Factors associated with long-term altered well-being after prophylactic bilateral salpingo-oophorectomy, namely, lower quality of life, altered sexual functioning, greater anxiety, and more endocrine symptoms, were identified.
Keywords: Quality of life, BRCA, Hereditary breast and ovarian cancer, Prophylactic oophorectomy, Menopause, Sexual function
Learning Objectives
After completing this course, the reader will be able to:
Describe factors associated with decreased well-being after PBSO in order to prospectively identify patients at risk.
Provide pre-operative counseling and information to patients at risk of decreased well-being after PBSO.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
Prophylactic bilateral salpingo-oophorectomy (PBSO) might alter several components of well-being, such as sexual functioning and endocrine symptoms, in women at high risk for hereditary breast and/or ovarian cancer, compared with the general population. We searched for factors associated with altered long-term well-being in this population (lower quality of life [QOL], altered sexual functioning, greater anxiety, more endocrine symptoms).
Methods.
All high-risk women who had undergone PBSO during the past 15 years in a single cancer center were contacted by mail. Upon acceptance, they were sent five questionnaires: (a) general social questions, (b) the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30, (c) Sexual Activity Questionnaire, (d) Functional Assessment of Cancer Therapy – Endocrine Symptom, and (5) State-Trait Anxiety Inventory. Logistic analyses were used to identify factors associated with altered results. Because of multiple testing, only p-values ≤ .01 were considered significant.
Results.
One hundred twelve of 175 women (64%) returned the completed questionnaires at a mean duration (standard deviation) of 6.0 (5.1) years after PBSO. QOL was positively influenced by two baseline factors: a high educational level and occupying an executive position. However, younger age at PBSO was associated with lower social functioning and greater anxiety. At the time of the study, practicing a sport and the avoidance of weight gain (≥10%) were highly related to QOL, sexual pleasure, endocrine symptoms, and anxiety in the univariate analysis and predictive of better QOL and lower anxiety in the multivariate analysis.
Conclusions.
Younger women and women with a low educational level and no occupation appear to be at higher risk for altered long-term well-being. After surgery, practicing a sport and stable weight may help maintain overall well-being.
Introduction
Women carriers of a BRCA1 or BRCA2 gene mutation have a substantially higher risk for breast and/or ovarian cancer. Prophylactic bilateral salpingo-oophorectomy (PBSO) is the main preventive health strategy in these patients. Its objective is to reduce the risk for both ovarian and breast cancer [1]. PBSO reduces ovarian cancer risk in BRCA1 and BRCA2 mutation carriers by 79% and breast cancer risk by 51% [2]. Risk reduction is effective whatever the BRCA mutation or history of previous breast cancer [3]. Furthermore, despite previous discussions regarding the potential long-term health consequences of early menopause [4], PBSO was recently clearly shown to reduce all-cause mortality by 60%, breast cancer-specific mortality by 56%, and ovarian cancer-specific mortality by 79% [3].
It has been reported that PBSO does not affect overall quality of life (QOL) but might alter sexual functioning and increase several endocrine symptoms in women having undergone the procedure, compared with the general population [5]. In contrast, favorable effects of PBSO in terms of lower anxiety levels and a low perceived cancer risk have been reported [5]. However, data are lacking concerning risk factors for altered results after surgery. Identifying women likely to experience a lower overall well-being after PBSO could potentially facilitate preoperative counseling and pre- or postoperative management. For this purpose, we selected both physical determinants and factors that could potentially influence their psychological well-being or worries about cancer from the available literature [5–8].
The objective of this study was therefore to search for factors associated with long-term altered well-being, namely, lower QOL, altered sexual functioning, greater anxiety, and more endocrine symptoms after PBSO.
Methods
Population
All women who had undergone PBSO at a single institution during the past 15 years because of a hereditary risk for breast or ovarian cancer were invited to participate in the study. Information letters were sent out offering them the possibility of accepting or declining participation in the study. If they accepted, they received anonymous questionnaires and a postage-paid return envelope. They were first asked general questions to evaluate their socioeconomic level, physical activity, menopausal status, and marital status before and after PBSO. Medical data were retrieved from hospital medical records. To assess generic QOL, the French version of the Cancer Quality of Life Questionnaire C30 (QLQ-C30) of the European Organization for Research and Treatment (EORTC) was used. The Sexual Activity Questionnaire (SAQ) was used to measure sexual functioning [9, 10]. Endocrine symptoms were evaluated by the Endocrine Symptom subscale of the Functional Assessment of Cancer Therapy questionnaire (FACT-ES) [11]. The level of anxiety was evaluated by the State-Trait Anxiety Inventory (STAI) [12].
Statistical Analysis
Descriptive statistics (frequencies, means, and standard deviations [SDs]) were generated to characterize the sample in terms of sociodemographic and medical variables. Before the analysis, we selected 19 clinically relevant variables from previous articles [5–8] and from sociodemographic questionnaires as factors potentially associated with “poor” postoperative results. Continuous variables were dichotomized a priori at the median. These variables were either before surgery (baseline factors) or at the time of the study (current status). Before surgery, variables were age (≥50 years or <50 years), main occupation, educational level, professional function (executive or not), childbearing history, marital status, menopausal status, antihormonal treatment, hormone replacement therapy, body mass index (≥25 kg/m2 or <25 kg/m2), sexual activity, presence of a BRCA mutation (none, BRCA1, or BRCA2), personal history of breast cancer, and history of mastectomy. At the time of the study, variables were current age (≥50 years or <50 years), marital status, practice of hobbies (no, yes <20 hours per month, yes ≥20 hours per month), practice of sport (no, yes <8 hours per month, yes ≥8 hours per month), declared weight gain ≥10% since PBSO, and interval between surgery and questionnaire (≥4 years or <4 years). Outcome variables were selected a priori: the QLQ-C30 contains 30 items combined to derive 15 scales according to the EORTC guidelines. We focused on eight scales: five functional scales (physical, role, emotional, cognitive, and social functioning), two symptom scales (fatigue and insomnia), and the global health status/QOL. The scales' range is 0–100, with high scores corresponding to a healthy/high status for the global and functional scales, but to a high level of symptoms for the symptom scales. The EORTC SAS® program (SAS Institute Inc., Cary, NC) was used to score the QLQ-C30 items. The four SAQ dimensions and scores recommended by Fallowfield were retained (pleasure, discomfort, habit, and fatigue). Thus, a total of 14 criteria (the above-mentioned 12 items plus the FACT-ES menopause symptoms and STAI A anxiety item) were considered a priori and analyzed in relation to the 19 variables described above.
Univariate logistic analysis was used to identify factors associated with altered outcomes (defined as binary variables: worst quartile versus three other quartiles). A multivariate logistic regression analysis was conducted on factors that were significant in the univariate analysis. All p-values resulting from the logistic analyses are derived from χ2 tests. All reported p-values are two-sided; p-values <1% were considered significant to take into account the multiplicity of tests. Analyses were performed with SAS®, version 9.1 (SAS Institute Inc., Cary, NC).
Results
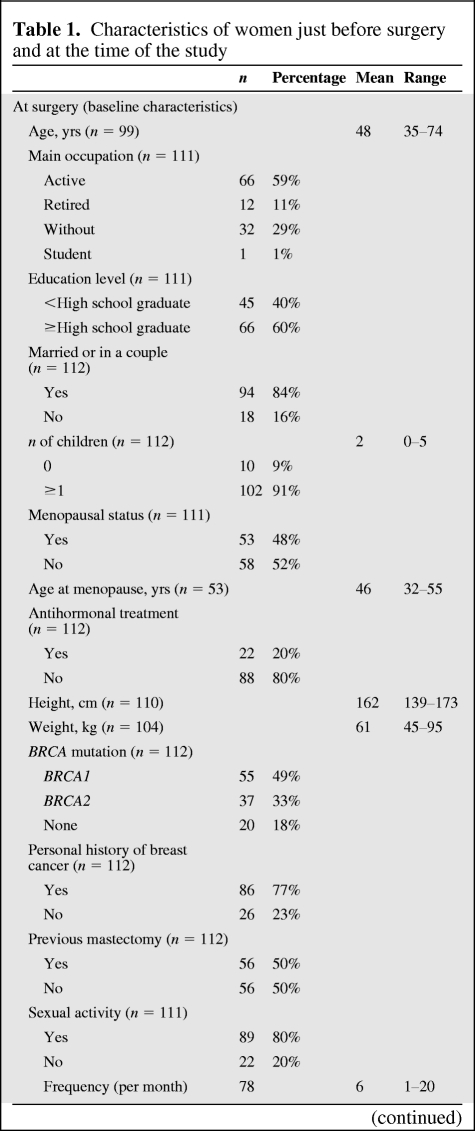
Among 175 women to whom questionnaires were sent in March 2009, 112 completed and returned the questionnaires by September 2009 (64%). PBSO had been performed via a laparoscopic approach in 99 women (88%) and via a laparotomy in 13 women (12%). The mean (SD) interval since PBSO was 6.0 (5.1) years. The mean age at PBSO was 48 (35–74) years. Fifty-five women (49%) were BRCA1 carriers, 37 (33%) were BRCA2 carriers, and 20 (18%) had no identified mutation. Eighty-six women (77%) had a personal history of breast cancer, 56 (50%) had previously undergone a mastectomy, and 23 (21%) had previously undergone a prophylactic bilateral mastectomy. The characteristics of women just before PBSO and at the time of the study are summarized in Table 1.
Table 1.
Characteristics of women just before surgery and at the time of the study
Descriptive Analysis: QOL, Sexual Functioning, Anxiety, and Endocrine Symptoms
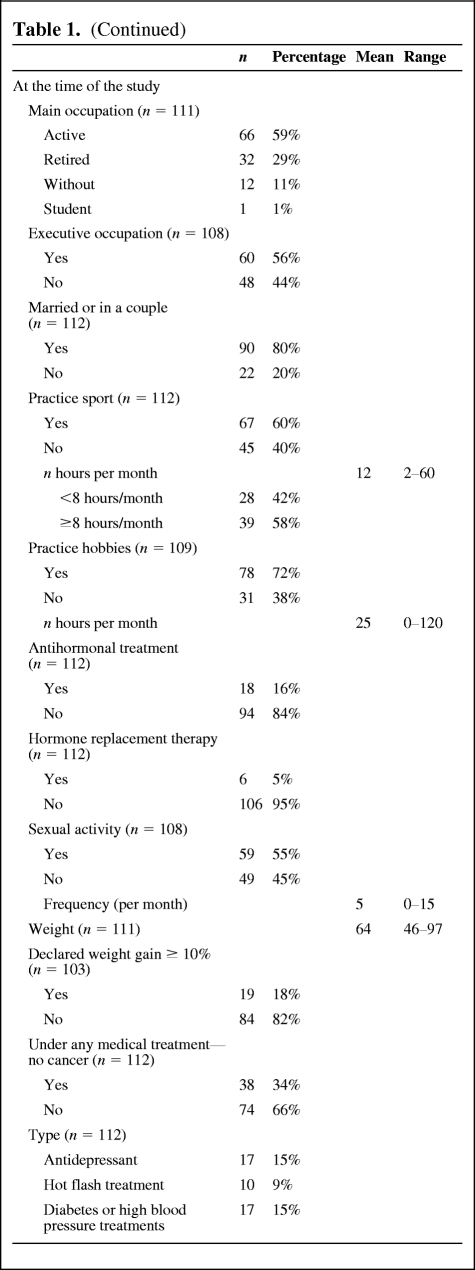
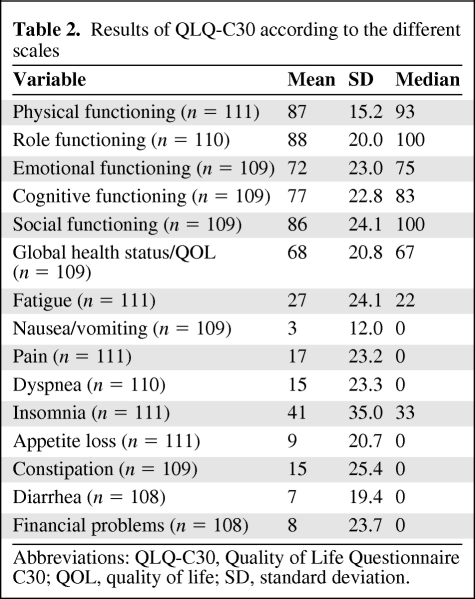
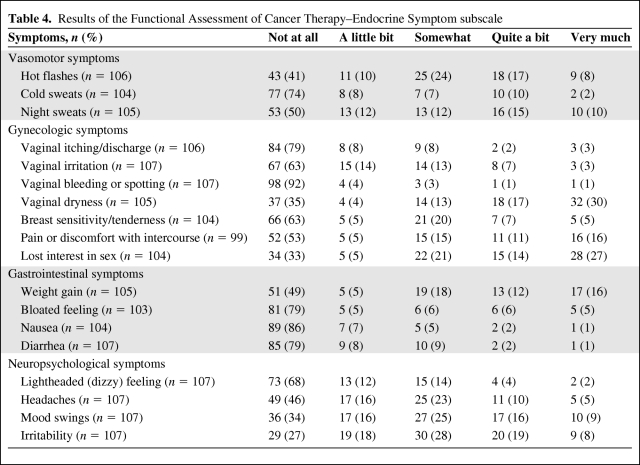
The results of the QLQ-C30 are reported in Table 2. Overall, global and specific QOL was not lower than the EORTC standard. The results of the SAQ are reported in Table 3. Fifty-nine patients (55%) were sexually active. The first reason explaining the sexual inactivity of the other 45% was a lack of interest in sex (42%). Long-term assessment of endocrine symptoms revealed a mean score of 18.2 (0–48). The most frequent complaints were vaginal dryness (47%) and loss of interest in sex (41%), as detailed in Table 4. Major vasomotor symptoms (quite a bit and very much) were reported by 25% of the women. The mean anxiety level score was 41.2 (20–73), which is considered a low anxiety level. The anxiety level was very low for 33%, low for 31%, intermediate for 20%, high for 13%, and very high for 4% of the women.
Table 2.
Results of QLQ-C30 according to the different scales
Abbreviations: QLQ-C30, Quality of Life Questionnaire C30; QOL, quality of life; SD, standard deviation.
Table 3.
Results of the SAQ
Abbreviations: SAQ, Sexual Activity Questionnaire; SD, standard deviation.
Table 4.
Results of the Functional Assessment of Cancer Therapy–Endocrine Symptom subscale
Factors Associated with QOL, Sexual Functioning, Anxiety, and Endocrine Symptoms
Baseline Factors
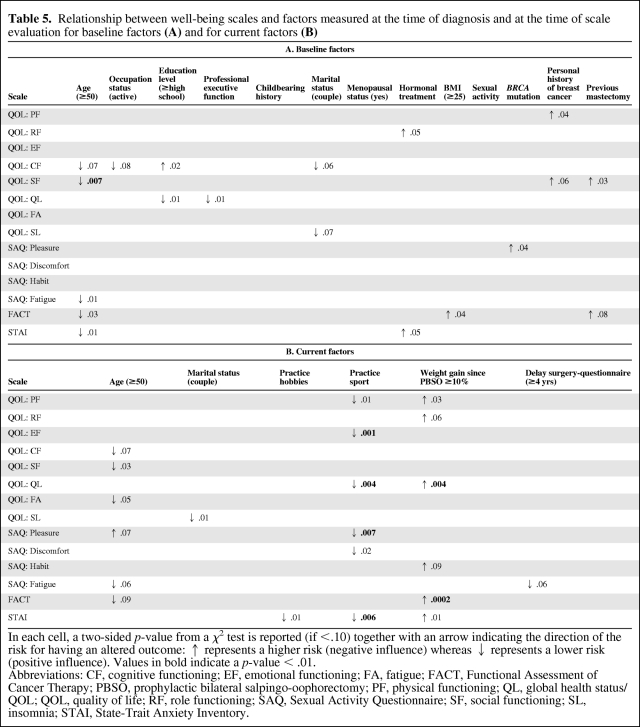
A high educational level (p = .01) and occupying an executive position (p = .01) at PBSO positively influenced the global health status (Table 5A). A younger age at PBSO was specifically associated with lower social functioning (p = .007) and greater anxiety (p = .01). None of the 10 other baseline factors tested were significantly associated with QOL parameters, including the BRCA status and a history of breast cancer. For example, a trend toward lower physical functioning and social functioning in the QLQ-C30 questionnaire was observed for women with a history of breast cancer, but this was not statistically significant (p = .04 and p = .06, respectively). A history of mastectomy did not influence sexual pleasure (p = .9) or social functioning (p = .03).
Table 5.
Relationship between well-being scales and factors measured at the time of diagnosis and at the time of scale evaluation for baseline factors (A) and for current factors (B)
In each cell, a two-sided p-value from a χ2 test is reported (if <.10) together with an arrow indicating the direction of the risk for having an altered outcome: ↑ represents a higher risk (negative influence) whereas ↓ represents a lower risk (positive influence). Values in bold indicate a p-value < .01.
Abbreviations: CF, cognitive functioning; EF, emotional functioning; FA, fatigue; FACT, Functional Assessment of Cancer Therapy; PBSO, prophylactic bilateral salpingo-oophorectomy; PF, physical functioning; QL, global health status/QOL; QOL, quality of life; RF, role functioning; SAQ, Sexual Activity Questionnaire; SF, social functioning; SL, insomnia; STAI, State-Trait Anxiety Inventory.
Current Factors
Practicing a sport at the time of the study appeared to be highly related to a better global health status (p = .004), physical functioning (p = .01) and emotional functioning (p = .001), as well as greater sexual pleasure (p = .007), and less anxiety (p = .006) (Table 5B). Weight gain was associated with a lower global health status (p = .004), greater anxiety (p = .01), and greater endocrine symptoms (p = .0002). Practicing hobbies at the time of the study was associated with less anxiety (p = .01).
Multivariate Analysis
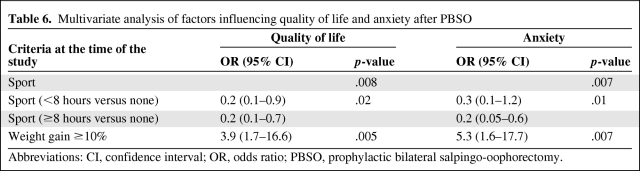
Because only QOL and anxiety had more than one statistically significant (p < .01) correlation, multivariate analyses were performed exclusively for these two scales. In the multivariate analyses, sport and the absence of weight gain were both highly predictive of a better global health status (p = .008 and p = .005, respectively) and lower anxiety (p = .007 and p = .007, respectively). Furthermore, >8 hours of sport was also significantly associated with a better global health status and lower anxiety than with <8 hours of sport (p = .02 and p = .01, respectively) (Table 6).
Table 6.
Multivariate analysis of factors influencing quality of life and anxiety after PBSO
Abbreviations: CI, confidence interval; OR, odds ratio; PBSO, prophylactic bilateral salpingo-oophorectomy.
Discussion
In this long-term observational study among women who had undergone PBSO for a high risk for breast and ovarian cancer, overall QOL results did not differ from the standard reference EORTC results [5, 8]. Likewise, the anxiety level appeared to be reasonably low. This is in line with previous reports in such populations of women who opted for prophylactic surgery [5, 13–15]. However, this population appeared to have high endocrine symptom scores and lower sexual functioning. This has also been more or less described in other publications [5, 7, 8, 15, 16].
We showed that a high educational level and occupying an executive position at the time of PBSO positively influenced QOL. In the literature, a history of cancer was the only risk factor reported for a lower QOL [6]. In the present study, a history of breast cancer tended to result in lower physical functioning and social functioning on the QLQ-C30 questionnaire, but this was not statistically significant (p = .04 and p = .06, respectively). This could be explained by the high proportion (77%) of women with a history of cancer in our study. Similarly, a history of mastectomy tended to lead to lower social functioning on the QLQ-C30 questionnaire, but this was not statistically significant (p = .03). We think that this information could be really clinically relevant. Several women spontaneously complained of this condition in the questionnaire because of its psychological impact on their body image.
The women in our series reported fewer endocrine symptoms than those described in previous series [5, 7]. The main difference between our study and those series [5, 7] is the mean interval since PBSO, which were 1.9 years and 2.8 years of follow-up after PBSO, versus 6 years in our study. We assume that such symptoms may be alleviated over time. Finch et al. [17] recently showed that women who were premenopausal at the time of prophylactic oophorectomy experienced significantly higher rates of hot flashes, night sweats, and sweating than menopausal women. Moreover, they reported that premenopausal women with a previous diagnosis of breast cancer had significantly more vasomotor symptoms before surgery than premenopausal unaffected women [17]. In our study, neither the menopausal status before PBSO nor a previous diagnosis of breast cancer influenced endocrine symptoms (Table 6). However, a young age at surgery tended to have a greater influence on sexual function (p = .001) and endocrine symptoms (p = .003) in our study (Table 6).
Although a high educational level and occupying an executive position were positively associated with QOL, in contrast, a younger age at PBSO was associated with lower social functioning and greater anxiety. Preoperative counseling may therefore be particularly important in young women or women with a low educational level and no occupation. These women should be carefully informed of possible side effects and should receive psychological care before and after PBSO.
In this study, it was not possible to identify women at risk for altered results more accurately, through the construction of a mathematical predictor or a predictive score, because of its retrospective nature and because only a few baseline factors were statistically significant.
The two major results of this study concern the influence of sports practice and weight gain on QOL and overall well-being after PBSO. Practicing a hobby was also a determinant in the univariate analysis but was not significant in the multivariate analysis. Actually, 79% of women practicing a sport also had a hobby. It is impossible to know whether the practice of sport is the cause or a consequence of a good QOL, sexual pleasure, and low anxiety. Regardless, women consulting for PBSO should be aware of this information. Their lifestyle may have an impact on their tolerance of symptoms and their QOL [18].
A prospective comparative study investigated sociodemographic, medical, and psychosocial factors associated with the use of PBSO versus screening among BRCA1 and BRCA2 mutation carriers. They found that women with lower or intermediate education levels were more likely to undergo PBSO [19]. In our series, this baseline factor was associated with lower QOL. Women in the PBSO group also had a poorer general health perception, viewed ovarian cancer as an incurable disease, and believed more strongly in the benefits of surgery [19]. Finally, women reported high levels of satisfaction with their decision to undergo surgery in several studies [7, 20, 21]. This is in accordance with their overall good QOL. Moreover, Madalinska et al. [5] reported lower breast and ovarian cancer anxiety levels in the PBSO group, and 85% of those women said they would choose PBSO again.
Conclusion
The main contribution of our work is the identification of patients at potential risk for a more altered well-being after PBSO, such as younger women, women with a low education level, and women without an occupation. We were also able to show that practicing sports, hobbies, and stable weight are associated with better outcomes. These findings open the way for improving the preoperative counseling of these women and for constructing pre- and postoperative dedicated intervention programs.
Acknowledgments
The authors would like to thank Mrs. Lorna Saint-Ange for editing the manuscript.
Author Contributions
Conception/Design: Suzette Delaloge, Cyril Touboul, Catherine Uzan, Ariane Dunant, Sarah Dauchy
Provision of study material or patients: Suzette Delaloge, Catherine Uzan, Olivier Caron, Sébastien Gouy, Brigitte Bressac de Paillerets, Philippe Morice
Collection and/or assembly of data: Cyril Touboul, Catherine Uzan, Olivier Caron
Data analysis and interpretation: Suzette Delaloge, Cyril Touboul, Catherine Uzan, Jean Laurent Ichanté, Ariane Dunant, Brigitte Bressac de Paillerets, Philippe Morice
Manuscript writing: Suzette Delaloge, Cyril Touboul, Ariane Dunant
Final approval of manuscript: Suzette Delaloge, Cyril Touboul, Catherine Uzan, Jean Laurent Ichanté, Olivier Caron, Ariane Dunant, Sarah Dauchy, Sébastien Gouy, Brigitte Bressac de Paillerets, Philippe Morice
References
- 1.Kauff ND, Barakat RR. Risk-reducing salpingo-oophorectomy in patients with germline mutations in BRCA1 or BRCA2. J Clin Oncol. 2007;25:2921–2927. doi: 10.1200/JCO.2007.11.3449. [DOI] [PubMed] [Google Scholar]
- 2.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: Long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madalinska JB, Hollenstein J, Bleiker E, et al. Quality-of-life effects of prophylactic salpingo-oophorectomy versus gynecologic screening among women at increased risk of hereditary ovarian cancer. J Clin Oncol. 2005;23:6890–6898. doi: 10.1200/JCO.2005.02.626. [DOI] [PubMed] [Google Scholar]
- 6.Michelsen TM, Dørum A, Trope CG, et al. Fatigue and quality of life after risk-reducing salpingo-oophorectomy in women at increased risk for hereditary breast-ovarian cancer. Int J Gynecol Cancer. 2009;19:1029–1036. doi: 10.1111/IGC.0b013e3181a83cd5. [DOI] [PubMed] [Google Scholar]
- 7.Robson M, Hensley M, Barakat R, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89:281–287. doi: 10.1016/s0090-8258(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 8.Fang CY, Cherry C, Devarajan K, et al. A prospective study of quality of life among women undergoing risk-reducing salpingo-oophorectomy versus gynecologic screening for ovarian cancer. Gynecol Oncol. 2009;112:594–600. doi: 10.1016/j.ygyno.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkins L, Fallowfield LJ. Fallowfield's Sexual Activity Questionnaire in women with without and at risk of cancer. Menopause Int. 2007;13:103–109. doi: 10.1258/175404507781605578. [DOI] [PubMed] [Google Scholar]
- 10.Thirlaway K, Fallowfield L, Cuzick J. The Sexual Activity Questionnaire: A measure of women's sexual functioning. Qual Life Res. 1996;5:81–90. doi: 10.1007/BF00435972. [DOI] [PubMed] [Google Scholar]
- 11.Fallowfield LJ, Leaity SK, Howell A, et al. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: Validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55:189–199. doi: 10.1023/a:1006263818115. [DOI] [PubMed] [Google Scholar]
- 12.Spielberger CD. Palo Alto, CA: Consulting Psychologists Press; 1983. Manual for the State-Trait Anxiety Inventory (STAI) [Google Scholar]
- 13.Tiller K, Meiser B, Butow P, et al. Psychological impact of prophylactic oophorectomy in women at increased risk of developing ovarian cancer: A prospective study. Gynecol Oncol. 2002;86:212–219. doi: 10.1006/gyno.2002.6737. [DOI] [PubMed] [Google Scholar]
- 14.Elit L, Esplen MJ, Butler K, et al. Quality of life and psychosexual adjustment after prophylactic oophorectomy for a family history of ovarian cancer. Fam Cancer. 2001;1:149–156. doi: 10.1023/a:1021119405814. [DOI] [PubMed] [Google Scholar]
- 15.Miller S, Roussi P, Daly MB, et al. New strategies in ovarian cancer: Uptake and experience of women at high risk of ovarian cancer who are considering risk-reducing salpingo-oophorectomy. Clin Cancer Res. 2010;16:5094–5106. doi: 10.1158/1078-0432.CCR-09-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011;125:837–847. doi: 10.1007/s10549-010-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finch A, Metcalfe KA, Chiang JK, et al. The impact of prophylactic salpingo-oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecol Oncol. 2011;121:163–168. doi: 10.1016/j.ygyno.2010.12.326. [DOI] [PubMed] [Google Scholar]
- 18.McNeely ML, Campbell KL, Rowe BH, et al. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madalinska JB, van Beurden M, Bleiker EM, et al. Predictors of prophylactic bilateral salpingo-oophorectomy compared with gynecologic screening use in BRCA1/2 mutation carriers. J Clin Oncol. 2007;25:301–307. doi: 10.1200/JCO.2006.07.4922. [DOI] [PubMed] [Google Scholar]
- 20.Fry A, Busby-Earle C, Rush R, et al. Prophylactic oophorectomy versus screening: Psychosocial outcomes in women at increased risk of ovarian cancer. Psychooncology. 2001;10:231–241. doi: 10.1002/pon.512. [DOI] [PubMed] [Google Scholar]
- 21.Swisher EM, Babb S, Whelan A, et al. Prophylactic oophorectomy and ovarian cancer surveillance. Patient perceptions and satisfaction. J Reprod Med. 2001;46:87–94. [PubMed] [Google Scholar]