop lymphedema. The incidence of lymphedema has been estimated to be in the range of 6%–85%, with a higher rate found in women who are obese [47]. Patient reports of “heaviness” or swelling should be followed by a careful assessment and referral to a Lymphology Association of North America or National Lymphedema Network certified lymphedema therapist. Lymphedema may be treated with decongestive lymphatic therapy that includes manual lymph drainage, compression garments, remedial exercises, and skin care [48]. Some women may benefit from low-level laser therapy [49, 50]. Prevention and treatment of infection are paramount. Women with lymphedema can also experience functional impairment as well as decreased emotional well-being [51]. If the lymphedema is refractory to treatment or has a late onset (years after diagnosis and treatment) without apparent cause, tumor recurrence or the development of lymphangiosarcoma should be considered.
Locoregional Recurrence
Local recurrence may be the only form of disease recurrence and can cause severe symptoms that impact patients' quality of life. When this occurs, local recurrence should be treated aggressively with surgery and/or radiation with or without systemic therapy [52]. For example, if the initial treatment was breast-conserving surgery, a mastectomy or surgical resection may be performed, if possible. Radiation to the chest wall of supraclavicular, axillary, and intramammory lymph nodes may also be possible. If these approaches have been unsuccessful, a topical solution of 6% miltefosine was found to effectively treat cutaneous metastases in preliminary studies [53, 54]. Although the goal of treatment is to control the disease, this is not always possible. Fungating lesions may develop that can be painful or malodorous, can have drainage, and can bleed. Such lesions can be devastating to patients and families and limit social contact. Wound care is targeted to managing infection; oral or topical metronidazole (1% spray or gel) has been helpful in reducing odor and drainage [54, 55]. There is a range of dressings that can help manage drainage, odor, and bleeding while minimizing pain, including hydrocolloids, alginates, foams, and hydrogels. Consulting a wound care nurse can be helpful in working with the patient and family in the best dressing approach for the type of skin.
Dyspnea
Dyspnea, the subjective perception of impaired breathing, includes chest tightness, air hunger, suffocation, the sensation of breathlessness, and increased work in breathing [56]. Not all breast cancer patients with dyspnea have lung metastasis because 25% of cancer patients with dyspnea do not have documented lung involvement or other cardiopulmonary pathology [57]. Pulmonary carcinomatous lymphangitis is common in breast cancer patients [58, 59], and pulmonary infiltrates causing dyspnea may be associated with treatments (treatment-induced pneumonitis) such as radiation [60] or chemotherapy (especially with taxanes) [61].
Opioids are effective for the management of dyspnea, but are underused by physicians because of concerns about respiratory depression [62]. The American College of Physicians (ACP) recommends treatment with short-acting opioids, because several randomized controlled trials have shown the beneficial effects of systemic opioids in the control of dyspnea [63, 64]. Although small series describe relief of dyspnea with the administration of nebulized opioids [65], randomized controlled trials and a Cochrane review did not show that nebulized opioids are effective for dyspnea [66]. Benzodiazepines may be helpful in some patients when dyspnea is related to a panic disorder or severe anxiety, and corticosteroids may be effective in dyspnea related to carcinomatous lymphangitis or treatment-related pneumonitis [61, 67, 68].
The value of oxygen is uncertain. One study showed no association between dyspnea and oxygen saturation and found morphine to be superior to oxygen in relieving subjective dyspnea as well as hypoxia [69]. However, other studies have shown mixed results. Based on the level of evidence, the ACP has recommended oxygen for short-term relief of hypoxemia in patients with dyspnea at the end of life [70]. Cognitive behavioral therapy and breathing retraining approaches have been helpful in reducing breathlessness [71]. Because there is a lack of compelling evidence that oxygen use improves quality of life, patients who find oxygen use problematic should not be encouraged to continue its use.
Malignant pleural effusions are also a common cause of dyspnea in breast cancer patients, occurring in ∼10% of breast cancer patients [72]. Pleurodesis with doxycycline or talc may be indicated for symptom relief, as well as serial thoracenteses or even the placement of a PleurX catheter for palliative management in patients who are not suitable for pleurodesis [73, 74].
Bone Metastasis
Bone is among the most common sites of breast cancer metastasis, and metastasis there can result in pain, hypercalcemia, pathologic fracture, loss of mobility, and spinal cord compression. In a Danish population-based cohort study, 4% of all breast cancer patients were diagnosed with bone metastasis either at the time of their initial breast cancer diagnosis or during follow-up (median follow-up, 3.5 years), and of those patients with a bone metastasis, >40% had a skeletal-related event (pathological fracture, spinal cord compression, or pain requiring palliative therapy) [75].
Patients with symptomatic hypercalcemia of malignancy or severe hypercalcemia (>14 mg/dL) usually require immediate treatment with hydration with isotonic saline to euvolemia and a bisphosphonate [76]. The use of a loop diuretic is not necessary except to treat edema or volume overload [76]. In a pooled analysis of two randomized controlled trials, zoledronic acid (4 mg) resulted in a response rate superior to that with pamidronate (90 mg), and this is the recommended agent for initial treatment; for those with refractory hypercalcemia, 8 mg is recommended [77]. In addition to the above, for patients who are symptomatic with severe hypercalcemia, calcitonin may also be considered, recognizing that tachyphylaxis to this agent occurs within 48 hours [78]. Denosumab, a human monoclonal antibody against the nuclear factor κB ligand receptor, was shown, in a randomized clinical trial in breast cancer patients with bone metastases, to be superior to zoledronic acid in delaying or preventing pathologic fracture, radiation or surgery to bone, and spinal cord compression. Renal failure was rare with denosumab (0.2%), but hypocalemia occurred in 5.5% of patients [79].
The American Society of Clinical Oncology (ASCO) in 2011 updated their recommendations on the role of bone-modifying agents in MBC patients to prevent skeletal-related events. For breast cancer patients with bone metastasis, denosumab (120 mg s.c. every 4 weeks), pamidronate (90 mg i.v. over 2 hours every 3–4 weeks), or zoledronic acid (4 mg i.v. over 15 minutes every 3–4 weeks) is recommended. Caution should be exercised in patients with renal insufficiency and the U.S. Food and Drug Administration (FDA)-approved label should be referenced for creatinine clearance <60 mL/minute. For patients with renal insufficiency, for whom a bisphosphonate is not felt to be able to be safely used, the use of denosumab may be considered with caution. Patients and providers should be aware of the uncommon, but serious, adverse event of osteonecrosis of the jaw with the use of bone-modifying agents and avoid invasive dental procedures while on these agents [80].
Pain caused by bone metastasis should be addressed as necessary with NSAIDs, opioid and nonopioid analgesics, corticosteroids, adjuvant agents, interventional procedures, local radiation therapy, surgery, and systemic radiopharmaceuticals [80]. Surgical or radiation oncologic interventions may be used to prevent pathologic fractures or restore mobility and alleviate pain if these occur.
Spinal cord compression is an oncologic emergency that requires prompt neurosurgical and radiation oncologic consultation and the use of corticosteroids, usually in the form of i.v. dexamethasone. The initial recommended dose of dexamethasone varies, but starting with a bolus of 10 mg followed by 16 mg daily in divided doses has a lower risk for serious adverse events than treating with higher doses [81]. Neurosurgical or orthopedic consultation at the time the diagnosis is suspected is mandatory.
Gastrointestinal Symptoms
Both nausea (the sensation of needing to vomit) and vomiting (forceful expulsion of gastric contents) decrease cancer patients' quality of life and occur in 30% (vomiting) to 60% (nausea) of cancer patients [62]. Causes of nausea and vomiting include chemotherapy, radiation therapy, and other medications; infection; anxiety; constipation; bowel obstruction; organ failure; electrolyte disturbances; uremia; impaired gastric emptying; gastric/esophageal irritation; and brain metastases [62, 82]. Antiemetics may be administered orally, s.c., i.v., sublingually, or transdermally, and multiple agents may need to be used to achieve symptom control. Dexamethasone is useful for central nervous system metastasis or as an adjuvant antiemetic to other therapies. The serotonin 5-HT3 receptor antagonists and the substance P/neurokinin receptor antagonists are usually used for nausea and vomiting associated with chemotherapy or radiation, but may also be used in advanced cancer [83]. Metoclopromide, a dopamine antagonist, may be used to treat nausea associated with a noncomplete bowel obstruction (with octreotide), and lorazepam may be used to treat anticipatory or anxiety-related nausea; lorazepam also dissolves under the tongue for at home use if nausea and vomiting are present [62]. For cancer-related nausea, evidence supports the use of metoclopromide at a dose of 10 mg orally before meals and at bedtime or 10 mg orally, s.c., or i.v. every 4 hours as needed [84]. The piperazine phenothiazine antipsychotic prochlorperazine is also a useful antiemetic that may be administered orally, i.v., i.m., or rectally.
Along with nausea and vomiting, patients with advanced cancer frequently report constipation (at least 50%), especially if they are being treated with opioids [28, 62, 85]. Prophylaxis and prevention of constipation with the goal of a soft bowel movement every 1–2 days should be initiated with the start of opioids. A stool softener such as docusate sodium in combination with a laxative (lactulose, magnesium hydroxide, or magnesium citrate) or stimulant (senna or bisacodyl) may be a good starting point for relieving constipation. Bulking agents should be avoided in constipation caused by opioids. For constipation that is refractory to the above, rectal suppositories or enemas after 48 hours may be required. Methylnaltrexone, a μ-opioid antagonist, is FDA approved for opioid-induced constipation. The dose is weight based (12 mg for patients 62–114 kg) s.c. every other day. Methylnaltrexone is contraindicated in patients with bowel obstruction, and common adverse events include diarrhea, dizziness, hyperhidrosis, and, rarely, gastrointestinal perforation [62]. Table 6 summarizes the management of opioid-induced constipation.
At some point during the course of their disease, most patients with advanced breast cancer will have anorexia [85]. Anorexia and the physical changes that accompany it typically cause more distress to the patient's caregivers than to the patient herself [86]. Reversible causes of anorexia should be evaluated, such as constipation, uncontrolled pain, nausea, vomiting, depression, delirium, or stomatitis. Nutritional counseling, which may incorporate the use of high-calorie, high-protein drink supplements, may be effective [87]. A prospective study of dexamethasone in palliative care demonstrated an improvement in anorexia [88]. Megestrol acetate, at varying doses but usually at 800 mg daily, has been shown to stimulate appetite in patients with cancer [62, 89]. The adverse events of adrenal insufficiency and venous thromboembolism should be remembered when prescribing megestrol acetate. Dronabinol (synthetic delta-9-tetrahydrocannabinol) may also be used to treat anorexia, although in a randomized trial, megestrol acetate (800 mg) was statistically superior to dronabinol (5 mg) in improving appetite and weight gain [90]. Enteral tube feeding and parenteral nutrition do not improve comfort or survival in terminally ill cancer patients and may lead to complications that prolong suffering [91]. They should be discouraged and only considered as temporizing measures in patients with mechanical impediments to nutrition.
Breast cancer can metastasize to the peritoneum or liver, causing obstruction of the portal veins or liver failure leading to ascites. Large-volume therapeutic paracentesis may be performed for symptom relief, and peritoneal ports or catheters may be placed to assist with drainage, especially at home, but infection is a risk. Diuretics may also be used, especially if portal hypertension is present. Ascites resulting from breast cancer is a very poor prognostic sign and survival usually is short [92].
Overall Management
When breast cancer recurs, the goals of treatment often shift from one of cure to controlling the disease for as long as possible while palliating symptoms interfering with the patient's functional status and quality of life. This requires ongoing discussions with the patient and family about the goals of care. Balancing the side effects of treatment with potential control of the disease and symptom relief must be evaluated with every change in status. Identifying which patients may actually benefit enough to undergo treatment can be difficult because there is little evidence to provide direction to the clinician [93]. Unfortunately, 20% of cancer patients are still receiving chemotherapy in the last 2 weeks of life, with many hospice referrals occurring just days before death [94]. Patient-centered communication during this phase of care is focused on eliciting the patient's report of symptoms; communicating prognosis while maintaining hope; making decisions about anticancer treatments, life support, substituted judgment, and hospice care; responding to the emotions of the patient, family, and caregivers; and helping the patient navigate the transition to hospice care [95].
Palliative Care
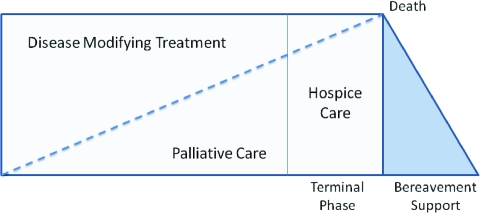
Integrating palliative care has become more important because patients can live with metastatic disease for long periods of time. Palliative care focuses on symptom management regardless of where the patient is in the cancer continuum. The focus of this article is the management of patients with MBC and is, in essence, about palliative care. A model for providing palliative care can be seen in Figure 1. In addition to improving quality of life, a structured palliative care approach was shown to improve survival in patients with metastatic lung cancer, and a similar approach should be considered for patients with breast cancer [96].
Figure 1.
Third-generation model of palliative and hospice care. From Mazanec P, Daly B, Ptorak E et al. A new model of palliative care for oncology patients with advanced disease. J Hosp Palliat Nurs 2009;11:324–331. Reprinted with permission from Wolters Kluwer Health.
Hospice
Whether it is months or years, treatments in the metastatic setting will eventually stop working. Discussing patients' preferences for end-of-life care should occur when discussing goals of care and advanced directives, and in truth, it is never too early to start these conversations. These conversations can be difficult conversations for providers, patients, and families, but they are crucial in clarifying patient and family preferences about care, place of death, and other wishes. Many patients interpret this to mean that there is no hope left, making oncologists reluctant to discuss these issues for a variety of reasons [97]. ASCO has made a booklet available to help introduce these topics [98]. Referring patients to hospice can be a difficult decision, but it often occurs too late in the patient's disease course when the benefits of participating are truncated.
Conclusions
The management of symptoms in women with MBC is challenging and is frequently made even more so by the potentially dire additive effects of tumor-related organ dysfunction and treatment toxicity. Managing the major or more common symptoms in patients with breast cancer metastases is discussed in detail in this paper. Optimal management requires a team approach that provides close and frequent communication among caregivers, patients, and families. Although incurable, some women with MBC can live for long periods of time. Maintaining the highest quality of life while optimizing disease control should be the mainstay of care.
Author Contributions
Conception/Design: Deborah K. Mayer, Hyman B. Muss, William Irvin Jr.
Manuscript writing: Deborah K. Mayer, Hyman B. Muss, William Irvin Jr.
Final approval of manuscript: Deborah K. Mayer, Hyman B. Muss, William Irvin Jr.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, Version 2.2011. [accessed August 10, 2011]. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 3.Surveillance, Epidemiology, and End Results (SEER) Cancer of the Breast. SEER Stat Fact Sheets 2008, based on November 2007 SEER data submission, posted to the SEER Web site 2008. [accessed August 10, 2011]. Available at http://seer.cancer.gov.
- 4.Beslija S, Bonneterre J, Burstein HJ, et al. Third consensus on medical treatment of metastatic breast cancer. Ann Oncology. 2009;20:1771–1785. doi: 10.1093/annonc/mdp261. [DOI] [PubMed] [Google Scholar]
- 5.Hayes D. Evaluation after primary therapy. In: Harris JR, Lippman ME, Morrow M, et al., editors. Diseases of the Breast. Philadelphia: Lippincott Williams & Wilkins; 2000. p. 709. [Google Scholar]
- 6.Hellman S, Harris J. Natural history of breast cancer. In: Harris JR, Lippman ME, Morrow M, et al., editors. Diseases of the Breast. Philadelphia: Lippincott Williams & Wilkins; 2000. p. 407. [Google Scholar]
- 7.Luoma ML, Hakamies-Blomqvist L. The meaning of quality of life in patients being treated for advanced breast cancer: A qualitative study. Psychooncology. 2004;13:729–739. doi: 10.1002/pon.788. [DOI] [PubMed] [Google Scholar]
- 8.Mayer M, Hunis A, Oratz R, et al. Lessons Learned From the Metastatic Breast Cancer Community. Semin Oncol Nurs. 2010;26:195–202. doi: 10.1016/j.soncn.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenne Sarenmalm E, Ohlén J, Jonsson T, et al. Coping with recurrent breast cancer: Predictors of distressing symptoms and health-related quality of life. J Pain Symptom Manage. 2007;34:24–39. doi: 10.1016/j.jpainsymman.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Aranda S, Schofield P, Weih L, et al. Mapping the quality of life and unmet needs of urban women with metastatic breast cancer. Eur J Cancer Care. 2005;14:211–222. doi: 10.1111/j.1365-2354.2005.00541.x. [DOI] [PubMed] [Google Scholar]
- 12.Goudas LC, Bloch R, Gialeli-Goudas M, et al. The epidemiology of cancer pain. Cancer Invest. 2005;23:182–190. [PubMed] [Google Scholar]
- 13.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 14.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 15.Dy SM, Asch SM, Naeim A, et al. Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26:3879–3885. doi: 10.1200/JCO.2007.15.9517. [DOI] [PubMed] [Google Scholar]
- 16.Taillibert S, Laigle-Donadey F, Chodkiewicz C, et al. Leptomeningeal metastases from solid malignancy: A review. J Neurooncol. 2005;75:85–99. doi: 10.1007/s11060-004-8101-x. [DOI] [PubMed] [Google Scholar]
- 17.Chang EL, Lo S. Diagnosis and management of central nervous system metastases from breast cancer. The Oncologist. 2003;8:398–410. doi: 10.1634/theoncologist.8-5-398. [DOI] [PubMed] [Google Scholar]
- 18.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454. doi: 10.1002/14651858.CD005454.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore RA, Straube S, Wiffen PJ, et al. Pregabalin for acute and chronic pain in adults. Cochrane Database of Syst Rev. 2009;(3):CD007076. doi: 10.1002/14651858.CD007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;(3):CD007938. doi: 10.1002/14651858.CD007938.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollingshead J, Dḧmke RM, Cornblath DR. Tramadol for neuropathic pain. Cochrane Database Syst Rev. 2006;3:CD003726. doi: 10.1002/14651858.CD003726.pub3. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network (NCCN) Adult Cancer Pain. [accessed August 10, 2011]. Available at http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf.
- 23.Hoskin PJ, Ford HT, Harmer CL. Hemibody irradiation (HBI) for metastatic bone pain in two histologically distinct groups of patients. Clin Oncol (R Coll Radiol) 1989;1:67–69. doi: 10.1016/s0936-6555(89)80037-8. [DOI] [PubMed] [Google Scholar]
- 24.Ural AU, Avcu F, Baran Y. Bisphosphonate treatment and radiotherapy in metastatic breast cancer. Med Oncol. 2008;25:350–355. doi: 10.1007/s12032-008-9044-4. [DOI] [PubMed] [Google Scholar]
- 25.Hoskin PJ. Bisphosphonates and radiation therapy for palliation of metastatic bone disease. Cancer Treat Rev. 2003;29:321–327. doi: 10.1016/s0305-7372(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 26.Hoskin PJ, Stratford MR, Folkes LK, et al. Effect of local radiotherapy for bone pain on urinary markers of osteoclast activity. Lancet. 2000;355:1428–1429. doi: 10.1016/s0140-6736(00)02144-9. [DOI] [PubMed] [Google Scholar]
- 27.Gangi A, Dietemann JL, Schultz A, et al. Interventional radiologic procedures with CT guidance in cancer pain management. Radiographics. 1996;16:1289–1304. doi: 10.1148/radiographics.16.6.8946536. discussion 1304–1306. [DOI] [PubMed] [Google Scholar]
- 28.McNicol E, Horowicz-Mehler N, Fisk RA, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: A systematic review. J Pain. 2003;4:231–256. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- 29.Leonard R, Hardy J, van Tienhoven G, et al. Randomized, double-blind, placebo-controlled, multicenter trial of 6% miltefosine solution, a topical chemotherapy in cutaneous metastases from breast cancer. J Clin Oncol. 2001;19:4150–4159. doi: 10.1200/JCO.2001.19.21.4150. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell SA, Beck SL, Hood LE, et al. Putting evidence into practice: Evidence-based interventions for fatigue during and following cancer and its treatment. Clin J Oncol Nurs. 2007;11:99–113. doi: 10.1188/07.CJON.99-113. [DOI] [PubMed] [Google Scholar]
- 31.Wanchai A, Armer JM, Stewart BR. Nonpharmacologic supportive strategies to promote quality of life in patients experiencing cancer-related fatigue. Clin J Oncol Nurs. 2011;15:203–214. doi: 10.1188/11.CJON.203-214. [DOI] [PubMed] [Google Scholar]
- 32.Svensson H, Brandberg Y, Einbeigi Z, et al. Psychological reactions to progression of metastatic breast cancer—an interview study. Cancer Nurs. 2009;32:55. doi: 10.1097/01.NCC.0000343374.09270.ff. [DOI] [PubMed] [Google Scholar]
- 33.Warren M. Uncertainty, lack of control and emotional functioning in women with metastatic breast cancer: A review and secondary analysis of the literature using the critical appraisal technique. Eur J Cancer Care. 2010;19:564–574. doi: 10.1111/j.1365-2354.2010.01215.x. [DOI] [PubMed] [Google Scholar]
- 34.Kissane DW, Grabsch B, Love A, et al. Psychiatric disorder in women with early stage and advanced breast cancer: A comparative analysis. Aust N Z J Psychiatry. 2004;38:320–326. doi: 10.1080/j.1440-1614.2004.01358.x. [DOI] [PubMed] [Google Scholar]
- 35.Desmarais JE, Looper KJ. Managing menopausal symptoms and depression in tamoxifen users: Implications of drug and medicinal interactions. Maturitas. 2010;67:296–308. doi: 10.1016/j.maturitas.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Kelly CM, Juurlink DN, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: A population based cohort study. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Santiago S, Zárate R, Haba-Rodríguez J, et al. CYP2D6*4 polymorphism as blood predictive biomarker of breast cancer relapse in patients receiving adjuvant tamoxifen. J Clin Oncol. 2007;25(18 suppl):590. [Google Scholar]
- 38.Goetz MP, Knox SK, Suman VJ, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101:113–121. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- 39.Aubert RE, Stanek EJ, Yao J, et al. Risk of breast cancer recurrence in women initiating tamoxifen with CYP2D6 inhibitors. J Clin Oncol. 2009;27(9 suppl):CRA508. [Google Scholar]
- 40.Rae JM, Drury S, Hayes DF, et al. Lack of correlation between gene variants in tamoxifen metabolizing enzymes with primary endpoints in the ATAC trial [abstract S1–7]. Presented at the San Antonio Breast Cancer Symposium; December 9, 2010; San Antonio, Texas. [Google Scholar]
- 41.Fiorentino L, Ancoli-Israel S. Insomnia and its treatment in women with breast cancer. Sleep Med Rev. 2006;10:419–429. doi: 10.1016/j.smrv.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lis CG, Gupta D, Grutsch JF. The relationship between insomnia and patient satisfaction with quality of life in cancer. Support Care Cancer. 2008;16:261–266. doi: 10.1007/s00520-007-0314-z. [DOI] [PubMed] [Google Scholar]
- 43.Page MS, Berger AM, Johnson LB. Putting evidence into practice: Evidence-based interventions for sleep-wake disturbances. Clin J Oncol Nurs. 2006;10:753–767. doi: 10.1188/06.CJON.753-767. [DOI] [PubMed] [Google Scholar]
- 44.Berger AM. Update on the state of the science: Sleep-wake disturbances in adult patients with cancer. Oncol Nurs Forum. 2009;36:E165–E177. doi: 10.1188/09.ONF.E165-E177. [DOI] [PubMed] [Google Scholar]
- 45.Michael S, Charikleia S, Konstantinos K. Lymphedema and breast cancer: A review of the literature. Breast Cancer. 2011;18:174–180. doi: 10.1007/s12282-010-0246-1. [DOI] [PubMed] [Google Scholar]
- 46.Nesvold IL, Reinertsen KV, Fosså SD, et al. The relation between arm/shoulder problems and quality of life in breast cancer survivors: A cross-sectional and longitudinal study. J Cancer Surviv. 2011;5:62–72. doi: 10.1007/s11764-010-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rourke LL, Hunt KK, Cormier JN. Breast cancer and lymphedema: A current overview for the healthcare provider. Womens Health (London) 2010;6:399–406. doi: 10.2217/whe.10.21. [DOI] [PubMed] [Google Scholar]
- 48.Poage E, Singer M, Armer J, et al. Demystifying lymphedema: Development of the lymphedema putting evidence into practice card. Clin J Oncol Nurs. 2008;12:951–964. doi: 10.1188/08.CJON.951-964. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed Omar MT, El Morsy AM, Abd-El-Gayed Ebid A. Treatment of post-mastectomy lymphedema with laser therapy: Double blind placebo control randomized study. J Surg Res. 2011;165:82–90. doi: 10.1016/j.jss.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 50.Lau RWL, Cheing GLY. Managing postmastectomy lymphedema with low-level laser therapy. Photomed Laser Surg. 2009;27:763–769. doi: 10.1089/pho.2008.2330. [DOI] [PubMed] [Google Scholar]
- 51.Vassard D, Olsen MH, Zinckernagel L, et al. Psychological consequences of lymphoedema associated with breast cancer: A prospective cohort study. Eur J Cancer. 2010;46:3211–3218. doi: 10.1016/j.ejca.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 52.Shikama N, Sekiguchi K, Nakamura N. Management of locoregional recurrence of breast cancer. Breast Cancer. 2010 May 7; doi: 10.1007/s12282-010-0206-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Leonard R, Hardy J, van Tienhoven G, et al. Randomized, double-blind, placebo-controlled, multicenter trial of 6% miltefosine solution, a topical chemotherapy in cutaneous metastases from breast cancer. J Clin Oncol. 2001;19:4150–4159. doi: 10.1200/JCO.2001.19.21.4150. [DOI] [PubMed] [Google Scholar]
- 54.Adderley U, Smith R. Topical agents and dressings for fungating wounds. Cochrane Database Syst Rev. 2007;(2):CD003948. doi: 10.1002/14651858.CD003948.pub2. [DOI] [PubMed] [Google Scholar]
- 55.Paul JC, Pieper BA. Topical metronidazole for the treatment of wound odor: A review of the literature. Ostomy Wound Manage. 2008;54:18–27. [PubMed] [Google Scholar]
- 56.Thomas JR, von Gunten CF. Management of dyspnea. J Support Oncol. 2003;1:23–32. doi: 10.1007/978-1-59745-291-5_1. discussion 32–34. [DOI] [PubMed] [Google Scholar]
- 57.Reuben DB, Mor V. Dyspnea in terminally ill cancer patients. Chest. 1986;89:234–236. doi: 10.1378/chest.89.2.234. [DOI] [PubMed] [Google Scholar]
- 58.Lower EE, Baughman RP. Pulmonary lymphangitic metastasis from breast cancer. Lymphocytic alveolitis is associated with favorable prognosis. Chest. 1992;102:1113–1117. doi: 10.1378/chest.102.4.1113. [DOI] [PubMed] [Google Scholar]
- 59.Goldsmith HS, Bailey HD, Callahan EL, et al. Pulmonary lymphangitic metastases from breast carcinoma. Arch Surg. 1967;94:483–488. doi: 10.1001/archsurg.1967.01330100047007. [DOI] [PubMed] [Google Scholar]
- 60.Gibson PG, Bryant DH, Morgan GW, et al. Radiation-induced lung injury: A hypersensitivity pneumonitis? Ann Intern Med. 1988;109:288–291. doi: 10.7326/0003-4819-109-4-288. [DOI] [PubMed] [Google Scholar]
- 61.Nagata S, Ueda N, Yoshida Y, et al. Severe interstitial pneumonitis associated with the administration of taxanes. J Infect Chemother. 2010;16:340–344. doi: 10.1007/s10156-010-0058-4. [DOI] [PubMed] [Google Scholar]
- 62.Shoemaker LK, Estfan B, Induru R, et al. Symptom management: An important part of cancer care. Cleve Clin J Med. 2011;78:25–34. doi: 10.3949/ccjm.78a.10053. [DOI] [PubMed] [Google Scholar]
- 63.Abernethy AP, Currow DC, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327:523–528. doi: 10.1136/bmj.327.7414.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at the end of life: A systematic review. Ann Intern Med. 2008;148:147–159. doi: 10.7326/0003-4819-148-2-200801150-00010. [DOI] [PubMed] [Google Scholar]
- 65.Coyne PJ, Viswanathan R, Smith TJ. Nebulized fentanyl citrate improves patients' perception of breathing, respiratory rate, and oxygen saturation in dyspnea. J Pain Symptom Manage. 2002;23:157–160. doi: 10.1016/s0885-3924(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 66.Polosa R, Simidchiev A, Walters EH. Nebulised morphine for severe interstitial lung disease. Cochrane Database Syst Rev. 2002;(3):CD002872. doi: 10.1002/14651858.CD002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Navigante AH, Cerchietti LC, Castro MA, et al. Midazolam as adjunct therapy to morphine in the alleviation of severe dyspnea perception in patients with advanced cancer. J Pain Symptom Manage. 2006;31:38–47. doi: 10.1016/j.jpainsymman.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 68.Ripamonti C, Fulfaro F, Bruera E. Dyspnoea in patients with advanced cancer: Incidence, causes and treatments. Cancer Treat Rev. 1998;24:69–80. doi: 10.1016/s0305-7372(98)90072-x. [DOI] [PubMed] [Google Scholar]
- 69.Clemens KE, Quednau I, Klaschik E. Use of oxygen and opioids in the palliation of dyspnoea in hypoxic and non-hypoxic palliative care patients: A prospective study. Support Care Cancer. 2009;17:367–377. doi: 10.1007/s00520-008-0479-0. [DOI] [PubMed] [Google Scholar]
- 70.Qaseem A, Snow V, Shekelle P, et al. Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:141–146. doi: 10.7326/0003-4819-148-2-200801150-00009. [DOI] [PubMed] [Google Scholar]
- 71.DiSalvo WM, Joyce MM, Tyson LB, et al. Putting evidence into practice: Evidence-based interventions for cancer-related dyspnea. Clin J Oncol Nurs. 2008;12:341–352. doi: 10.1188/08.CJON.341-352. [DOI] [PubMed] [Google Scholar]
- 72.Apffelstaedt JP, Van Zyl JA, Muller AG. Breast cancer complicated by pleural effusion: Patient characteristics and results of surgical management. J Surg Oncol. 1995;58:173–175. doi: 10.1002/jso.2930580307. [DOI] [PubMed] [Google Scholar]
- 73.Bielsa S, Hernàndez P, Rodriguez-Panadero F, et al. Tumor type influences the effectiveness of pleurodesis in malignant effusions. Lung. 2011;189:151–155. doi: 10.1007/s00408-011-9283-6. [DOI] [PubMed] [Google Scholar]
- 74.Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546–551. doi: 10.1016/j.ejso.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 75.Jensen AØ, Jacobsen JB, Nørgaard M, et al. Incidence of bone metastases and skeletal-related events in breast cancer patients: A population-based cohort study in Denmark. BMC Cancer. 2011;11:29. doi: 10.1186/1471-2407-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hosking DJ, Cowley A, Bucknall CA. Rehydration in the treatment of severe hypercalcaemia. Q J Med. 1981;50:473–481. [PubMed] [Google Scholar]
- 77.Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: A pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19:558–567. doi: 10.1200/JCO.2001.19.2.558. [DOI] [PubMed] [Google Scholar]
- 78.Wisneski LA. Salmon calcitonin in the acute management of hypercalcemia. Calcif Tissue Int. 1990;46(suppl):S26–S30. doi: 10.1007/BF02553290. [DOI] [PubMed] [Google Scholar]
- 79.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 80.Van Poznak CH, Temin S, Yee GC, et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29:1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 81.George R, Jeba J, Ramkumar G, et al. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev. 2008;(4):CD006716. doi: 10.1002/14651858.CD006716.pub2. [DOI] [PubMed] [Google Scholar]
- 82.Stephenson J, Davies A. An assessment of aetiology-based guidelines for the management of nausea and vomiting in patients with advanced cancer. Support Care Cancer. 2006;14:348–353. doi: 10.1007/s00520-005-0897-1. [DOI] [PubMed] [Google Scholar]
- 83.Currow DC, Coughlan M, Fardell B, et al. Use of ondansetron in palliative medicine. J Pain Symptom Manage. 1997;13:302–307. doi: 10.1016/s0885-3924(97)00079-1. [DOI] [PubMed] [Google Scholar]
- 84.Dy SM, Apostol CC. Evidence-based approaches to other symptoms in advanced cancer. Cancer J. 2010;16:507–513. doi: 10.1097/PPO.0b013e3181f45877. [DOI] [PubMed] [Google Scholar]
- 85.Donnelly S, Walsh D. The symptoms of advanced cancer. Semin Oncol. 1995;22(suppl 3):67–72. [PubMed] [Google Scholar]
- 86.Yamagishi A, Morita T, Miyashita M, et al. The care strategy for families of terminally ill cancer patients who become unable to take nourishment orally: Recommendations from a nationwide survey of bereaved family members' experiences. J Pain Symptom Manage. 2010;40:671–683. doi: 10.1016/j.jpainsymman.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 87.Adams LA, Shepard N, Caruso RA, et al. Putting evidence into practice: Evidence-based interventions to prevent and manage anorexia. Clin J Oncol Nurs. 2009;13:95–102. doi: 10.1188/09.CJON.95-102. [DOI] [PubMed] [Google Scholar]
- 88.Mercadante S, Fulfaro F, Casuccio A. The use of corticosteroids in home palliative care. Support Care Cancer. 2001;9:386–389. doi: 10.1007/s005200000218. [DOI] [PubMed] [Google Scholar]
- 89.Loprinzi CL, Ellison NM, Schaid DJ, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst. 1990;82:1127–1132. doi: 10.1093/jnci/82.13.1127. [DOI] [PubMed] [Google Scholar]
- 90.Jatoi A, Windschitl HE, Loprinzi CL, et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: A North Central Cancer Treatment Group study. J Clin Oncol. 2002;20:567–573. doi: 10.1200/JCO.2002.20.2.567. [DOI] [PubMed] [Google Scholar]
- 91.Winter SM. Terminal nutrition: Framing the debate for the withdrawal of nutritional support in terminally ill patients. Am J Med. 2000;109:723–726. doi: 10.1016/s0002-9343(00)00609-4. [DOI] [PubMed] [Google Scholar]
- 92.Stephenson J, Gilbert J. The development of clinical guidelines on paracentesis for ascites related to malignancy. Palliat Med. 2002;16:213–218. doi: 10.1191/0269216302pm509oa. [DOI] [PubMed] [Google Scholar]
- 93.Anders CK, Peppercorn J. Treating in the dark: Unanswered questions on costs and benefits of late line therapy for metastatic breast cancer. Cancer Investig. 2009;27:13–16. doi: 10.1080/07357900802484944. [DOI] [PubMed] [Google Scholar]
- 94.Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol. 2008;26:3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Epstein R, Street R., Jr . NIH Publication No. 07–6225. Bethesda, MD: National Cancer Institute; 2007. Patient-Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering; pp. 1–203. [Google Scholar]
- 96.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 97.Behl D, Jatoi A. What do oncologists say about chemotherapy at the very end of life? Results from a semiqualitative survey. J Palliat Med. 2010;13:831–835. doi: 10.1089/jpm.2009.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.American Society of Clinical Oncology. Advanced Cancer Care Planning: What Patients and Their Families Need to Know About Their Choices When Facing Serious Illness. [accessed August 10, 2011]. Available at http://www.cancer.net/patient/Coping/Advanced%20Cancer%20Care%20Planning/Advanced_Cancer_Care_Planning.pdf.
- 99.Mazanec P, Daly B, Ptorak E, et al. A new model of palliative care for oncology patients with advanced disease. J Hosp Palliat Nurs. 2009;11:324–331. [Google Scholar]
- 100.National Comprehensive Cancer Network (NCCN) NCCN Guidelines – Fatigue, version 1/2011. [accessed August 10, 2011]. Available at http://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf.
- 101.National Cancer Institute. Sleep Disorders (PDQ®) Management. [accessed August 10, 2011]. Available at http://www.cancer.gov/cancertopics/pdq/supportivecare/sleepdisorders/HealthProfessional/page4#Section_74.