The study describes hepatocellular carcinoma tumor characteristics and different therapeutic approaches, evaluates patient survival time from HCC diagnosis, and identifies clinical prognostic predictors in patients with and without HIV infection.
Keywords: Hepatocellular carcinoma, Highly active antiretroviral therapy, HIV, Hepatitis C virus, AIDS
Abstract
Purpose.
Hepatocellular carcinoma (HCC) is an increasing cause of mortality in HIV-infected patients in the highly active antiretroviral therapy (HAART) era. The aims of this study were to describe HCC tumor characteristics and different therapeutic approaches, to evaluate patient survival time from HCC diagnosis, and to identify clinical prognostic predictors in patients with and without HIV infection.
Patients and Methods.
A multicenter observational retrospective comparison of 104 HIV-infected patients and 484 uninfected patients was performed in four Italian centers. HCC was staged according to the Barcelona Clinic Liver Cancer (BCLC) criteria.
Results.
Tumor characteristics of patients with and without HIV were significantly different for age, Eastern Cooperative Oncology Group performance status (PS) score ≤1, and etiology of chronic liver disease. Despite the similar potentially curative option rate and better BCLC stage at diagnosis, the median survival time was significantly shorter in HIV+ patients. HIV+ patients were less frequently retreated at relapse.
Independent predictors of survival were: BCLC stage, potentially effective HCC therapy, tumor dimension ≤3 cm, HCC diagnosis under a screening program, HCC recurrence, and portal vein thrombosis. Restricting the analysis to HIV+ patients only, all positive prognostic factors were confirmed together with HAART exposure.
Conclusion.
This study confirms a significantly shorter survival time in HIV+ HCC patients. The less aggressive retreatment at recurrence approach does not balance the benefit of younger age and better BCLC stage and PS score of HIV+ patients. Thus, considering the prognosis of HIV+ HCC patients, effective screening techniques, programs, and specific management guidelines are urgently needed.
Introduction
With the availability of highly active antiretroviral treatment (HAART) in 1996, a remarkable decrease in HIV-related morbidity and mortality was observed [1, 2]. This decline was associated with a relative significant increase in morbidity and mortality related to many different non–HIV-related diseases, such as chronic liver diseases (CLDs) [3, 4]. End-stage liver diseases (ESLDs), which are primarily related to complications of chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) coinfection, as well as hepatotoxicity associated with antiretroviral therapy and alcohol use [5–7], now account for up to 50% of deaths among people with HIV infection. It is expected that mortality associated with HCV infection will continue to increase over the next 25 years [8, 9]. In a national survey on deaths among HIV-infected patients, liver diseases represented the third most frequent underlying cause of death [9].
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver, and according to the World Health Organization report, the fourth most common cause of death [10–12]. The risk for HCC is sevenfold higher in HIV-infected than in HIV-uninfected patients [13, 14]. Since the introduction of HAART, no decrease in the incidence of HCC has been observed, unlike for other HIV-associated cancers [11, 13, 15].
In the general population, HCC occurs several decades after the initial infection with HCV or HBV [11, 16, 17]. Although it was suspected that HIV infection alone may be a risk factor for HCC, this hypothesis seems to have been excluded in large retrospective cohort studies [15]. On the other hand, in HIV+ patients, coinfection with HCV or HBV is common, and a significantly higher risk for developing HCC as a result of chronic viral hepatitis is well documented. Little is still known about the interactions between HIV and HBV and/or HCV over the long term: HIV coinfection seems to accelerate disease progression and to reduce the efficacy of anti-HCV and anti-HBV therapies. However, it is unclear whether HIV infection directly increases the likelihood of HCC in patients with viral hepatitis [18–21]. In addition to potential indirect effects on HCC risk through improvements in immune reconstitution and survival, HAART is known to have some direct hepatotoxic effects, especially among HIV-infected patients chronically infected with HBV or HCV [7, 22].
In addition to the elevated risk for developing HCC, individuals with HIV infection may have higher HCC-related morbidity and mortality. Some studies showed that HIV–HCV coinfected patients develop liver cirrhosis more quickly than HCV monoinfected individuals, and that in these patients, HCC is more aggressive [21, 23–25]. Nevertheless, the clinical course of HCC in an HIV-infected setting is not well defined yet, because most previous studies had small sample sizes and many HIV patients were not undergoing HAART [20, 24, 26]. A large case–control study provided evidence that, at diagnosis, HCC was more advanced (infiltrating or metastatic) in HIV-infected patients than in non-HIV patients; moreover, in the HIV-infected group, HCC occurrence did not seem to be related to HIV disease stage [20]. More recently, an Italian–Spanish study found a shorter survival time for HIV-infected patients with HCC than for HIV-uninfected controls [23].
Despite these available studies, many questions about the outcomes of HCC in HIV-infected patients still remain open: the effect of HAART as a risk/protective factor at the onset/development of HCC, the role of viral hepatitis coinfection, the HIV infection profile (CD4+ cell counts and HIV viral load), the clinical characteristics of the tumor, and, finally, the possible differences between HIV-infected and HIV-uninfected patients in terms of the efficacy of HCC treatment.
The aims of this study were to describe HCC tumor characteristics and different therapeutic approaches, evaluate patient survival time from HCC diagnosis, and identify clinical prognostic predictors in patients with and without HIV infection.
Patients and Methods
A retrospective multicenter study was conducted in four Italian centers for HIV-infected patients. Controls were recruited from the oncology or internal medicine units of the same hospitals. Cases and controls were recruited consecutively over a 13-year period from January 1, 1997 to March 31, 2010. Patient data comprised data on demographics, mortality, characteristics and staging of the HCC at diagnosis, serum level of α-fetoprotein (AFP), viral status (HIV, HCV, and HBV), laboratory values concerning liver function (aspartate aminotransferase–alanine aminotransferase ratio, total bilirubin, albumin, prothrombin time, and platelet count), Child-Turcotte-Pugh scores, presumptive date of initial HBV or HCV infection, history of alcohol abuse (defined as alcohol intake ≥60 g/day for males and ≥40 g/day for females), and HCC treatment (at first diagnosis and, if performed, at HCC progression).
CLD was diagnosed in patients with HCV or hepatitis B surface antigen (HBsAg)+ serology and persistent liver enzyme elevation for ≥6 months. For the vast majority of the patients, data on acute hepatitis were not available.
Patients included in the study were diagnosed as having liver cirrhosis according to the following criteria: (a) biopsy proven, (b) esophageal varices with endoscopic documentation, (c) thrombocytopenia (≤100,000/mm3) with ultrasound (US) signs of portal hypertension.
Portal vein thrombosis (PVT) was detected using US with Doppler examination and/or computed tomography.
HIV–HCV coinfected patients were those with anti-HCV antibodies or plasma HCV RNA detected in any test. HIV-infected patients with chronic HBV infection were those seropositive for HBsAg and/or HB e antigen (HBeAg). With regard to HIV-infected patients, both virological and immunological characteristics as well as antiretroviral treatment were recorded.
Patients were considered as undergoing HAART if exposed to antiretroviral drugs for ≥6 months; HIV viral load was stratified as detectable or undetectable (<50 copies/mL), and CD4 strata were <200/μL, 200–350/μL, and >350/μL.
HCC Diagnosis and Treatment
Screening and diagnosis of HCC were performed according to the American Association for the Study of Liver Disease guidelines [16]. We considered patients as being under an HCC screening program if there was evidence of US and AFP evaluations every 6 months in their charts.
The Barcelona Clinic Liver Cancer (BCLC) staging system [27] was used for HCC staging: we chose this scoring system because of recent evidence indicating its good predictive value for survival in non-HIV patients with HCC [16, 17, 28]. The BCLC stage was therefore used in this study in all statistical analyses.
HCC treatment was considered potentially curative if locoregional treatments (radiofrequency ablation/ethanol injection [RFA/PEI] or RFA combined with transarterial chemoembolization [TACE]) or surgical options (liver resection and liver transplantation) were considered. TACE was considered an effective but not curative strategy.
Treatment was considered unproven or ineffective if systemic chemotherapy (used before sorafenib was available) and biological drugs (i.e., sorafenib) were used.
In cases of multiple modality HCC treatment, the most effective in terms of survival was recorded; for example, RFA over TACE over systemic chemotherapy. Thus, for example, we considered patients treated with an RFA plus TACE combination regimen as receiving a “curative” treatment, because RFA is considered to be the better treatment [16, 17]. Patients treated with an RFA plus TACE regimen were those whose tumor size and/or lesion number slightly exceeded Milano's criteria (intermediate stage; mean size, 4.2 ± 1.5 cm; 1–3 lesions) [16, 17].
Statistical Methods
Continuous variables were compared using Student's t-test (for normally distributed variables) or the Mann–Whitney U-test (in cases of non-normally distributed variables); categorical variables were compared using χ2 analysis or Fisher's exact test when appropriate. Survival was compared using Kaplan–Meier analysis and log-rank testing (Bonferroni's correction was used as appropriate). Multivariate Cox proportional hazards analysis tested all factors for independent association with survival that were associated with survival on univariate analysis at p < .10 and that did not have significant collinearity [29]. In all survival analyses, we considered only patients having a follow-up period of ≥3 months (n = 435).
Statistical analyses were performed using PASW/SPSS®, version 18.0 (SPSS, Inc., Chicago, IL) and MedCalc®, version 11.2.1 (MedCalc Software, Mariakerke, Belgium) statistical software.
Results
Between January 1997 and March 2010, 588 white patients with HCC were enrolled in the present study: 104 (17.7%) patients were HIV infected and 484 (82.3%) were uninfected. In the HIV-infected patients, at HCC diagnosis, the CD4+ cell count was ≤200/mL in 25 cases (24%), ≤400/mL in 45 cases (43.3%), and >400/mL in 34 cases (32.7%). In all 104 HIV-infected individuals, HCC was diagnosed after 1996: 93 (89%) were on HAART at HCC diagnosis, 61 (58.6%) had an undetectable HIV viral load (<50 copies/mL), and 20 (19.2%) had low-level HIV viremia (<10,000 copies/mL). Only 21 (20.2%) HIV-infected patients had previously been diagnosed with an AIDS-defining illness. Among the patients on HAART, 13 (14.6%) were on three nucleotide reverse transcriptase inhibitors (NRTIs), 22 (24.7%) were on two NRTIs plus one non-nucleoside reverse transcriptase inhibitor, and 54 (60.7%) were on two NRTIs plus one boosted protease inhibitor.
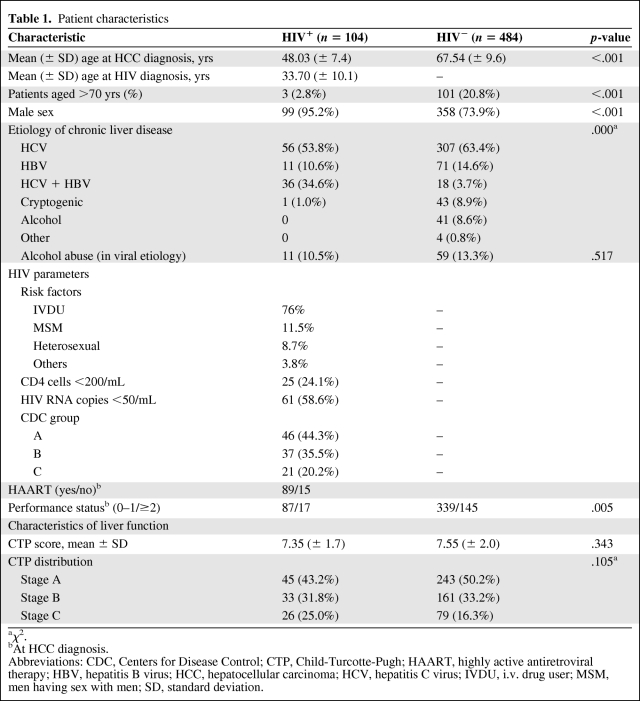
Comparison of Baseline Values
Patient characteristics are summarized in Table 1. HIV-infected patients were predominantly male, about 20 years younger than their non–HIV-infected counterparts, abused alcohol less often (10.5% versus 13.5%), and more often had chronic HBV or HCV as the underlying prevalent etiology (99% versus 81.6%). Among the HIV-uninfected controls, the main nonviral causes of HCC were alcohol abuse and cryptogenic, mostly as suspected evolution of nonalcoholic fatty liver disease (about 9% each).
Table 1.
Patient characteristics
aχ2.
bAt HCC diagnosis.
Abbreviations: CDC, Centers for Disease Control; CTP, Child-Turcotte-Pugh; HAART, highly active antiretroviral therapy; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IVDU, i.v. drug user; MSM, men having sex with men; SD, standard deviation.
HCV genotypes were available in 92 HIV-infected patients with the following distribution: genotype 1, 57.7%; genotype 2, 25%; genotype 3, 14.1%; and genotype 4, 3.2%. HCV genotypes were available for 325 HIV uninfected patients with the following distribution: genotype 1, 60.7%; genotype 2, 23.4%; genotype 3, 13.8%; and genotype 4, 2.1%. There was no significant difference in the HCV genotype distribution between HIV-infected and uninfected patients (χ2, 0.558; p = .91) .
For HBV infection, most of the patients in both the HIV-infected and uninfected groups were HBeAg−: 57.4% (27 of 47) and 59.5% (53 of 89), respectively. This difference is not statistically significant (p = .79).
We only had data for a few patients on the quantitative HCV RNA or HBV DNA polymerase chain reaction determination at the time of HCC diagnosis (these biological markers are not recommended by clinical guidelines). HCV RNA was available only in HIV-infected patients; most of them (55.4%) had HCV RNA <500,000 IU/mL.
HBV DNA was available for 47 HIV-infected patients: HBV DNA was undetectable in 38.3%, low (<2,000 IU/mL if HbeAg− or <20,000 IU/mL if HbeAg+) in 23.4%, and high (>2,000 IU/mL if HbeAg− or >20,000 IU/mL if HbeAg+) in 23.4%. All patients with high HBV viral load were not on HAART.
At HCC diagnosis, HIV-infected patients had a better performance status (PS) score than their uninfected counterparts.
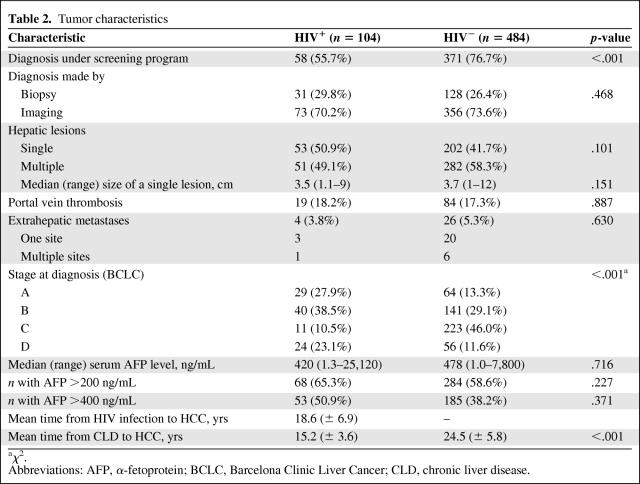
HCC Tumor Characteristics
Tumor characteristics are shown in Table 2. No differences were found in terms of the HCC diagnostic tool used (liver biopsy versus imaging) between the two groups. The HCC diagnosis was achieved through screening programs more frequently in HIV-uninfected than in HIV-infected patients [30, 31]. No significant differences among centers concerning screening modality, diagnosis, survival, and HIV status were observed.
Table 2.
Tumor characteristics
aχ2.
Abbreviations: AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CLD, chronic liver disease.
The HCC presentation (multiple versus single node) at diagnosis was similar in the two groups, as was the prevalence of detectable vascular invasion, PVT, and extrahepatic metastasis.
HCC Tumor Staging
The staging of HCC was different between the two groups (Table 2). In fact, over half of the HIV-uninfected patients had advanced-stage HCC: BCLC stage C or D was present in 57.6% of HIV-uninfected patients versus 33.6% HIV-infected patients. Conversely, the prevalence of BCLC stage A in HIV-infected patients was double that of HIV-uninfected patients (27.9% versus 13.3%, respectively).
No significant difference in the median serum AFP level was found between the two groups.
The time from a CLD diagnosis to HCC discovery was significantly shorter in the HIV-infected group than in their uninfected counterparts (15.2 years versus. 24.5 years).
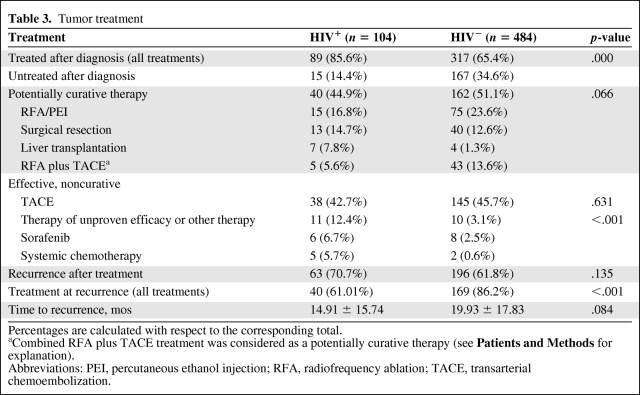
HCC Treatment
Overall, a higher number of HIV-infected patients received treatment for HCC (85.6 versus 65.4%). HCC treatment was divided into three groups: potentially curative, proven effective but not curative, and unproven or ineffective therapy (Table 3). The first group included locoregional treatments (RFA/PEI or RFA combined with TACE) together with surgical options (liver resection and liver transplantation). The latter group included systemic chemotherapy (used before sorafenib was available) and biological drugs (i.e., sorafenib). In HIV-uninfected patients, RFA and TACE were commonly used (69.4% versus 59.5% for uninfected and infected patients, respectively), whereas in HIV-infected patients surgical options were preferred (22.4%, versus 13.9%). Interestingly, the orthotopic liver transplant (OLT) option was significantly more prevalent in HIV-infected than in HIV-uninfected patients (7.9% versus 1.3%). The overall survival rates for patients treated with OLT were 90%, 80%, and 70% at 6 months, 1 year, and 5 years, respectively. Because of the small number of OLT-treated patients, we did not consider comparing survival after OLT between HIV-infected and uninfected patients.
Table 3.
Tumor treatment
Percentages are calculated with respect to the corresponding total.
aCombined RFA plus TACE treatment was considered as a potentially curative therapy (see Patients and Methods for explanation).
Abbreviations: PEI, percutaneous ethanol injection; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Overall, potentially curative therapies were equally administered in both groups (44.9% versus 51.1%; p = .066). Similarly, no significant difference for the effective but not curative option (i.e., TACE) was observed between the two groups (42.7% versus 45.7%; p = .631). HIV-infected patients underwent therapies of unproven efficacy more often (12.4% versus 3.1%, respectively; p < .001).
Interestingly, the recurrence rate after therapy was similar between the two groups, regardless of the treatment option (70.7% versus 61.8%; p = .135). However, the rate of retreatment (all treatments) at recurrence was dramatically higher in the HIV-uninfected group than in their HIV-infected counterparts (86.2% versus 61.01%; p < .001), despite the number of potentially treatable patients—at HCC recurrence, there were 56 patients (40 were treated) with BCLC stage A–C disease in the HIV-infected group and 173 patients (169 were treated) with BCLC stage A–C disease in the HIV-uninfected group.
At recurrence, curative options were used in 47.9% of cases in the HIV-uninfected group, compared with 42.5% of the HIV-infected group. TACE was used at recurrence in 52.5% of patients treated in the HIV-infected group, compared with 43.7% of those treated in the HIV-uninfected group. Time to recurrence was similar between groups: 14.91 ± 15.74 months versus 19.30 ± 17.83 months (p = .084), for the HIV-infected versus HIV-uninfected patients, respectively.
Tolerance of different treatment regimens (OLT, RFA, TACE, TACE plus RFA, etc.) used in HIV-infected patients was remarkably similar between the two groups. Moreover, in HIV-infected patients treated with sorafenib, treatment was stopped in three of six patients as a result of secondary effects (grade 3 hypophosphatemia, grade 2 hypertension, and grade 2 mucositis).
Survival
The 435 patients considered in the survival analysis (97 in the HIV-infected group and 338 in the non–HIV-infected group) underwent follow-up for at least 3 months. One hundred fifty-eight deaths (36.3%)—42.3% and 34.6%, respectively, in the HIV-infected and uninfected groups—were recorded. All were described as liver-related death.
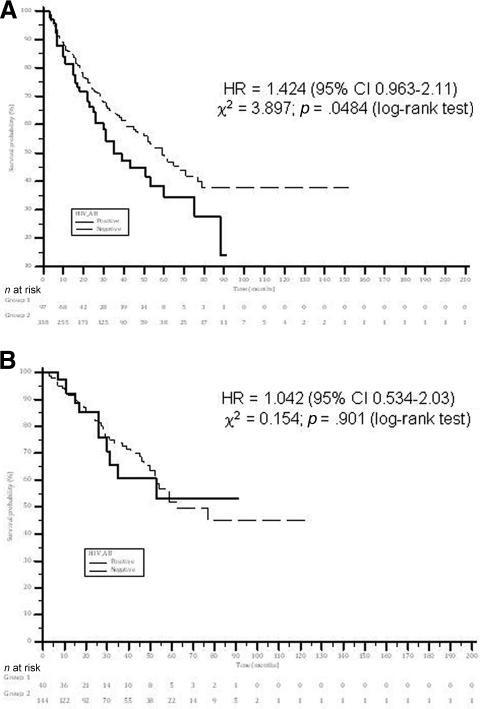
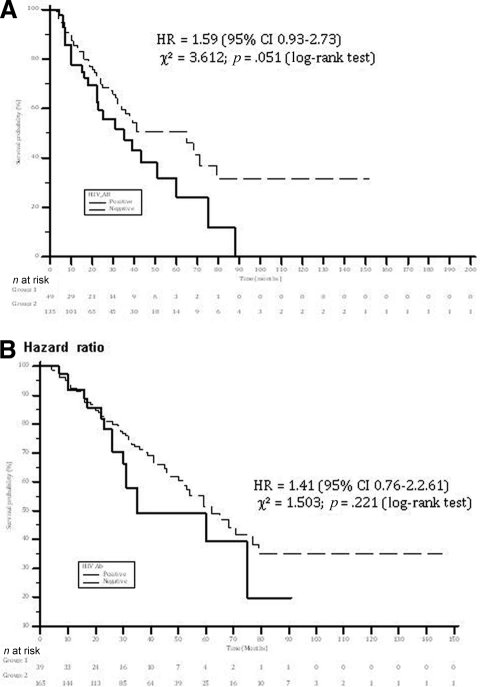
The median survival time was significantly shorter in HIV-infected patients than in HIV-uninfected patients—35 months (95% confidence interval [CI], 22.6–47.3 months) versus 59 months (95% CI, 46.3–70.6 months), respectively (log-rank p = .048). The 6-month, 1-year, 2-year, and 5-year survival rates were 92.3% versus 93.3%, 81.5% versus 85.8%, 66.6% versus 72.2%, and 34% versus 49.1%, for HIV-infected patients versus HIV-uninfected patients, respectively (Fig. 1A).
Figure 1.
Survival rates in HIV+ and HIV− patients with hepatocellular carcinoma. All patients had at least 3 months of follow-up. (A): All patients. (B): Patients treated with curative treatment. Patients treated with combined radiofrequency ablation and transarterial chemoembolization were considered to be treated with curative treatment. The difference in survival rates did not change significantly after exclusion of these patients from the analysis.
Abbreviations: AB, antibody; CI, confidence interval; HR, hazard ratio.
In patients with HCV infection (HIV+HCV+, n = 85; HIV−HCV+, n =241), coinfected patients had a significant shorter survival time—35 months (95% CI, 23.7–46.2 months) versus 65 months (95% CI, 46.9–83.1 months), respectively (log-rank p = 0.036). In the subgroups with HBV infection (HIV+Hasbro+, n = 53; HIV−Hasbro−, n = 59), survival rates were similar—51 months (95% CI, 27.2–74.8 months) versus 53 months (95% CI, 34.7–71.2, months) (log-rank p = .844).
The median survival time was not different between HIV-infected and uninfected patients treated with potentially curative therapy—52 months (95% CI, 40.5–68.9 months) in HIV-infected patients versus 62 months (95% CI, 42.7–81.3 months) in uninfected patients (p = .901) (Fig. 1B).
Stratifying by specific treatment type, the median survival time also was not different between groups. In particular, the survival durations in patients treated with surgery were 53 months (95% CI, 39.5–85.1 months) versus 59 months (95% CI, 37.1–87.1 months) (p = 0.575), respectively, for HIV-infected and uninfected patients. In patients treated with RFA/PEI, these were 51 months (95% CI, 43.5–72.1 months) versus 53 months (95% CI, 45.2–71.3 months) (p = .673) and in patients treated with TACE plus RFA, they were 45 months (95% CI, 32.3–58.1 months) versus 43 months (95% CI, 31.1–68.1 months) (p = .428), respectively, for HIV-infected and uninfected patients.
Conversely, a significant difference was observed in survival between groups when considering patients who did not receive potentially curative therapies—35 months (95% CI 15.2–54.8 months) versus 65 months (95% CI, 37.4–92.7 months) (p = .051) for HIV-infected versus uninfected patients, respectively. The 6-month, 1-year, 2-year, and 5-year survival rates were 93.0% versus 94.7%, 77.6% versus 85.6%, 59.3% versus 69.7%, and 23.9% versus 50.1% for HIV-infected patients versus HIV-uninfected patients, respectively (Fig. 2A). TACE-treated subgroups did not show a significant difference in survival. The median survival times were 38 months (95% CI, 16.2–48 months) and 41 months (95 CI, 18.2–45 months) (p = .087) for HIV-infected and uninfected patients, respectively. The 5-year survival rates for patients treated with TACE were 31% for the HIV-uninfected group and 48% for HIV-infected patients.
Figure 2.
Survival rates in HIV+ and HIV− patients with hepatocellular carcinoma. All patients had at least 3 months of follow-up. (A): Patients treated with noncurative treatments. (B): Patients retreated (all treatments) at recurrence.
Abbreviations: AB, antibody; CI, confidence interval; HR, hazard ratio.
No significant differences were observed in survival rates between HIV-infected and HIV-uninfected patients who underwent retreatment (of any kind) at HCC recurrence (Fig. 2B).
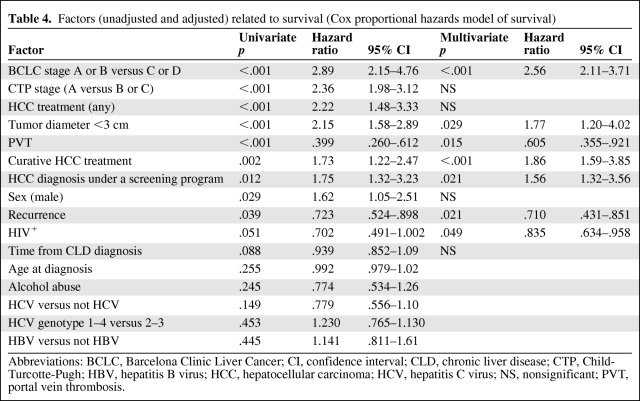
Cox univariate regression analysis found 11 factors to be significantly associated with survival and two showing a trend for association, with p < .1 (Table 4). Alcohol abuse, age at HCC diagnosis, and HCV or HBV presence were not predictive of survival. Multivariate Cox regression analysis identified seven factors to be independently associated with survival: BCLC stage, tumor dimension ≤3 cm, proven effective HCC therapy, recurrence after therapy, the presence of vascular invasion, diagnosis of HCC under a screening program, and HIV antibody positivity (Table 4). Treatment at recurrence (considered as “any treatment”) was also significantly related to survival in the univariate regression analysis (hazard ratio [HR], 1.95; 95% CI, 1.21–3.16; p = .006). Interestingly, when this parameter was inserted into the multivariate Cox model, it remained as an independent factor associated with survival, excluding HIV status from the model (HR, 1.85; 95% CI, 1.28–2.95; p = .008).
Table 4.
Factors (unadjusted and adjusted) related to survival (Cox proportional hazards model of survival)
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; CLD, chronic liver disease; CTP, Child-Turcotte-Pugh; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; NS, nonsignificant; PVT, portal vein thrombosis.
In HIV-infected patients, we did not find any significant association between low HCV viral load and survival on univariate analysis (odds ratio [OR], 1.035; 95% CI, 0.541–1.980; p = .917). Conversely, we observed a significant association for low HBV viral load and survival (OR, 0.634; 95% CI, 0.248–0.618; p = .041).
CD4 count at diagnosis of HCC was not independently associated with survival (p-values were nonsignificant for all CD4 strata).
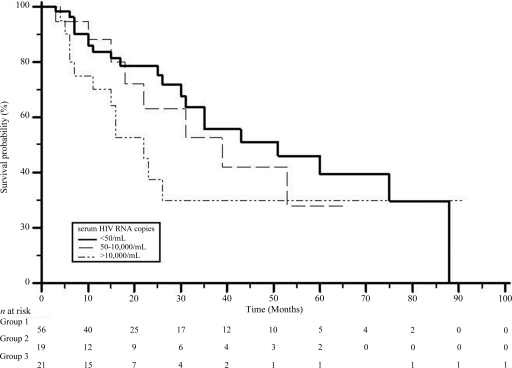
In HIV-infected individuals, the median survival duration was longer in those with undetectable HIV RNA than in those with a higher viremia level (Fig. 3).
Figure 3.
Survival rates in HIV+ patients with hepatocellular carcinoma with respect to HIV RNA status. All patients had at least 3 months of follow-up. Statistics are reported in the text.
In HIV-infected patients, the following factors were independently associated with survival: BCLC stage A or B versus stage C or D (HR, 2.51; 95% CI, 2.13–2.95; p = .001), HAART (HR, 1.25; 95% CI, 1.09–2.11; p = .024), proven effective HCC therapy (HR, 1.78; 95% CI, 1.38–2.25; p = .001), tumor dimension ≤3 cm (HR, 1.56; 95% CI, 1.14–2.55; p = .018), and HCC diagnosis made under a screening program (HR, 1.32; 95% CI, 1.18–2.65; p = .021). Even in HIV-infected patients, retreatment at recurrence was significantly associated with survival both on univariate (HR, 1.65; 95% CI, 1.21–2.86; p = .035) and multivariate (HR, 1.58; 95% CI, 1.20–3.01; p = .038) analysis.
Discussion
In the last decade, along with the dramatic reduction in AIDS-related mortality and morbidity as an effect of the introduction of HAART, ESLD has become one of the leading causes of mortality and morbidity (reaching 50% in some centers) in patients infected with HIV and coinfected with HCV or HBV [5, 15, 32]. According to the available epidemiological data, deaths related to CLD complications, mainly HCC, significantly increased from 15% to 25% in the short period from 2000 to 2005 in western countries. This suggests that HCC must be considered as an important emerging cause of death in HIV+ patients [26, 32]. However, our knowledge of HCC outcomes in HIV-infected patients is still poor, especially regarding HIV and HCC treatment.
In this study, we provide an analysis of the presentation and outcomes of 104 consecutive Italian HIV+ patients with HCC from the Italian Cooperative Group on AIDS and Tumors.
To our knowledge, this is the largest cohort study comparing HIV-infected with HIV-uninfected HCC patients who all had an HCC diagnosis made in the HAART era. To date, only a few relatively small retrospective studies have been published on this topic, and they involved, as a whole, <200 HIV-infected patients [13, 20, 23, 24, 26, 32].
In agreement with these previous reports, we found that, in most cases of HIV-infected patients, HCC was diagnosed in patients with well-controlled HIV disease and a good PS. The age at HCC diagnosis was younger in HIV-infected patients than in uninfected patients, and HCV coinfection was the major risk factor, with a worse prognosis in terms of shorter median survival time than for HIV-uninfected patients, despite good HIV infection control. As expected, HIV+ patients in our series also had a shorter mean time of evolution from CLD to HCC [24, 26].
Many different important findings emerge from our results. First, HIV-infected patients who developed HCC were coinfected with HCV or HBV in the great majority of cases. Second, in HIV-infected patients, HCC was diagnosed mostly at an early stage (66% at BCLC stage A or B), and for this reason it was amenable for curative approaches. In spite of this result, the median survival time of the HIV-infected cohort was shorter (Fig. 1) than that of the HIV-uninfected cohort, in whom HCC was diagnosed at a more advanced stage (58% at BCLC stage C or D) and was hence less frequently amenable to curative approaches. A possible explanation for this different outcome between the two groups obviously relies on a more aggressive tumor biology for HCC in the presence of HIV infection [23–25]. This hypothesis may be supported by the observation that, in HIV coinfected patients, HCC seems to develop more rapidly than in their uninfected counterparts. Nevertheless, it is important to remark how these data may be significantly biased by the natural difficulty of obtaining an accurate estimate of the actual start date of chronic liver viral infection. Moreover, in our series, HIV+ patients had a better tumor stage at diagnosis, and no significant difference was observed between the two groups in terms of either time to tumor recurrence or the tumor recurrence rate after HCC treatment.
Third, HIV-infected patients on HAART at HCC diagnosis had a better prognosis than patients not on HAART.
A possible explanation relies on both the HAART effect on HBV infection and its impact in downregulating liver inflammation with a reduction in liver fibrosis progression [18, 33–35]. A significant association between HBV replication status at HCC diagnosis and HCC outcome on univariate analysis was found: all HBV patients with high serum HBV DNA levels were not on HAART and, not surprisingly, HAART excluded HBV replication status as an independent predictor of survival in the regression analysis.
Fourth, no response rate difference was found between the two groups with regard to potentially curative treatments either at diagnosis or at recurrence, although in the case of HCC disease progression after treatment, HIV-infected patients were retreated significantly less frequently than HIV-uninfected patients.
HIV+ patients in our study had a significant longer median survival duration (35 months) than that reported by Puoti et al. [23] (6 months), In that study, comparing 41 HIV+ HCC patients with HIV− HCC patients from two cohorts (n = 381 and n = 701), they also reported that HIV infection was independently associated with a shorter survival time (HR, 1.63; 95% CI, 1.10–2.40; p = .015). The difference observed may be explained by two fundamental aspects: (a) the higher percentage of HIV+ HCC patients diagnosed at an earlier stage in our cohort (66.4% versus 46%) and (b) the higher percentage (94.3%) of our HIV+ patients whose HCC was treated with potentially curative/effective therapeutic approaches than in the Puoti et al. [23] series (only 40%). Our survival rates are also significantly different from those reported in the study by Brau et al. [24]. In this case, the median survival time for HIV+ HCC patients was significantly longer in our study (35 months versus 6.9 months, respectively). The discrepancy may be explained by the significant difference in HCC stage at diagnosis and by the different approaches in terms of screening (66.4% versus 50%) and therapy between the two cohorts [24].
In our opinion, the other available studies have different designs and are too limited to allow for further comparisons or consideration [13, 20, 26, 32].
Other relevant data emerging from our study concern the treatment received by HIV-infected patients. In about one third of our HIV-infected patients, HCC was treated with a potentially curative option. These results are in contrast to those from other case series, in which no adequate treatment for HCC disease or no treatment at all (even for those with a nonadvanced BCLC stage) was used in most HIV-infected patients [13, 20, 23, 24, 26, 32]. Also, in our study, HCC treatment rates (expressed in terms of curative and noncurative options) were quite similar in the two groups, making a more adequate comparison possible.
Nevertheless, regarding potentially curative options, the HIV-infected patients in our series were more frequently treated with surgical options for HCC than the HIV-uninfected patients; this difference may be explained by the younger age, better PS, and better HCC stage at diagnosis of the HIV-infected group. It is to be noted that HIV+ patients treated with surgical options had outcomes, in terms of postoperative surgical complications and survival, similar to HIV-uninfected patients, according to previous reports [36, 37], and that the progression-free survival (PFS) time was not significantly different between the two groups.
Even though HCC patients presented with similar clinical and biological characteristics at diagnosis, were similarly treated, and had the same PFS probability after the first treatment, we noticed a significantly worse overall survival outcome in HIV-infected patients. How can we explain these results? Our data suggest that this worse prognosis for HIV+ HCC patients may be a result of a significant and unexplained lower propensity to retreat HIV-infected patients at HCC recurrence, rather than to an intrinsic biological aggressiveness of HCC (see above) or to a lack of therapeutic intervention at diagnosis. In our series, considering patients eligible for retreatment at HCC recurrence, HIV-infected patients were treated significantly less frequently than their uninfected counterparts. Besides HAART, our data show that independent prognostic factors for HCC outcome in HIV-infected patients were nearly the same as in HIV-uninfected patients, because treatment at recurrence and early HCC diagnosis (i.e., diagnosis under screening program) were significantly less frequently performed in HIV-infected patients.
The retrospective design and the possible heterogeneity in diagnosing and managing patients among the participating centers were the limitations of our study that we tried to overcome by recruiting cases and controls from the same hospitals (no significant differences in overall results were observed after stratifying results by centers). Another possible concern was with the allocation of the “combined approach” (RFA combined with TACE) for HCC to the “curative options.” This option, even if promising, does not yet have a well-established role in HCC treatment [38, 39]. Nevertheless, no significant difference in the survival rate results between groups was observed in our series, even after the exclusion of patients treated with a therapeutic approach from among the curative options (data not shown).
At present, no universal guidelines for managing HCC in clinical practice in HIV-infected patients are available [16–17, 40, 41]. Considering that our data did not really show any significant differences between HIV-infected and HIV-uninfected patients in terms of HCC response to the therapy available at present, different therapeutic strategies for HCC in the HIV setting may possibly be advocated to improve the integration of HIV and HCC treatments, and HCC therapeutic trials specifically designed for HIV-infected patients are needed. On the other hand, considering the key roles in survival of early diagnosis of HCC and retreatment for HCC recurrence, the extension of regular screening programs for HCC to HIV-infected patients according to proposed guidelines [16, 28, 40–43], together with a greater proclivity for treatment and retreatment options in cases of an HCC diagnosis or recurrence, may represent an important and urgent breakthrough in facing this emerging problem.
In conclusion, this study confirms a significantly shorter HCC survival time in patients with HIV infection. The less aggressive retreatment at recurrence approach does not balance the benefit of younger age and better BCLC stage and PS score of HIV-infected patients.
Acknowledgments
The authors gratefully acknowledge their colleagues, Vincenzo Montesarchio, Massimilano Lanzafame, Lorenza Guella, Roberta Cinelli, Marcello Tavio, Beatrice Autino, Vincenzo Raise, Annalisa Ridolfo, Lisa Malincarne, Clara Schiantarelli, and Rossella Fisichella, who helped gather data on patients.
Author Contributions
Conception/Design: Massimiliano Berretta, Umberto Tirelli, Paolo Ventura
Financial support: Paolo de Paoli
Provision of study material or patients: Massimiliano Berretta, Elisa Garlassi, Bruno Cacopardo, Arben Lleshi, Paolo Ventura
Collection and/or assembly of data: Massimiliano Berretta, Elisa Garlassi, Arben Lleshi, Paolo Ventura
Data analysis and interpretation: Giovanni Guaraldi, Stefania Cocchi, Paolo Ventura
Manuscript writing: Massimiliano Berretta, Umberto Tirelli, Paolo Ventura
Final approval of manuscript: Massimiliano Berretta, Elisa Garlassi, Bruno Cacopardo, Alessandro Cappellani, Giovanni Guaraldi, Stefania Cocchi, Paolo de Paoli, Arben Lleshi, Immacolata Izzi, Augusta Torresin, Pietro Di Gangi, Antonello Pietrangelo, Mariachiara Ferrari, Alessandra Bearz, Salvatore Berretta, Guglielmo Nasti, Fabrizio Di Benedetto, Luca Balestreri, Umberto Tirelli, Paolo Ventura
References
- 1.UNAIDS: Global Summary of the HIV and AIDS Epidemic. 2007. Available at http://www.unaids.org.
- 2.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 5.Macías J, Melguizo I, Fernàndez-Rivera FJ, et al. Mortality due to liver failure and impact on survival of hepatitis virus infections in HIV-infected patients receiving potent antiretroviral therapy. Eur J Clin Microbiol Infect Dis. 2002;21:775–781. doi: 10.1007/s10096-002-0823-0. [DOI] [PubMed] [Google Scholar]
- 6.Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356:1445–1454. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sulkowski MS, Thomas DL, Chaisson RE, et al. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 8.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48:353–367. doi: 10.1016/j.jhep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Deuffic-Burban S, Poynard T, Sulkowski MS, et al. Estimating the future health burden of chronic hepatitis C and human immunodeficiency virus infections in the United States. J Viral Hepat. 2007;14:107–115. doi: 10.1111/j.1365-2893.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 10.Gomaa AI, Khan SA, Toledano MB, et al. Hepatocellular carcinoma: Epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300–4308. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch FX, Ribes J, Diaz M, et al. Primary liver cancer: Worldwide incidence and trends. Gastroenterology. 2004;127(suppl 1):S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Jong-wook L. Global health improvement and WHO: Shaping the future. Lancet. 2003;362:2083–2088. doi: 10.1016/S0140-6736(03)15107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clifford GM, Rickenbach M, Polesel J, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008;22:2135–2141. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 14.Smukler AJ, Ratner L. Hepatitis viruses and hepatocellular carcinoma in HIV-infected patients. Curr Opin Oncol. 2002;14:538–542. doi: 10.1097/00001622-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Lewden C, Salmon D, Morlat P, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: Emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–130. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 17.El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 18.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 19.Salmon D, Robain M, Rockstroh JK, et al. Therapeutic management of hepatitis and HIV infection in co-infected patients: Results of a survey performed before the 2005 Consensus Conference. J Hepatol. 2006;44(1 suppl):S2–S5. doi: 10.1016/j.jhep.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Giordano TP, Kramer JR, Souchek J, et al. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: A cohort study, 1992–2001. Arch Intern Med. 2004;164:2349–2354. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 21.Pineda JA, Romero-Gómez M, Díaz-García F, et al. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41:779–789. doi: 10.1002/hep.20626. [DOI] [PubMed] [Google Scholar]
- 22.Reisler K. High hepatotoxicity rate seen among HAART patients. AIDS Alert. 2001;16:118–119. [PubMed] [Google Scholar]
- 23.Puoti M, Bruno R, Soriano V, et al. Hepatocellular carcinoma in HIV-infected patients: Epidemiological features, clinical presentation and outcome. AIDS. 2004;18:2285–2293. doi: 10.1097/00002030-200411190-00009. [DOI] [PubMed] [Google Scholar]
- 24.Bräu N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: A U.S.-Canadian multicenter study. J Hepatol. 2007;47:527–537. doi: 10.1016/j.jhep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 25.García-Samaniego J, Rodríguez M, Berenguer J, et al. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:179–183. doi: 10.1111/j.1572-0241.2001.03374.x. [DOI] [PubMed] [Google Scholar]
- 26.Bruno R, Sacchi P, Filice C, et al. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis: An emerging issue. J Acquir Immune Defic Syndr. 2002;30:535–536. doi: 10.1097/00126334-200208150-00011. [DOI] [PubMed] [Google Scholar]
- 27.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 28.Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: Comparison of 7 staging systems in an American cohort. Hepatology. 2005;41:707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 29.Hosmer DW, Lemeshow SS. 2nd ed. Hoboken, NJ: Wiley; 2000. Applied Logistic Regression; pp. 1–392. [Google Scholar]
- 30.Colombo M. Screening and diagnosis of hepatocellular carcinoma. Liver Int. 2009;29(suppl 1):143–147. doi: 10.1111/j.1478-3231.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- 31.Pascual S, Irurzun J, Zapater P, et al. Usefulness of surveillance programmes for early diagnosis of hepatocellular carcinoma in clinical practice. Liver Int. 2008;28:682–689. doi: 10.1111/j.1478-3231.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- 32.Salmon-Ceron D, Rosenthal E, Lewden C, et al. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalité 2005 study. J Hepatol. 2009;50:736–745. doi: 10.1016/j.jhep.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Miailhes P, Trabaud MA, Pradat P, et al. Impact of highly active antiretroviral therapy (HAART) on the natural history of hepatitis B virus (HBV) and HIV coinfection: Relationship between prolonged efficacy of HAART and HBV surface and early antigen seroconversion. Clin Infect Dis. 2007;45:624–632. doi: 10.1086/520752. [DOI] [PubMed] [Google Scholar]
- 34.Asmuth DM, Busch MP, Laycock ME, et al. Hepatitis B and C viral load changes following initiation of highly active antiretroviral therapy (HAART) in patients with advanced HIV infection. Antiviral Res. 2004;63:123–131. doi: 10.1016/j.antiviral.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Vogel M, Rockstroh JK. Liver disease: The effects of HIV and antiretroviral therapy and the implications for early antiretroviral therapy initiation. Curr Opin HIV AIDS. 2009;4:171–175. doi: 10.1097/COH.0b013e328329c602. [DOI] [PubMed] [Google Scholar]
- 36.Benedetto F, De Ruvo N, Berretta M, et al. Don't deny liver transplantation to HIV patients with hepatocellular carcinoma in the highly active antiretroviral therapy era. J Clin Oncol. 2006;24:e26–e27. doi: 10.1200/JCO.2006.06.1374. [DOI] [PubMed] [Google Scholar]
- 37.Di Benedetto F, De Ruvo N, Berretta M, et al. Hepatocellular carcinoma in HIV patients treated by liver transplantation. Eur J Surg Oncol. 2008;34:422–427. doi: 10.1016/j.ejso.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Takaki H, Yamakado K, Nakatsuka A, et al. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas 5 cm or smaller: Risk factors for local tumor progression. J Vasc Interv Radiol. 2007;18:856–861. doi: 10.1016/j.jvir.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 39.Ventura P, De Santis M, Romagnoli E, et al. Radiofrequency ablation combined with transcatheter chemoembolization in the treatment of advanced hepatocellular carcinoma. Gut. 2008;57(suppl II):A153. [Google Scholar]
- 40.Bruno R, Puoti M, Sacchi P, et al. Management of hepatocellular carcinoma in human immunodeficiency virus-infected patients. J Hepatol. 2006;44(1 suppl):S146–S150. doi: 10.1016/j.jhep.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 41.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 42.Hoofnagle JH. Hepatocellular carcinoma: Summary and recommendations. Gastroenterology. 2004;127(suppl 1):S319–S323. doi: 10.1053/j.gastro.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 43.Management of hepatocellular carcinoma: From prevention to molecular targeted therapy. Oncology; Proceedings of the 3rd International Kobe Liver Symposium on HCC with an International Liver Cancer Association (ILCA) Scientific Session; June 6–7, 2009; Hyogo, Japan. 2010. pp. 1–190. [PubMed] [Google Scholar]