Differences in depression severity and rates of positive screens for major depressive disorder among newly diagnosed patients with stage IV non-small cell lung cancer and known epidermal growth factor receptor (EGFR) genotype were examined. EGFR mutations were associated with lower depression severity and lower rates of probable major depressive disorder.
Keywords: Non-small cell, Genotyping, Epidermal growth factor receptor, Depression, Major depressive disorder
Abstract
Introduction.
Depression appears to be associated with worse survival from cancer, but underlying mechanisms for this association are unknown. In the present study, we explored the degree to which tumor genotype may be associated with depression in patients with non-small cell lung cancer (NSCLC). We examined differences in depression severity and rates of positive screens for major depressive disorder among newly diagnosed patients with stage IV NSCLC and known epidermal growth factor receptor (EGFR) genotype.
Methods.
Newly diagnosed patients (n = 53) with metastatic NSCLC attending an initial thoracic oncology consultation completed self-report questionnaires regarding demographics, smoking behavior, and depression before meeting with their oncologist. Biopsy samples were subsequently genotyped, including screening for EGFR mutations. We conducted a retrospective chart review to obtain clinical data, including tumor stage, performance status, and EGFR genotype.
Results.
Twelve patients (22.6%) tested positive for EGFR mutation. No EGFR mutation–positive cases met the screening criteria for major depressive disorder, in comparison with 29.3% of patients with wild-type EGFR (p = .03). Mutations of EGFR were also associated with lower depression severity than with wild-type EGFR, independent of gender, performance status, and smoking history (p < .05). This finding persisted for both the cognitive–affective and somatic domains of depression symptoms.
Conclusions.
EGFR mutations were associated with lower depression severity and lower rates of probable major depressive disorder in patients with metastatic NSCLC, based on mood screening performed before results of genotyping were known. Findings support further work to explore the directionality of the associations and potential biological pathways to depression.
Introduction
Depression appears to be associated with worse survival in cancer patients [1, 2]. Several mechanisms for this association have been suggested, such as greater rates of health risk behaviors and chronic stress activation among depressed patients [3, 4]. However, depression may also possibly be linked biologically to more aggressive or treatment-resistant tumors. Although recent advances in genetic testing and molecular identification of tumor cells have resulted in better responses to targeted cancer treatments, these factors have yet to be examined in studies of depression and cancer survival.
Tumor genotyping is becoming increasingly common in the standard clinical care of patients with metastatic non-small cell lung cancer (NSCLC), a population in which we previously found a significant association between depression and worse survival [5]. In our clinical experience, we noted that patients with stage IV NSCLC and epidermal growth factor receptor (EGFR) mutations were rarely referred for psychiatric evaluations for depression, in contrast to those with wild-type EGFR. Because individuals with EGFR tumor mutations have a longer survival duration with and without targeted therapies [6–10], we hypothesized that these patients might be less depressed given their awareness of the favorable prognosis. However, a biological connection between EGFR mutation status and depression could exist, which requires further study. Although some evidence shows that cancer-related symptoms are associated with certain genetic polymorphisms in individuals [11–14], the relationships of such symptoms to the genetics of the tumor itself have not yet been explored. After our institution began routine tumor genotyping for all patients with stage IV NSCLC in 2009, we had the opportunity to test these hypotheses prospectively by assessing for depression in newly diagnosed patients before the results of the genotyping were known.
Thus, if EGFR mutation status is associated with depression even before the results of NSCLC tumor genotyping are known, a biologic connection likely exists between depression and tumors with worse prognoses. In that case, EGFR genotype may partially explain the observed relationship between depression and survival in patients with metastatic NSCLC.
Materials and Methods
Procedure
In 2006–2010, we invited all new patients in the multidisciplinary thoracic oncology clinic at the Massachusetts General Hospital Cancer Center to participate in a study of cancer-related symptoms, including depression. Eligible and willing patients signed internal review board–approved informed consent forms prior to participation. Patients completed self-report questionnaires regarding physical and psychological symptoms at the initial clinic consultation; trained study staff extracted additional demographic and clinical data from electronic medical records. The rate of enrollment of new patients was approximately 85%. Our institutional review board approved the study procedures prior to initiation.
In March 2009, the Massachusetts General Hospital Cancer Center began implementing routine clinical genotyping of all stage IV NSCLC biopsies. For patients who enrolled in the cancer-related symptoms study following this implementation, we obtained genotyping results from the electronic medical records once they became available, per participant consent.
In the current analysis, we used demographic, clinical, and symptom data as well as genotyping results from the first 70 consecutive patients with stage IV NSCLC who participated in the symptoms study and had genotyping ordered. Routine genotyping for the clinic population allowed us to avoid potential sampling bias associated with testing specific at-risk groups [15]. Also, because genotyping results were typically available 3–5 weeks following the initial oncology consultation, this lag period ensured that participants completed the self-report assessments of depression before knowing their EGFR genotype. Because depression treatment (i.e., antidepressant medication or psychotherapy) could obscure an association between EGFR mutation status and depression severity, we excluded patients in depression treatment at the time of the initial clinic visit.
Measures
Depression
As part of the symptom study, we assessed for depression using the nine-item depression scale of the Patient Health Questionnaire. This self-report instrument was developed to screen for major depressive disorder in primary care using the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders IV [16]. Investigators have since validated and used the measure in studies of patients with cancer [17, 18]. In the current study, scores were evaluated as continuous values to assess symptom severity and categorically to identify probable cases of major depressive disorder (i.e., scores ≥10). This cutoff has shown good sensitivity (88%) and specificity (88%) for clinician-diagnosed major depressive disorder in medical populations [19]. Also, per prior recommendations, cases with two or fewer missing scale items were retained for analysis by substituting missing values with the average score of the nonmissing items, whereas cases with more than missing items were excluded from the analysis [20].
Genotype Profile
EGFR mutation status was clinically tested with the multiplexed polymerase chain reaction–based SNaPshot platform (Applied Biosystems, Foster City, CA), which we have adapted to detect recurrent mutations in 13 key cancer genes in a U.S. Clinical Laboratory Improvement Amendments–certified fashion. Clinical pathology laboratory technicians conducted the genotyping and returned results for entry into patients' electronic medical records. For the current study, we reviewed the records to identify EGFR mutation status, our predictor of interest, as well as two other mutations that may impact response to treatment for NSCLC: KRAS mutations and ALK translocations [21, 22].
Demographic, Clinical, and Behavioral Characteristics
As part of the medical record review, we confirmed patient age, gender, race, tumor stage, and performance status at the time of initial clinic consultation. Oncology clinicians had assessed each patient using the Eastern Cooperative Oncology Group performance status anchors, which are commonly reported in cancer clinical trials [23]. Performance status anchors represent levels of functional impairment, with level 0 indicating full activity without restriction and level 4 indicating complete disability. Participants also completed self-report assessments of smoking history, including frequency and amount of tobacco use, as well as a lung cancer–specific quality of life assessment using the lung cancer subscale of the Functional Assessment of Cancer Therapy–Lung [24]. We used a revised subscale, excluding three items (change in weight, appetite, and ability to think clearly) that overlap with known symptoms of depression. The remaining four items (shortness of breathing, coughing, tightness in chest, and ease of breathing) were summed, with higher scores indicating better quality of life.
Statistical Analysis
Using standard statistical software (SPSS, version 16, SPSS Inc., Chicago, IL), we first performed two-sided independent samples t-tests (for continuous variables) and χ2 tests or Fisher's exact tests (for categorical variables) to evaluate preliminary differences between individuals with wild-type EGFR and those with EGFR mutations in demographic, clinical, and behavioral characteristics (depression severity and rate of probable major depressive disorder cases). In the event of any significant group difference in depression severity, we planned multiple linear regression analyses to evaluate whether or not the difference persisted after controlling for gender, performance status, and smoking history. Because of the sample size, we restricted the panel of control variables to these three factors given their associations with EGFR mutation [15, 25] and depression in patients with cancer [26–29], as demonstrated in prior work. The threshold for statistical significance was p ≤ .05 for all analyses.
Results
Of the first 70 consecutive patients with stage IV NSCLC who completed the psychosocial assessment at their initial clinic consultation and provided biological samples for genotyping, we excluded eight cases for the following reasons: insufficient tumor specimen for testing (n = 5), incomplete depression data (n = 2), and patient knowledge of mutation status from testing prior to the initial visit (n = 1). An additional nine cases were excluded after identifying that these patients were under treatment for depression at the time of initial consultation; no other cases were identified as having a history of depression or current depression treatment. Although the small number of patients under depression treatment precluded extensive group comparisons, preliminary analysis using Fisher's exact test indicated that the rate of depression treatment did not differ significantly by EGFR mutation status—two of 14 (14.3%) patients with EGFR mutations and seven of 48 (14.6%) patients with wild-type EGFR (p = 1.00).
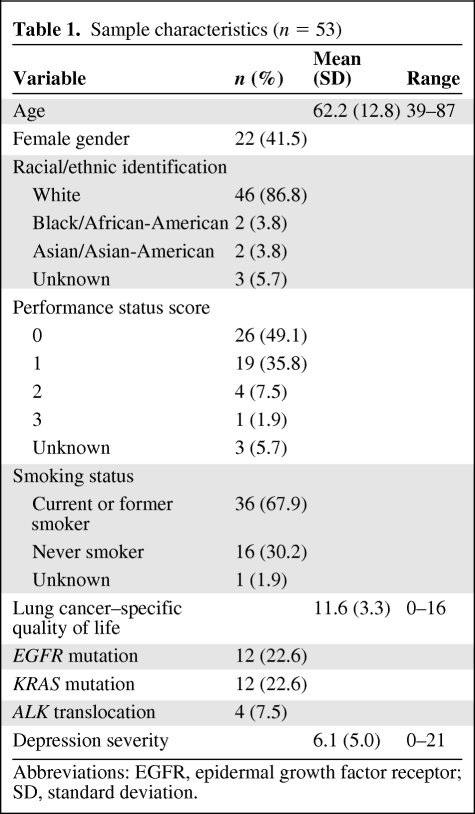
A final sample of 53 cases was included in the current analysis, consistent with sample sizes in prior studies of biological correlates of depression in cancer [30, 31]. The mean age of patients was 62.2 years (standard deviation [SD], 12.8 years) (Table 1). Over half of the participants were male (58.5%), and the majority identified as non-Hispanic white (86.8%). Approximately two thirds (67.9%) reported a history of smoking (at least one pack-year). Per the medical record notes of oncology clinicians, almost half (49.1%) of the participants were fully active with no restrictions (performance status score, 0). The mean depression score for the sample was 6.1 (SD, 5.0; range, 0–21), with 12 participants (22.6%) screening positive for probable major depressive disorder.
Table 1.
Sample characteristics (n = 53)
Abbreviations: EGFR, epidermal growth factor receptor; SD, standard deviation.
Associations of EGFR Mutation Status with Demographic, Clinical, and Depression Variables
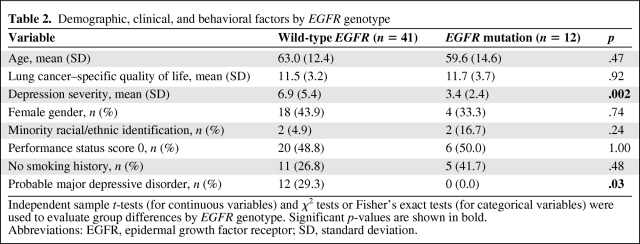
The results of genotyping indicated that 12 patients (22.6%) harbored EGFR mutations. Participants with an EGFR mutation, versus wild-type EGFR patients, did not differ significantly by age, gender, race (non-Hispanic white versus minority groups), performance status, smoking history (never-smoker versus ever-smoker), or lung cancer–specific quality of life (Table 2). However, participants with wild-type EGFR had a significantly higher depression severity than those with an EGFR mutation (wild-type EGFR mean, 6.9; SD, 5.4; mutant EGFR mean, 3.4; SD, 2.4; t = 3.25, p = .002). Additionally, the rate of positive major depressive disorder screens was 29.3% (12 of 41) for wild-type EGFR patients versus 0.0% (none of 12) for those with an EGFR mutation (Fisher's exact test, p = .03).
Table 2.
Demographic, clinical, and behavioral factors by EGFR genotype
Independent sample t-tests (for continuous variables) and χ2 tests or Fisher's exact tests (for categorical variables) were used to evaluate group differences by EGFR genotype. Significant p-values are shown in bold.
Abbreviations: EGFR, epidermal growth factor receptor; SD, standard deviation.
Of note, KRAS mutations and ALK translocations were mutually exclusive from each other and from EGFR mutations, occurring in 22.6% and 7.8% of the sample, respectively. The mean depression severity scores were 6.0 (SD, 6.1) in the KRAS mutation group (probable major depressive disorder cases, 16.7%) and 7.3 (SD, 4.9) in the ALK translocation group (probable major depressive disorder cases, 50%). KRAS mutation status (i.e., mutation versus wild-type) was not associated with a significant difference in the rate of probable major depressive disorder cases (Fishers exact test, p = .71) or in depression severity (t = 0.09; p = .93). The small number of ALK translocations precluded meaningful tests of differences.
Independent Association of EGFR Mutation Status with Depression Severity
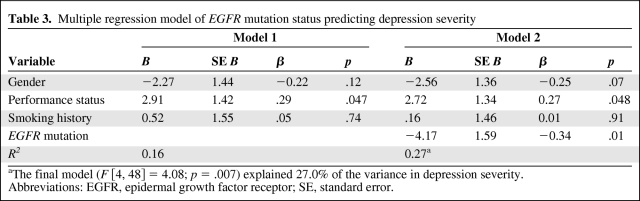
In a multiple linear regression model of depression severity that controlled for gender, performance status, and smoking history (never-smoker versus ever-smoker), EGFR mutations remained significantly associated with a lower depression severity (unstandardized β = −4.17; t = −2.63; p = .01) (Table 3). Among the three control variables, only performance status was also associated with depression severity, such that those with a performance status score greater than zero had significantly higher depression severity than those with a performance status score of zero (unstandardized β = 2.72; t = −2.03; p < .05). The overall model was significant (F [4, 48] = 4.08; p = .007) and explained 27.0% of the variance in depression severity, of which 11.5% was accounted for by EGFR mutation status.
Table 3.
Multiple regression model of EGFR mutation status predicting depression severity
aThe final model (F [4, 48] = 4.08; p = .007) explained 27.0% of the variance in depression severity.
Abbreviations: EGFR, epidermal growth factor receptor; SE, standard error.
We conducted post hoc analyses to examine whether or not the association of EGFR mutation status with depression severity would persist for both the cognitive–affective (depressed mood, anhedonia, guilt, suicidality) and somatic (disrupted sleep, appetite, energy, and concentration, as well as psychomotor retardation/amplification) symptom domains. After controlling for gender, performance status, and smoking history, we observed the same pattern of results across the two domains. Specifically, EGFR mutations remained associated not only with lower scores for the cognitive–affective symptom composite (unstandardized β = −1.71; t = −2.25; p = .03), accounting for 9.4% of the variance in this domain, but also with lower scores for the somatic symptom composite (unstandardized β = −2.46; t = −2.31; p = .03), explaining 8.9% of the variance in this domain.
Discussion
We have shown that, among 53 patients with stage IV NSCLC at their initial oncology clinic presentation prior to genotype testing, none of the patients whose tumors harbored EGFR mutations met the screening criteria for major depressive disorder, compared with almost one third of those with wild-type EGFR tumors. Patients with EGFR mutations also had significantly lower depression symptom severity scores than those with wild-type EGFR tumors, independent of gender, smoking history, and performance status. This association persisted for both the cognitive–affective and somatic domains of depression, indicating that the link between mutation status and depression was not limited to physical symptoms. In addition, the correlation might possibly explain an association between depression and survival in patients with advanced NSCLC. However, we were unable to test this hypothesis directly because many in the sample sought second opinion consultations only and received cancer care outside our institution, precluding longitudinal follow-up for survival data.
Several factors support our preliminary findings. First, recruitment from a clinic that routinely conducts genotype screening reduced sampling bias associated with testing specific groups who are at greater likelihood of demonstrating an EGFR mutation, such as women and those with no or minimal smoking history [15, 25]. Furthermore, participants completed the depression assessment approximately 3–5 weeks prior to receipt of genotyping results, ensuring that depression scores were not influenced by patient or provider knowledge of mutation status. Because EGFR mutations are associated with a better prognosis [6–10], learning about a wild-type EGFR status may possibly increase the risk for psychological distress over the course of the disease, an alternative hypothesis that we were able to rule out based on the timing of our assessments. Finally, other genetic mutations associated with response to treatments for NSCLC (i.e., KRAS and ALK) were not associated with depression, supporting the specificity of the relationship with EGFR status.
Despite the strengths of the study, the current cross-sectional design precludes conclusions about causality. Although EGFR mutations may reduce the risk for depression, chronic depression may possibly be associated with a higher risk for developing wild-type EGFR tumors. Epidemiological data have yielded mixed results for depression being associated with a higher risk for lung cancer, but these data did not subdivide the lung cancers by EGFR genotype [32, 33]. Nonetheless, results from large clinical trials of advanced NSCLC patients have shown that EGFR tyrosine kinase inhibitors have beneficial effects on mood. Specifically, investigators have demonstrated that EGFR tyrosine kinase inhibitors significantly improve quality of life, including emotional well-being, among individuals with advanced lung cancer regardless of tumor response or EGFR mutation status [34].
Biological plausibility for an association between depression and NSCLC EGFR genotype might be provided by the role of EGFR outside carcinogenesis. EGFR is critical in the suprachiasmatic nucleus of the hypothalamus, the master pacemaker controlling circadian rhythms. Activation of EGFR in the suprachiasmatic nucleus by tumor growth factors may lead to the development of cancer-related symptoms through circadian rhythm disturbances [35]. Transforming growth factor (TGF)-α is a ligand of EGFR, stimulating the signaling cascade that leads to both tumor development and growth in NSCLC as well as circadian rhythm disruptions in the suprachiasmatic nucleus. In preclinical animal models, the administration of exogenous TGF-α resulted in circadian rhythm disturbances and seemingly depressive behaviors (i.e., less activity and less eating) [36]. Similarly, among patients with metastatic colorectal cancer, researchers have found an association between the serum level of TGF-α and circadian rhythm disturbances [37]. In that study, colorectal cancer patients with higher serum levels of TGF-α also had higher depression scores on the Hospital Anxiety and Depression Scale, although that difference did not reach statistical significance. Recent studies have found that high levels of TGF-α in the serum of patients with NSCLC are predictive of nonresponse to EGFR tyrosine kinase inhibitors [38, 39]. Interestingly, some reports show that patients with NSCLC tumors with EGFR mutations have nondetectable serum levels of TGF-α, which could possibly reduce their risk for depression [40–43]. Thus, the observed improvement in mood and quality of life in patients with NSCLC unscreened for EGFR mutations who receive tyrosine kinase inhibitors could be a result of blocking the activation of EGFR in the suprachiasmatic nucleus by TGF-α, though this hypothesis requires further study.
Alternative pathways are plausible. Somatic depression symptoms are also hallmarks of sickness behavior, a syndrome that includes hyperalgesia, fatigue, and disturbances in appetite, sleep, cognitive functioning, and psychomotor behavior [44], but does not include not depressed cognitions, anhedonia, or changes in mood. Current findings thus support the association of mutation status with depression but do not rule out an association of mutation status with the induction of sickness behavior. Future work should test the role of TGF-α in concert with inflammatory cytokines that have been implicated in sickness behavior [45–47].
Although the findings of this study are provocative, a number of limitations deserve mention. We did not administer diagnostic interviews, the gold standard for evaluating major depressive disorder. However, the depression scale of the Patient Health Questionnaire is based on the Diagnostic and Statistical Manual of Mental Disorders IV criteria for major depressive disorder and assesses the same symptoms as structured diagnostic interviews, with high specificity and sensitivity for this disorder as diagnosed via interview [16, 19]. In addition, we did not collect past histories of depression, which may have helped clarify the directionality of the observed association. Although the results support that EGFR mutation status is associated with greater depression severity independently of three key risk factors for both EGFR mutation status and depression, the current sample size precluded a more extensive panel of control variables. Post hoc power calculations indicated 70.6% power to detect the current difference in depression severity between patients with EGFR mutations and wild-type EGFR patients (effect size = .84) at α = .05%. However, further work is still needed to replicate the findings using diagnostic interviews for past and present major depressive disorder in larger samples that can support the examination of more potential confounders. Finally, EGFR mutation status explained only a small portion of the variance in depression scores, highlighting the point that multiple risk factors likely account for the development of depression in patients with stage IV NSCLC, some of which may have much greater importance.
Conclusion
Despite current limitations, this study warrants further investigation of associations between cancer-related symptoms and tumor genotypes. The identification of associations would have broad implications for the treatment of cancer-related symptoms. In addition to the use of genotyping for the selection of anticancer therapies, such tests could help detect symptoms like depression that would otherwise be commonly missed during routine oncology visits [48, 49]. Greater awareness that a patient with a particular tumor genotype may be more likely to have depression could guide clinicians to be more sensitive to the presence of potential symptoms. Additionally, existing placebo-controlled trials of antidepressants for depression in individuals with cancer have not shown benefit, suggesting that the pathophysiology of depression comorbid with cancer may differ from that in patients without cancer [50–52]. Clarifying the underlying mechanisms of the associations between symptoms and tumor genotypes would undeniably aid in the development of more effective interventions that target the molecular pathways of symptoms, greatly reducing suffering from cancer. The treatment of cancer-related symptoms would enter the realm of targeted therapies, similar to treatments for the cancer itself.
Acknowledgment
This work was supported by NIH grant CA115908 (Pirl). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Conception/Design: William F. Pirl, Jennifer S. Temel
Provision of study material or patients: William F. Pirl, Heather Bemis, Emily Gallagher, Jennifer S. Temel
Collection and/or assembly of data: William F. Pirl, Heather Bemis, Emily Gallagher
Data analysis and interpretation: William F. Pirl, Lara Traeger, Joseph A. Greer, Inga Lennes, Jennifer S. Temel, Rebecca Heist, Lecia Sequist
Manuscript writing: William F. Pirl, Lara Traeger, Joseph A. Greer, Heather Bemis, Emily Gallagher, Inga Lennes, Jennifer S. Temel, Rebecca Heist, Lecia Sequist
Final approval of manuscript: William F. Pirl, Lara Traeger, Joseph A. Greer, Heather Bemis, Emily Gallagher, Inga Lennes, Jennifer S. Temel, Rebecca Heist, Lecia Sequist
References
- 1.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 2.Pinquart M, Duberstein PR. Depression and cancer mortality: A meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 4.Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 5.Pirl WF, Temel JS, Billings A, et al. Depression after diagnosis of advanced non-small cell lung cancer and survival: A pilot study. Psychosomatics. 2008;49:218–224. doi: 10.1176/appi.psy.49.3.218. [DOI] [PubMed] [Google Scholar]
- 6.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 7.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 8.Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: Results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5273. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YJ, Park IK, Park MS, et al. Activating mutations within the EGFR kinase domain: A molecular predictor of disease-free survival in resected pulmonary adenocarcinoma. J Cancer Res Clin Oncol. 2009;135:1647–1654. doi: 10.1007/s00432-009-0611-7. [DOI] [PubMed] [Google Scholar]
- 10.Wu M, Zhao J, Song SW, et al. EGFR mutations are associated with prognosis but not with the response to first-line chemotherapy in the Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2010;67:343–347. doi: 10.1016/j.lungcan.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Aouizerat BE, Dodd M, Lee K, et al. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biol Res Nurs. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- 12.Collado-Hidalgo A, Bower JE, Ganz PA, et al. Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain Behav Immun. 2008;22:1197–1200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reyes-Gibby CC, Wu X, Spitz M, et al. Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncol. 2008;9:777–785. doi: 10.1016/S1470-2045(08)70197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jatoi A, Qi Y, Kendall G, et al. The cancer anorexia/weight loss syndrome: Exploring associations with single nucleotide polymorphism (SNPs) of inflammatory cytokines in patients with non-small cell lung cancer. Support Care Cancer. 2010;18:1299–1304. doi: 10.1007/s00520-009-0748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 17.Ell K, Xie B, Quon B, et al. Randomized controlled trial of collaborative care management of depression among low-income patients with cancer. J Clin Oncol. 2008;26:4488–4496. doi: 10.1200/JCO.2008.16.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psycooncology. 2009;18:14–22. doi: 10.1002/pon.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Löwe B, Schenkel I, Carney-Doebbeling C, et al. Responsiveness of the PHQ-9 to psychopharmacological depression treatment. Psychosomatics. 2006;47:62–67. doi: 10.1176/appi.psy.47.1.62. [DOI] [PubMed] [Google Scholar]
- 21.Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15:5216–5223. doi: 10.1158/1078-0432.CCR-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–1733. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 23.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 24.Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 25.Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: Biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 26.Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90:2297–2304. doi: 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurtz ME, Kurtz JC, Stommel M, et al. Physical functioning and depression among older persons with cancer. Cancer Pract. 2001;9:11–18. doi: 10.1046/j.1523-5394.2001.91004.x. [DOI] [PubMed] [Google Scholar]
- 28.LoConte NK, Else-Quest NM, Eickhoff J, et al. Assessment of guilt and shame in patients with non-small-cell lung cancer compared with patients with breast and prostate cancer. Clin Lung Cancer. 2008;9:171–178. doi: 10.3816/CLC.2008.n.026. [DOI] [PubMed] [Google Scholar]
- 29.Cooley ME, Sarna L, Brown JK, et al. Tobacco use in women with lung cancer. Ann Behav Med. 2007;33:242–250. doi: 10.1007/BF02879906. [DOI] [PubMed] [Google Scholar]
- 30.Musselman DL, Miller AH, Porter MR, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: Preliminary findings. Am J Psychiatry. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 31.Lutgendorf SK, Lamkin DM, DeGeest K, et al. Depressed and anxious mood and T-cell cytokine expressing populations in ovarian cancer patients. Brain Behav Immun. 2008;22:890–900. doi: 10.1016/j.bbi.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knekt P, Raitasalo R, Heliövaara M, et al. Elevated lung cancer risk among persons with depressed mood. Am J Epidemiol. 1996;144:1096–1103. doi: 10.1093/oxfordjournals.aje.a008887. [DOI] [PubMed] [Google Scholar]
- 33.Gross AL, Gallo JJ, Eaton WW. Depression and cancer risk: 24 years of follow-up of the Baltimore Epidemiologic Catchment Area sample. Cancer Causes Control. 2010;21:191–199. doi: 10.1007/s10552-009-9449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bezjak A, Tu D, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: Quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2006;24:3831–3837. doi: 10.1200/JCO.2006.05.8073. [DOI] [PubMed] [Google Scholar]
- 35.Rich TA. Symptom clusters in cancer patients and their relation to EGFR ligand modulation of the circadian axis. J Support Oncol. 2007;5:167–174. [PubMed] [Google Scholar]
- 36.Gilbert J, Davis FC. Behavioral effects of systemic transforming growth factor-alpha in Syrian hamsters. Behav Brain Res. 2009;198:440–448. doi: 10.1016/j.bbr.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rich T, Innominato PF, Boerner J, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–1763. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 38.Addison CL, Ding K, Zhao H, et al. Plasma transforming growth factor alpha and amphiregulin protein levels in NCIC Clinical Trials Group BR.21. J Clin Oncol. 2010;28:5247–5256. doi: 10.1200/JCO.2010.31.0805. [DOI] [PubMed] [Google Scholar]
- 39.Vollebergh MA, Kappers I, Klomp HM, et al. Ligands of epidermal growth factor receptor and the insulin-like growth factor family as serum biomarkers for response to epidermal growth factor receptor inhibitors in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1939–1948. doi: 10.1097/JTO.0b013e3181f77a39. [DOI] [PubMed] [Google Scholar]
- 40.Fukuyama T, Ichiki Y, Yamada S, et al. Cytokine production of lung cancer cell lines: Correlation between their production and the inflammatory/immunological responses both in vivo and in vitro. Cancer Sci. 2007;98:1048–1054. doi: 10.1111/j.1349-7006.2007.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masago K, Fujita S, Hatachi Y, et al. Clinical significance of pretreatment serum amphiregulin and transforming growth factor-alpha, and an epidermal growth factor somatic mutation in patients with advanced non-squamous, non-small cell lung cancer. Cancer Sci. 2008;99:2295–2301. doi: 10.1111/j.1349-7006.2008.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volante M, Saviozzi S, Rapa I, et al. Epidermal growth factor ligand/receptor loop and downstream signaling activation pattern in completely resected nonsmall cell lung cancer. Cancer. 2007;110:1321–1328. doi: 10.1002/cncr.22903. [DOI] [PubMed] [Google Scholar]
- 43.Yonesaka K, Zejnullahu K, Lindeman N, et al. Autocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancers. Clin Cancer Res. 2008;14:6963–6973. doi: 10.1158/1078-0432.CCR-08-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raison CL, Miller AH. Depression in cancer: New developments regarding diagnosis and treatment. Biol Psychiatry. 2003;54:283–294. doi: 10.1016/s0006-3223(03)00413-x. [DOI] [PubMed] [Google Scholar]
- 45.Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: The febrile response. Trends Neurosci. 1997;12:565–570. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- 46.Dantzer R, Bluthé RM, Gheusi G, et al. Molecular basis of sickness behavior. Ann N Y Acad Sci. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- 47.Kent S, Bret-Dibat JL, Kelley KW, et al. Mechanisms of sickness-induced decreases in food-motivated behavior. Neurosci Biobehav Rev. 1996;20:171–175. doi: 10.1016/0149-7634(95)00037-f. [DOI] [PubMed] [Google Scholar]
- 48.Passik SD, Dugan W, McDonald MV, et al. Oncologists' recognition of depression in their patients with cancer. J Clin Oncol. 1998;16:1594–1600. doi: 10.1200/JCO.1998.16.4.1594. [DOI] [PubMed] [Google Scholar]
- 49.Newell S, Sanson-Fisher RW, Girgis A, et al. How well do medical oncologists' perceptions reflect their patients' reported physical and psychosocial problems? Data from a survey of five oncologists. Cancer. 1998;83:1640–1651. [PubMed] [Google Scholar]
- 50.Razavi D, Allilaire JF, Smith M, et al. The effect of fluoxetine on anxiety and depression symptoms in cancer patients. Acta Psychiatr Scand. 1996;94:205–210. doi: 10.1111/j.1600-0447.1996.tb09850.x. [DOI] [PubMed] [Google Scholar]
- 51.Musselman DL, Somerset WI, Guo Y, et al. A double-blind, multicenter, parallel-group study of paroxetine, desipramine, or placebo in breast cancer patients (stages I, II, III, and IV) with major depression. J Clin Psychiatry. 2006;67:288–296. doi: 10.4088/jcp.v67n0217. [DOI] [PubMed] [Google Scholar]
- 52.Stockler MR, O'Connell R, Nowak AK, et al. Effect of sertraline on symptoms and survival in patients with advanced cancer, but without major depression: A placebo-controlled double-blind randomised trial. Lancet Oncol. 2007;8:603–612. doi: 10.1016/S1470-2045(07)70148-1. [DOI] [PubMed] [Google Scholar]