This retrospective study investigated whether overall survival has improved in the general population with metastatic non-small cell lung cancer over the last decade, and examined the uptake and impact of new chemotherapeutic and targeted agents introduced during this time.
Keywords: Non-small cell lung cancer, Metastatic, Stage IV, Survival, Systemic therapy
Abstract
Purpose.
Significant advances in the systemic management of metastatic non-small cell lung cancer (NSCLC) have occurred over the past decade, with options now including multiple lines of chemotherapy, epidermal growth factor receptor inhibitors, and antiangiogenic agents. Improvements in overall survival have been demonstrated in randomized controlled trials comparing these newer agents with best supportive care or standard therapy. This study examined uptake of these therapies in general practice and their impact on survival.
Methods.
This retrospective cohort study compared demographic, treatment, and survival data among 987 patients diagnosed with stage IV NSCLC at two institutions in 1998, 2003, and 2008. Cohorts were selected based on intervals when doublet chemotherapy, second-line chemotherapy, and targeted agents were incorporated into the standard treatment regimen.
Results.
The proportion of patients receiving systemic therapy increased over time (20% in 1998, 42% in 2008). Overall survival improved significantly across cohorts (p < .001), with 2-year survival rates of 0.3% in 1998, 4% in 2003, and 15% in 2008. In a multivariate survival analysis, the 2003 and 2008 cohorts were independently associated with longer survival, as was the use of one or more lines of systemic therapy. Elderly patients (aged ≥70 years) were also more likely to receive systemic therapy over time, with longer overall survival (p < .001).
Conclusion.
Over the past decade, there has been an increasing use of systemic therapy in stage IV NSCLC patients, including the elderly. This has been associated with significantly longer overall survival.
Introduction
Lung cancer is the most common cancer worldwide and the leading cause of cancer death. The vast majority of patients have non-small cell lung cancer (NSCLC), comprising approximately 85% of all lung cancer cases. Over one half of newly diagnosed patients already have metastatic disease (stage IV) upon presentation [1].
Over the past two decades, there have been significant changes in the management of metastatic NSCLC, whereby surgical therapy is limited and overall survival remains poor [1]. In the 1990s, multiple meta-analyses demonstrated modestly longer survival times with systemic therapy than with best supportive care (BSC) alone [2]. The search for more active agents led to the identification of newer third-generation chemotherapeutic agents such as paclitaxel, docetaxel, vinorelbine, and gemcitabine. Combining these with a platinum agent was shown to result in longer survival times and better symptom control [3, 4].
Further progress was made in the 2000s, beginning with second-line therapy with single-agent docetaxel [5, 6]. Also introduced was the antifolate pemetrexed in the second-line setting [7], along with recent evidence that cisplatin plus pemetrexed may be effective in the nonsquamous population in the first-line setting [8]. The past decade also saw the development of molecular-targeted therapies such as tyrosine kinase inhibitors. In particular, the epidermal growth factor receptor (EGFR) inhibitors (erlotinib and gefitinib) have been found to be effective as monotherapies in the second-line setting [9, 10] and in EGFR mutation–positive tumors in the first-line setting [11]. Finally, discovery of angiogenesis as a promising drug target led to the development of bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF) that may be of benefit in combination with platinum-based doublet chemotherapy in patients with nonsquamous NSCLC [12].
Although these newer systemic therapies have been shown, in randomized controlled trials, to lead to longer overall survival times than with BSC or standard therapies, patients in such trials are highly selected and tend to have better performance status scores and fewer comorbidities. Furthermore, elderly patients (aged ≥70 years) are grossly underrepresented in clinical trials [13], even though retrospective subset analyses of several trials suggest that chemotherapy may have similar efficacy and tolerability in the elderly [14]. Thus, results from clinical trials may not necessarily be reflected in clinical practice.
This retrospective study investigated whether overall survival has improved in the general population with metastatic NSCLC over the last decade, and examined the uptake and impact of new chemotherapeutic and targeted agents introduced during this time.
Patients and Methods
Study Design
We undertook a retrospective chart review of patients diagnosed with stage IV NSCLC during three different time cohorts from two comprehensive cancer care institutions (Princess Margaret Hospital and Sunnybrook Odette Cancer Centre), located in Toronto, Canada. The three time cohorts spanned one decade and were selected based on intervals when doublet chemotherapy, second-line chemotherapy, and targeted agents were incorporated into the standard treatment: (a) January 1, 1998 to December 31, 1998, (b) January 1, 2003 to December 31, 2003, and (c) January 1, 2008 to December 31, 2008.
Patients were included if they had stage IV disease at initial presentation or if they experienced first recurrence with distant metastases during the time cohort. Patients with mixed non-small cell and small cell lung cancer were excluded, as were patients with another primary malignancy, with the exception of nonmelanoma skin cancer.
The paper or electronic chart for each patient was reviewed individually, and data extraction was performed using a standardized electronic data collection form linked to a Microsoft Excel database. In addition to demographics, the information recorded included the initial date of diagnosis of stage IV disease and sites of metastasis at diagnosis, as well as the dates and sites of subsequent metastases. The original date of the lung cancer diagnosis (prior to stage IV) was noted when applicable. Details of systemic therapy, radiation therapy, and surgical interventions were recorded. Vital status was ascertained from institutional health records, clinical follow-up, or by searching the Ontario Cancer Registry, with follow-up through to August 19, 2010. Survival was calculated from the date of diagnosis with stage IV disease. Patients who were lost to follow-up or for whom the date of death could not be confirmed were censored at the time of last contact in the survival analysis.
This study was approved by the University Health Network Research Ethics Board, the Cancer Registry Data Access Committee, and the Sunnybrook Health Sciences Centre Research Ethics Board.
Statistical Methods
Continuous factors were compared among the three cohorts using analysis of variance, and χ2 tests of association were used for categorical factors. Kaplan–Meier curves and survival estimates were constructed to compare overall survival among the three cohorts and to compare sociodemographic and clinical factors in a bivariate manner across each of the cohorts separately. Multivariate Cox proportional hazards models were used to model time to death on all factors simultaneously. All factors entered into the model were measured at the time of diagnosis of stage IV disease and thus considered time invariant, with the exception of number of lines of systemic therapy. This latter factor was entered into the model as a time-varying covariate. All analyses were conducted using SAS, version 9.2 (SAS Institute, Inc., Cary, NC); p-values < .05 were considered statistically significant.
Results
Patient Characteristics
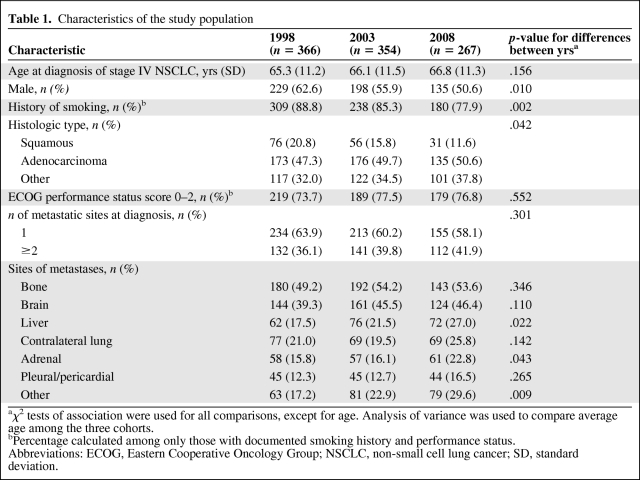
In total, 987 patients (456 from Princess Margaret Hospital and 531 from Sunnybrook Odette Cancer Centre) were diagnosed with stage IV NSCLC during the time periods of interest and included in the study—366 patients in 1998, 354 patients in 2003, and 267 patients in 2008. Table 1 shows the characteristics of the three cohorts. The cohorts were similar in age at diagnosis as well as performance status (when documented). However, the proportion of males decreased significantly over time (p = .010), as did the proportion of patients with a history of smoking (p = .002). The predominant subtype was adenocarcinoma, and the proportion of patients with the squamous subtype decreased over time (p = .042). Bone and brain were the most common sites of metastases, occurring in approximately half of all patients. Approximately 40% of patients had more than one site of metastasis at the time of diagnosis with stage IV disease, a proportion that did not vary significantly among cohorts.
Table 1.
Characteristics of the study population
aχ2 tests of association were used for all comparisons, except for age. Analysis of variance was used to compare average age among the three cohorts.
bPercentage calculated among only those with documented smoking history and performance status.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NSCLC, non-small cell lung cancer; SD, standard deviation.
Treatments
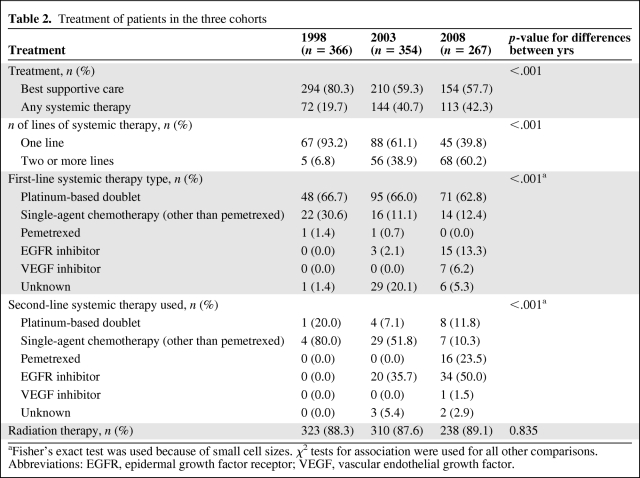
The treatments received by patients in each of the time cohorts are presented in Table 2. The vast majority of patients diagnosed in 1998 were given BSC only, with only 20% receiving any systemic therapy. The proportion receiving systemic therapy rose significantly to 41% in 2003 and to 42% in 2008 (p < .001). Of the patients who received systemic therapy, the percentage receiving two or more lines of treatment rose from 7% in 1998 to 39% in 2003 and to 60% in 2008. No patients diagnosed in 1998 received three or more lines of therapy, compared with 23% of treated patients diagnosed in 2008. Furthermore, 6% of treated patients in the 2008 cohort received four or five lines. In contrast, the use of radiation therapy remained constant across cohorts (p = .835). Radiation therapy was used for palliation of symptoms, and the most common targets were bone, brain, and thorax.
Table 2.
Treatment of patients in the three cohorts
aFisher's exact test was used because of small cell sizes. χ2 tests for association were used for all other comparisons.
Abbreviations: EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor.
Platinum-based doublet chemotherapy was the most commonly used first-line systemic therapy across cohorts, the choice in almost two thirds of all treated patients. Single-agent chemotherapy was the next most common first-line approach; however, it declined in use as EGFR inhibitors became more widely used in 2008. In the second-line setting, single-agent chemotherapy was used in 80% of treated patients in 1998. This diminished in the later cohorts and was replaced by greater use of EGFR inhibitors and pemetrexed. In 2008, EGFR inhibitors were used as second-line systemic therapy in one half of patients who received second-line systemic therapy, and pemetrexed was used in approximately one quarter.
Outcomes
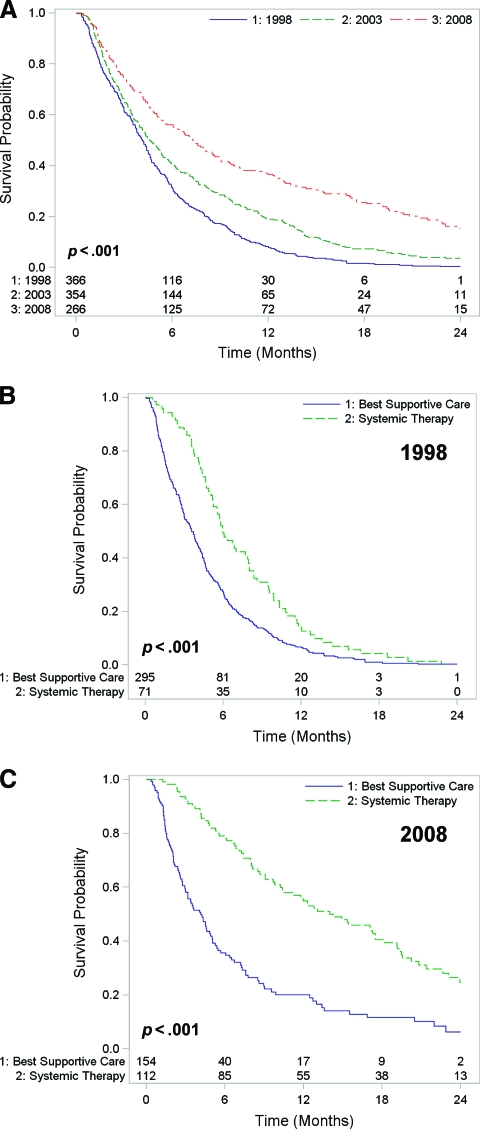
The survival of patients with stage IV NSCLC improved significantly over time (log-rank p < .001). The Kaplan–Meier survival curves for the three cohorts are shown in Figure 1A. The 1-year survival rate was 8% in the 1998 cohort, but this increased to 19% in 2003 and to 37% in 2008. Similarly, the survival rate at 2 years was only 0.3% in the 1998 cohort, compared with 4% in 2003 and 15% among patients diagnosed in 2008. The median overall survival times were 4.2 months (interquartile range [IQR], 1.9–7.0 months) in the 1998 cohort, 4.6 months (IQR, 2.2–9.8 months) in the 2003 cohort, and 5.1 months (IQR, 2.1–12.9 months) in the 2008 cohort.
Figure 1.
Overall survival in stage IV NSCLC. (A): The overall survival time of patients improved across cohorts (p < .001). (B, C): Overall survival time of patients who received systemic therapy versus best supportive care in the 1998 cohort (B) and the 2008 cohort (C). The survival advantage associated with systemic therapy increased across cohorts (p = .002).
The survival duration of patients receiving systemic therapy was significantly better than the survival time of patients receiving BSC (p < .001), and the survival advantage also increased significantly across cohorts (p = .002) (Fig. 1B, 1C). Although the 2-year survival rate in the 1998 cohort was <1% both for patients treated with systemic therapy and for those who received BSC, the corresponding 2-year survival rates were 8% for systemic therapy and 1% for BSC in 2003, and 25% for systemic therapy and 6% for BSC in the 2008 cohort. The median survival times of patients receiving systemic therapy versus BSC were, respectively, 6.0 months versus 3.6 months for the 1998 cohort, 9.6 months versus 2.9 months for the 2003 cohort, and 14.0 months versus 4.2 months for the 2008 cohort.
Analysis of Effects on Survival
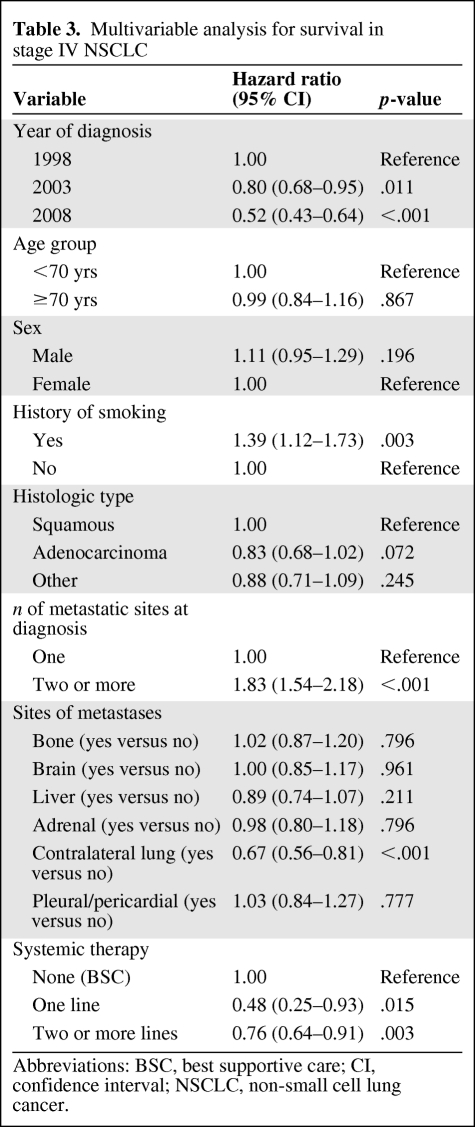
Table 3 shows the results of the multivariate survival analysis. Both the 2003 and 2008 cohorts had better survival, with hazard ratios (HRs) of 0.80 (p = .011) and 0.52 (p < .001), respectively. The use of systemic therapy was also identified as an independent predictor of survival. The HR for patients who received one line of systemic therapy was 0.48 (p = .015), whereas those who received two or more lines of systemic therapy had an HR of 0.76 (p = .003).
Table 3.
Multivariable analysis for survival in stage IV NSCLC
Abbreviations: BSC, best supportive care; CI, confidence interval; NSCLC, non-small cell lung cancer.
As expected, having two or more different sites of metastasis at diagnosis was associated with a worse outcome. Having a history of smoking was also associated with a higher risk for death. Contralateral lung metastases were associated with a significantly lower risk for death than with other sites. There was no difference in survival outcomes between the two institutions (HR, 1.04; p = .541).
Treatment and Survival of the Elderly
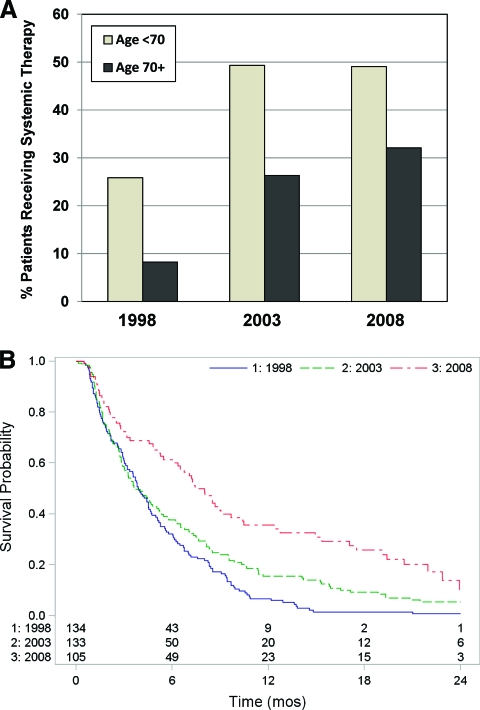
Age <70 years was not found to be a significant independent predictor of survival in the multivariate analysis (Table 3). The treatment and outcomes of elderly patients (aged ≥70 years) were therefore examined separately. Similar to the overall study population, patients in the elderly subset were also more likely to receive systemic therapy over time (Table 4). The vast majority of elderly patients diagnosed with stage IV NSCLC in 1998 were given BSC only, with a mere 8% receiving any systemic therapy. The proportion receiving systemic therapy increased significantly over time to 26% in the 2003 cohort and to 32% in the 2008 cohort (p < .001). Of the elderly patients in the 1998 cohort who received systemic therapy, none received more than one line of treatment. In contrast, 34% of patients in the 2003 cohort and 59% of patients in the 2008 cohort received two or more lines of systemic therapy; 5% of the 2008 cohort received three to five lines. Figure 2A compares the increase in the use of systemic therapy over time in the younger (age <70 years) and elderly (age ≥70 years) subpopulations.
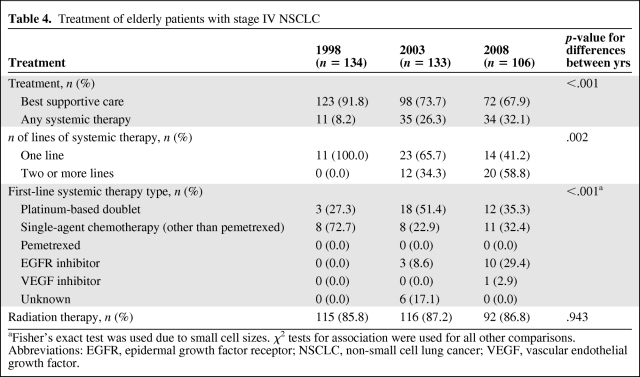
Table 4.
Treatment of elderly patients with stage IV NSCLC
aFisher's exact test was used due to small cell sizes. χ2 tests for association were used for all other comparisons.
Abbreviations: EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; VEGF, vascular endothelial growth factor.
Figure 2.
Use of systemic therapy and survival in the elderly. (A): An increase in the use of systemic therapy over time occurred in both the younger (age <70 years) and elderly (age ≥70 years) subgroups of patients with stage IV NSCLC. (B) Overall survival of elderly patients diagnosed with stage IV NSCLC in 1998, 2003, and 2008.
Abbreviation: NSCLC, non-small cell lung cancer.
The choice of first-line systemic therapy in the elderly differed somewhat from that of the overall study population (Table 4). In the 1998 cohort, the majority (73%) of elderly patients who received systemic therapy were given single-agent chemotherapy, with only 27% receiving platinum-based doublets. This is in contrast to the overall population, in which two thirds of patients (67%) in the 1998 cohort received platinum-based doublets (compare Table 4 with Table 2). Interestingly, the use of platinum-based doublets in the elderly rose in the 2003 cohort to 51% of treated patients, but decreased again in the 2008 cohort to 35%, as a result of greater use of EGFR inhibitors as first-line therapy (in 29% of treated patients).
Associated with the increasing use of systemic therapy across cohorts were significant longer survival times in the elderly population (p < .001), as shown in Figure 2B. The 2-year survival rate increased from only 0.7% in the 1998 cohort to 5% in the 2003 cohort and to 10% in the 2008 cohort.
Discussion
This study demonstrates a significant improvement over the last decade in the overall survival times of patients with stage IV NSCLC. This improvement was associated with increasing use of systemic therapy in these patients, including multiple lines of therapy, as well as the adoption of new agents and regimens such as platinum-based doublets including third-generation chemotherapeutic agents, second-line chemotherapy agents including docetaxel and pemetrexed, and EGFR inhibitors. Although the survival benefits of individual agents or regimens have previously been demonstrated in randomized controlled trials involving carefully selected patients, the current study is one of the first to do so in a large cohort of patients with metastatic NSCLC from the general population.
The use of systemic therapy in stage IV NSCLC patients doubled from 20% of patients in the 1998 cohort to 42% of patients in the 2008 cohort. The most commonly used first-line regimen was platinum-based doublet therapy, used in approximately two thirds of patients receiving systemic treatment. This practice was consistent across time cohorts, and reflects the substantial evidence that platinum-based doublet chemotherapy improves overall survival and palliates the symptoms of metastatic lung cancer [3, 4]. The evidence for second-line therapy that emerged in the 2000s was paralleled by a marked increase in the number of lines of systemic therapy given to patients across cohorts in our study population (from only 7% in 1998 receiving two or more lines, to 39% receiving two or more lines in 2003 and to 60% receiving two or more lines in 2008).
The introduction of the oral EGFR inhibitors in the early to mid-2000s was a significant advance that was reflected in our study population, with >10% of treated patients diagnosed in 2008 receiving either erlotinib or gefitinib in the first-line setting. In the second-line setting, 36% of treated patients diagnosed in 2003 and 50% of treated patients diagnosed in 2008 received EGFR inhibitors. As the evidence grows for the benefit of EGFR inhibitors in patients harboring EGFR mutations, we anticipate that the use of these therapies in the first-line setting in appropriately selected patients will only continue to increase. The small numbers of patients receiving VEGF inhibitors such as bevacizumab reflects the fact that these agents are not currently funded by Cancer Care Ontario for the treatment of metastatic NSCLC; those who received these drugs did so exclusively as part of a clinical trial.
The progress made in systemic therapy over the past decade was associated with a significant improvement in survival of patients in our study, with 2-year survival rates increasing from 0.3% in the 1998 cohort to 15% among patients diagnosed with stage IV NSCLC in 2008. Compared with those diagnosed in 1998, there was a 20% and a 48% lower risk for death among patients diagnosed in 2003 and 2008, respectively. In contrast to the improved survival rates, the median survival time did not change dramatically across cohorts (4.2 months in 1998 to 5.1 months in 2008), suggesting that the majority of patients still do poorly, even though a subset of patients are faring much better. Although we are not able, in this retrospective study, to attribute the improvement in survival to a specific therapeutic agent or regimen, the greater use of systemic therapy certainly played a role. Thus, patients who received one line of systemic therapy had approximately half the risk for death in a 2-year follow-up period of those who received BSC only, whereas those receiving two or more lines had a 24% lower risk than those who received BSC. Improvements in supportive care, most notably advances in antiemetics, and timing of palliative care may also have contributed to improvements in overall survival over time [15].
Interestingly, the elderly subset of our study population was also more likely to receive systemic therapy over time, and this was paralleled by a longer overall survival duration, with an increase in the 2-year survival rate from 0.7% in the 1998 cohort to 10% in the 2008 cohort. Despite the aging of our population and the higher incidence of cancer in the elderly, older patients remain underrepresented in clinical trials, and therefore the evidence in this area is lacking. Our findings lend support to the opinion that all patients with an adequate performance status may benefit from systemic therapy regardless of chronological age.
Finally, in our study population, we observed some notable changes in the epidemiology of metastatic NSCLC over time, with more women and nonsmokers being diagnosed, as well as a decrease in the frequency of the squamous subtype. It has been proposed that the improved survival of lung cancer patients over time might be attributed in part to the increased incidence of lung cancer in women, and the observation that women with NSCLC generally have better survival outcomes than men [16, 17]. However, in our survival analysis, female gender was not a significant predictor of longer survival. Similarly, adenocarcinoma was not an independent prognostic factor for survival, consistent with the results of previous studies [18, 19]. However, this situation may well change because certain therapies (e.g., EGFR inhibitors, pemetrexed) appear to be more effective for the nonsquamous subtypes, and as we recognize that targeted agents are maximally effective when used in patients with cancers that harbor the targeted genetic alterations. With the advent of personalized medicine and development of new targeted therapies, histological characteristics and predictive biomarkers may become increasingly valuable predictors of survival.
One of the potential limitations of this study is the issue of stage migration. Thus, better detection of metastatic disease through advances in diagnostic imaging could have contributed to an increase in survival rates. It should be noted that the use of positron emission tomography scanning during the study period was not routinely available in Canada outside a clinical trial, even in 2008, whereas computed tomography scanning was widely available over the entire study period. We did see a statistically significant increase in the frequency of metastases detected in the liver and adrenal glands: 18% of patients had liver metastases in 1998 compared with 27% of patients in 2008 (p = .022), and adrenal metastases were seen in 16% of patient in 1998 versus 23% of patients in 2008 (p = .043). However, in our multivariate analysis, having liver or adrenal metastases was not a significant predictor of survival (Table 3). Although there was a trend toward an increasing number of metastatic sites at diagnosis over time, this did not reach statistical significance. The proportion in each cohort of patients with bone or brain metastases (the most common metastatic sites) also did not increase significantly over time. There was no association between the presence of brain metastases and the lack of use of systemic therapy (data not shown).
Referral bias represents another potential limitation of this study, because the institutions included in this study were academic tertiary centers providing comprehensive cancer care services. Thus, one might postulate that there could have been higher use of systemic therapy in the study population than in the general population. To the contrary, our figures for the use of systemic therapy (20% in 1998 and 42% in 2008) were slightly lower than those reported in a recent retrospective study conducted in the U.S., which showed that 49% of patients diagnosed with stage IV NSCLC in 2000–2007 received chemotherapy [20]. There are multiple reasons why patients with metastatic NSCLC might not receive systemic therapy, including the decision not to treat because of a poor performance status or comorbidities, death prior to treatment, patient preference, as well as physician referral patterns [21].
With regard to the latter, we do know that a certain proportion of patients seen at tertiary centers (such as those in this study) are not candidates for systemic therapy and are referred there for palliative radiation only. Others referred for radiation only may have been receiving systemic therapy delivered by a medical oncologist at a community hospital without radiation facilities. Unless the details of their systemic therapy were adequately documented in the chart, these patients would have been considered to have received no systemic therapy. Thus, the most likely effect of referral bias in our study, if any, would have been to underestimate the proportion of patients receiving systemic therapy across all cohorts.
Although it would have been ideal to perform a true population-based outcome study rather than an institution-based study to circumvent the issue of referral bias [22], the Ontario Cancer Registry does not consistently record staging of disease (in particular, date of diagnosis of stage IV disease) and details of patient treatment, such that this type of study would be impossible in the metastatic NSCLC setting. A retrospective chart review was the best format to answer our question, for ensuring the accuracy of the initial date of diagnosis of stage IV disease, the sites of metastasis at diagnosis, as well as the dates and sites of subsequent metastases (by directly reviewing the imaging reports). Details of systemic therapy, radiation therapy, and surgical interventions could readily be ascertained from the chart. Other strengths of our study included the large study population of almost a thousand patients from two separate institutions, and the three distinct time cohorts spanning one decade.
Conclusion
This retrospective study showed that there has been a significant improvement over the past decade in the survival outcomes of patients with metastatic NSCLC, including those aged ≥70 years. This improvement is associated with the increased use of systemic therapy in these patients, and suggests that the benefits seen in clinical trials involving new agents and regimens may be extrapolated to the unselected general population with stage IV NSCLC. With continued research and development of new therapies and targeted agents, it is hoped that the next decade will see further improvements in survival and quality of life for patients with metastatic NSCLC.
Author Contributions
Conception/Design: Joanne L. Yu, Sunil Verma
Provision of study material or patients: Sunil Verma, Natasha B. Leighl
Collection and/or assembly of data: Joanne L. Yu, Christine Simmons, Dolly Han, Sophie Hogeveen
Data analysis and interpretation: Joanne L. Yu, Sunil Verma, J. Charles Victor
Manuscript writing: Joanne L. Yu, Sunil Verma, J. Charles Victor
Final approval of manuscript: Joanne L. Yu, Sunil Verma, Christine Simmons, Natasha B. Leighl, J. Charles Victor, Dolly Han, Sophie Hogeveen
References
- 1.Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute, based on November 2009 SEER data submission, posted to the SEER Web site, 2010; 1975–2007. [accessed February 27, 2011]. Available at http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 2.Souquet PJ, Chauvin F, Boissel JP, et al. Polychemotherapy in advanced non small cell lung cancer: A meta-analysis. Lancet. 1993;342:19–21. doi: 10.1016/0140-6736(93)91882-m. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–4291. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 6.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 7.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 8.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 10.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 trial) [corrected] J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 12.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Gridelli C, Shepherd FA. Chemotherapy for elderly patients with non-small cell lung cancer: A review of the evidence. Chest. 2005;128:947–957. doi: 10.1378/chest.128.2.947. [DOI] [PubMed] [Google Scholar]
- 15.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 16.Moore R, Doherty D, Chamberlain R, et al. Sex differences in survival in non-small cell lung cancer patients 1974–1998. Acta Oncol. 2004;43:57–64. [PubMed] [Google Scholar]
- 17.Patel JD, Bach PB, Kris MG. Lung cancer in US women: A contemporary epidemic. JAMA. 2004;291:1763–1768. doi: 10.1001/jama.291.14.1763. [DOI] [PubMed] [Google Scholar]
- 18.al-Kattan K, Sepsas E, Townsend ER, et al. Factors affecting long term survival following resection for lung cancer. Thorax. 1996;51:1266–1269. doi: 10.1136/thx.51.12.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hespanhol V, Queiroga H, Magalhaes A, et al. Survival predictors in advanced non-small cell lung cancer. Lung Cancer. 1995;13:253–267. doi: 10.1016/0169-5002(95)00497-1. [DOI] [PubMed] [Google Scholar]
- 20.Rasco DW, Yan J, Xie Y, et al. Looking beyond surveillance, epidemiology, and end results: Patterns of chemotherapy administration for advanced non-small cell lung cancer in a contemporary, diverse population. J Thorac Oncol. 2010;5:1529–1535. doi: 10.1097/JTO.0b013e3181e9a00f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wassenaar TR, Eickhoff JC, Jarzemsky DR, et al. Differences in primary care clinicians' approach to non-small cell lung cancer patients compared with breast cancer. J Thorac Oncol. 2007;2:722–728. doi: 10.1097/JTO.0b013e3180cc2599. [DOI] [PubMed] [Google Scholar]
- 22.Booth CM, Mackillop WJ. Translating new medical therapies into societal benefit: The role of population-based outcome studies. JAMA. 2008;300:2177–2179. doi: 10.1001/jama.300.18.2177. [DOI] [PubMed] [Google Scholar]