The present study was done to establish a prognostic model for patients and trials using an oxaliplatin-based or irinotecan-based first-line chemotherapy in metastatic colorectal cancer. Serum lactate dehydrogenase level was the main prognostic factor in predicting survival, followed by World Health Organization performance status. Three risk groups for death depending on these two baseline parameters were identified.
Keywords: Colorectal cancer, Prognostic model, Chemotherapy
Learning Objectives
After completing this course, the reader will be able to:
Describe prognostic factors in metastatic colorectal cancer.
Estimate prognostic score with a simple model using only PS and LDH as parameters.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
The present study was done to establish a prognostic model for patients and trials using an oxaliplatin-based or irinotecan-based first-line chemotherapy in metastatic colorectal cancer.
Patients and Methods.
Eight hundred three patients treated with FOLFOX or FOLFIRI in three prospective trials were randomly separated into learning (n = 535) and validation (n = 268) samples. Eleven baseline variables were evaluated in univariate and multivariate analysis as prognostic factors for overall survival, and a prognostic score was developed.
Results.
Independent prognostic factors identified in multivariate analysis for overall survival were performance status (PS) (p < .001), serum lactate dehydrogenase (LDH) (p < .001), and number of metastatic sites (p = .005). A prognostic score based on these three variables was found efficient (Harrell's C index 0.61). This new model was improved by selecting only PS and LDH (Harrell's C index 0.64). Three risk groups for death could be identified: a low-risk group (n = 184; median overall survival [OS] 29.8 months), an intermediate-risk group (n = 223; median OS 19.5 months), and a high-risk group (n = 128; median OS 13.9 months). Median survival for the low-, intermediate-, and high-risk groups were 26.8, 21.1, and 16.5 months, respectively, in the validation sample (Harrell's C index 0.63).
Conclusions.
Serum LDH level was the main prognostic factor in predicting survival, followed by WHO PS. We identified three risk groups for death depending on these two baseline parameters. This simple prognostic model can be useful for clinician's use and patient stratification in future clinical trials.
Introduction
Colorectal cancer is the third most common cancer and the second cause of cancer death in the United States [1]. Consequently, metastatic colorectal cancer remains a major public health priority. Chemotherapy is the cornerstone treatment in the case of unresectable metastatic disease.
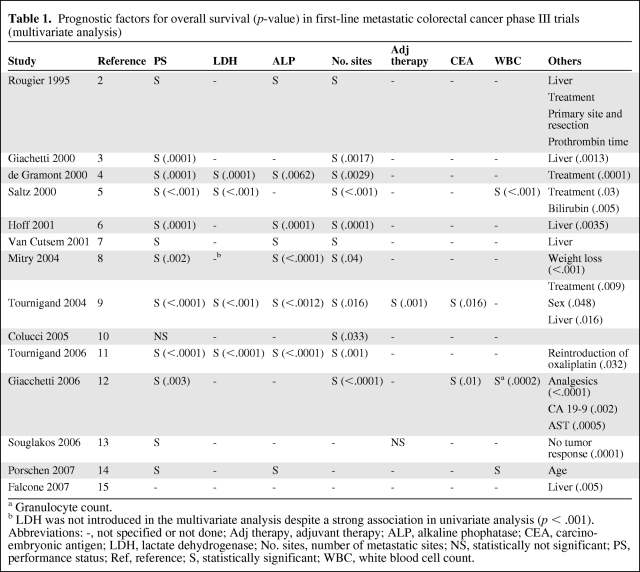
Heterogeneity in survival rates can be mainly explained by patient and tumor characteristics, host response factors, treatment strategy (drugs, regimen, surgery of metastasis). Of note, several prognostic factors have been found in previous clinical trials [2–15]. These were patient characteristics such as performance status (PS), age, sex, weight loss, biological variables such as white blood cell count (WBC), serum alkaline phosphatase (ALP) level, serum lactate dehydrogenase (LDH) level, serum carcino-embryonic antigen (CEA) level, or tumor characteristics like the number of metastatic sites or liver involvement (Table 1).
Table 1.
Prognostic factors for overall survival (p-value) in first-line metastatic colorectal cancer phase III trials (multivariate analysis)
a Granulocyte count.
b LDH was not introduced in the multivariate analysis despite a strong association in univariate analysis (p < .001).
Abbreviations: -, not specified or not done; Adj therapy, adjuvant therapy; ALP, alkaline phophatase; CEA, carcino-embryonic antigen; LDH, lactate dehydrogenase; No. sites, number of metastatic sites; NS, statistically not significant; PS, performance status; Ref, reference; S, statistically significant; WBC, white blood cell count.
Three risk groups for death were recognized in patients receiving 5-fluorouracil-based chemotherapy for metastatic colorectal cancer, depending on four baseline prognostic parameters: PS, WBC count, ALP, and number of metastatic sites (Köhne model) [16]. The low-risk group was defined as patients with World Health Organization (WHO) PS 0–1 and 1 metastatic site. The intermediate-risk group was defined as patients with either WHO PS 0–1, >1 metastatic site, and ALP level <300, or WHO PS >1, 1 metastatic site, and WBC <10,000/mm3. Finally, the high-risk group had either WHO PS 0–1, >1 metastatic site, and ALP level >300, or WHO PS >1, >1 metastatic site, and WBC <10,000/mm3, or WHO PS >1 and WBC >10,000/mm3. In recent trials, WHO PS and LDH level at baseline were strongly associated with survival (Table 1).
The aim of this study was (a) to identify prognostic factors in patients with metastatic colorectal cancer receiving an oxaliplatin-based or an irinotecan-based first-line chemotherapy, (b) to develop a prognostic scoring system based on the identified prognostic factors, and (c) to evaluate the efficiency of the previous model in this population.
Patients and Methods
Trials
Individual patient data from three randomized trials including patients with previously untreated metastatic colorectal cancer were updated and pooled. The C97-3 study compared the FOLFIRI1 regimen as first-line chemotherapy until progression or limiting toxicity followed by the FOLFOX6 regimen as second-line therapy, to the reverse sequence [9].
The OPTIMOX1 study compared the FOLFOX4 regimen as first-line chemotherapy until disease progression or limiting toxicity, to an oxaliplatin stop-and-go strategy with 3 months of FOLFOX7 regimen followed by a maintenance therapy with simplified LV5FU2 until disease progression or 12 cycles, then reintroduction of the same FOLFOX7 regimen [11].
The OPTIMOX2 study compared the oxaliplatin stop-and-go strategy to a complete stop-and-go strategy after six cycles of modified FOLFOX7 regimen [17]. Of note, none of these trials used targeted therapies in combination with first-line chemotherapy.
Data
To be eligible, the Köhne model had to be applicable and the baseline LDH value had to be available. The following data were analyzed for individual patients in all trials: patient identifier, center identifier, randomization date, treatment assigned by randomization, age, sex, performance status, primary tumor site (colon, rectum, or both), site of metastases, ALP level at baseline, LDH level at baseline, CEA level at baseline, date of first and last cycle of first-line chemotherapy, overall response status, date of first progression, progression status, survival status, and date of last news or death. Baseline WBC was available for patients with PS >1 because it was required to calculate the Köhne score.
Statistical Considerations
Progression-free survival was defined as the time interval from randomization to first disease progression or death from any cause if disease progression did not occur. Alive patients without progression were censored at the last follow-up. Progression was defined as assessed in each individual trial. Overall survival (OS) was defined as the time interval from randomization to death from any cause. Alive patients were censored at the last follow-up.
The median follow-up was estimated using the reverse Kaplan-Meier method [18]. Survival endpoints were estimated using the Kaplan-Meier method [19]. Patients were randomly separated with trial stratification into a learning sample (2/3) and a validation sample (1/3) to check overfitting [20].
The association between prognostic factors and survival was explored using univariate Cox analyses stratified on trials for all potential prognostic factors. Eleven baseline variables were evaluated in a univariate analysis: age (≤65 versus >65), sex, PS (0 versus 1 versus 2), number of metastatic sites (1 versus >1), liver involvement (yes versus no), primitive tumor (colon versus rectum versus both), synchronous versus metachronous disease, prior adjuvant chemotherapy (yes versus no), serum LDH level, serum ALP level, and serum CEA level. These variables were treated as qualitative data using cutoff for continuous variables in anticipation of elaborating a practical clinical tool. The Köhne scoring system was also explored. WBC was not considered for univariate and multivariate analysis because it was not available for all patients. Proportional hazards assumption was graphically assessed. Factors were considered for inclusion in the model as potentially associated with OS if the univariate p value was ≤.05. The Cox regression model stratified on trials was used for multivariate analysis of prognostic factors for OS [21].
A prognostic score was developed based on the identified prognostic factors in the multivariate analysis. The prognostic value for a given patient was the combination of the different variables weighted with the regression coefficients included in the multivariate analysis. To establish a practical and easy-to-compute score, the score was calibrated so that each variable contributed 1 or 2 units. The score was then calculated for each patient by adding the points corresponding to each prognostic factor retained in the multivariate model. The predictive accuracy of the new scoring system was examined and compared to the Köhne scoring system by calculating Harrell's C discrimination index extended for survival data [22]. Results of Harrell's C index vary from 0.5 (no discrimination for predicting OS) to 1.0 (perfect discrimination). Calibration was checked based on plotting results for patients with similar risk score categories (high risk /intermediate risk/low risk) and thus compared the mean predicted probability with the mean observed outcome (i.e., OS at 36 months) [23]. Bootstrap (n = 150) was used to take into account overfitting regarding prediction and Harrell's C index. All statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC) and STATA 11.0 (StataCorp LP, College Station, TX) software or R software (2.13.0, Design package).
Results
Population
Among the 1,042 patients included in the three original trials, 803 were eligible. LDH values at baseline were not available in 134 patients, and the Köhne model could not be applied for 105 additional patients (WBC not available in 29 patients, number of metastatic sites not available for 2 patients, and ALP value not available for 74 patients).
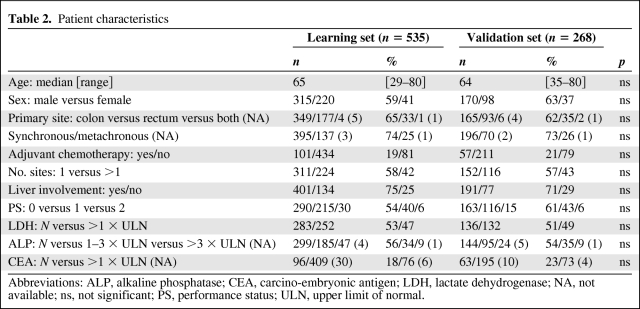
The characteristics of the 803 studied patients were similar across trials (supplemental online Table 1). The patient characteristics in learning sample (n = 535) and validation sample (n = 268) are shown in Table 2. There was no statistical imbalance between the two samples. Six percent of patients had PS2 in both samples. There were 252 patients (47%) and 132 patients (49%) with increased LDH level at baseline in the learning and validation samples, respectively, and 224 patients (42%) and 116 patients (43%) with 2 or more metastatic sites at baseline in the learning and validation samples, respectively. Prior adjuvant chemotherapy without oxaliplatin or irinotecan was given in 158 patients (20%).
Table 2.
Patient characteristics
Abbreviations: ALP, alkaline phosphatase; CEA, carcino-embryonic antigen; LDH, lactate dehydrogenase; NA, not available; ns, not significant; PS, performance status; ULN, upper limit of normal.
Follow-up
The median follow-up times for the learning and validation samples were 43.8 months (95% confidence interval [CI], 42.9–47.9) and 46.3 months (95% CI, 43.0–52.4) in the C97-3 trial, 35.3 months (95% CI, 32.1–38.8) and 35.6 months (95% CI, 31.6–43.6) in the OPTIMOX1 trial, and 38.5 months (95% CI, 31.3–46.4) and 39.1 months (95% CI, 32.6–46.7) in the OPTIMOX2 trial, respectively.
Prognostic Factors
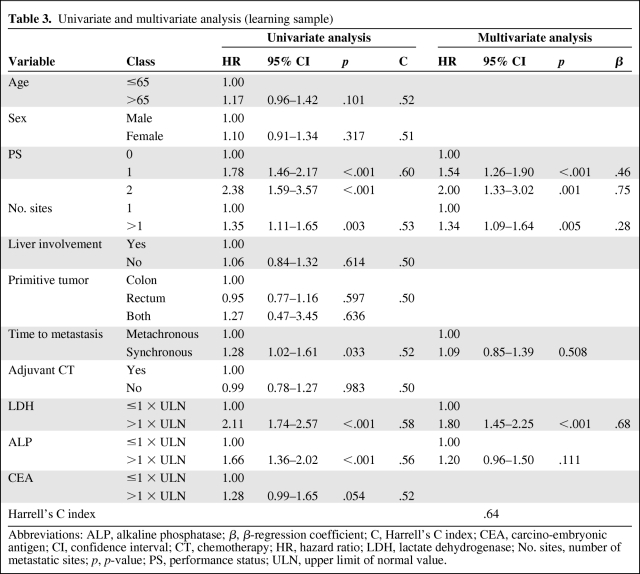
Five variables were identified as prognostic factors in the univariate analysis: PS (p < .001), LDH (p < .001), ALP (p < .001), number of sites (p = .003), and time to metastasis (p = .033). These five variables were introduced in the multivariate analysis. The CEA level at baseline (normal versus increased value) was not statistically significant (p = .054) (Table 3, Figure 1).
Table 3.
Univariate and multivariate analysis (learning sample)
Abbreviations: ALP, alkaline phosphatase; β, β-regression coefficient; C, Harrell's C index; CEA, carcino-embryonic antigen; CI, confidence interval; CT, chemotherapy; HR, hazard ratio; LDH, lactate dehydrogenase; No. sites, number of metastatic sites; p, p-value; PS, performance status; ULN, upper limit of normal value.
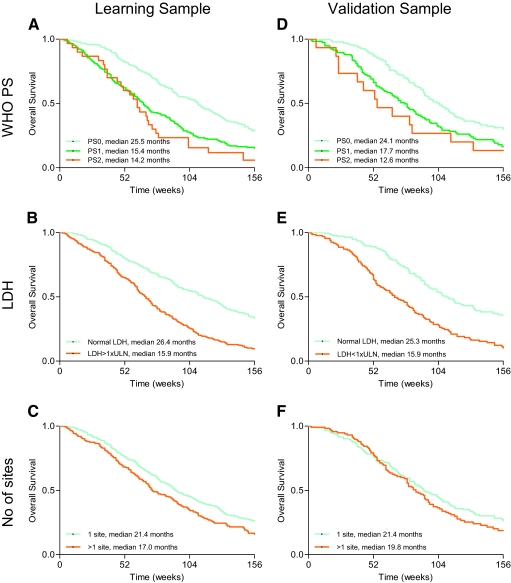
Figure 1.
Overall survival according to independent prognostic factors in learning sample (A–C) and validation sample (D–F). (A): WHO PS (learning sample), (B): serum LDH level (learning sample), (C): number of metastatic sites (learning sample), (D): WHO PS (validation sample), (E): serum LDH level (validation sample), and (F): number of metastatic sites (validation sample). Abbreviations: LDH, lactate dehydrogenase; PS, performance status; WHO, World Health Organization.
Independent prognostic factors for decreased overall survival in the multivariate analysis were as follows: abnormal serum LDH level (hazard ratio [HR] = 1.80; 95% CI, 1.45–2.25, p < .001), impaired performance status (PS1: HR = 1.54, 95% CI, 1.26–1.90, p < .001; and PS2: HR = 2.00, 95% CI, 1.33–3.02, p = .001), and >1 metastatic site (HR = 1.34, 95% CI, 1.09–1.64, p = .005). The Harrell's C index was 0.64 (Table 3).
Prognostic Model
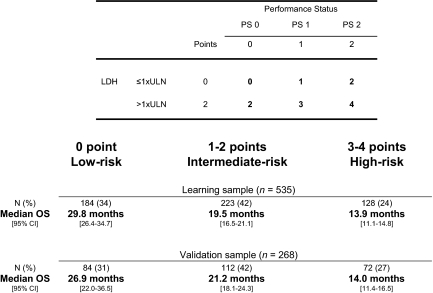
A new prognostic scoring system was based on the three independent prognostic factors identified in the multivariate analysis: LDH, PS, and the number of metastatic sites. For LDH ≤1 × upper limit of normal (ULN), PS0, and only 1 metastatic site: 0 point each; for PS1 and >1 site: 1 point each; for LDH >1 × ULN and PS2: 2 points each. A score of 0–5 was calculated for each patient. Three risk groups were identified in predicting survival: a low-risk group (0 point), an intermediate-risk group (1–3 points), and a high-risk group (4–5 points).
The median OS in the learning sample was 30.1 months (95% CI = 26.7–37.1) in the low-risk group (n = 118, 22%), 19.6 months (95% CI = 17.9–21.3) in the intermediate-risk group (n = 348, 65%), and 13.1 months (95% CI = 9.4–14.5) in the high-risk group (n = 69, 13%). According to low risk, the univariate HR was 1.92 (95% CI = 1.49–2.49) for the intermediate-risk group and 4.51 (95% CI = 3.22–6.33) for the high-risk group. The Harrell's C index for this model was 0.61. Optimism-corrected Harrell C index was 0.59. Calibration was satisfactory (data not shown).
The median OS in the validation sample was 36.7 months (95% CI, 23.7–58.1) in the low-risk group (n = 49, 18%), 19.8 months (95% CI, 17.1–22.1) in the intermediate-risk group (n = 187, 70%), and 12.7 months (95% CI, 10.0–19.2) in the high-risk group (n = 32, 12%). According to low risk, univariate HR was 2.17 (95% CI, 1.45–3.25) for the intermediate-risk group and 3.24 (95% CI, 1.91–5.48) for the high-risk group. The Harrell's C index for this model was 0.60. Optimism-corrected Harrell C index was 0.56. Calibration was not satisfactory (data not shown).
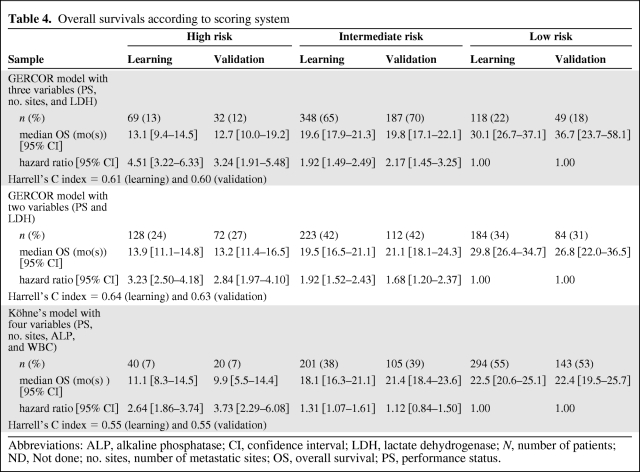
A prognostic score based on the two most important prognostic factors (LDH and PS) was also developed. For LDH ≤1 × ULN and PS0: 0 point each; for PS1: 1 point; for LDH >1 × ULN and PS2: 2 points each. A score from 0 to 4 was calculated for each patient. Three risk groups for mortality were identified: a low-risk group (0 point), an intermediate-risk group (1–2 points), and a high-risk group (3–4 points) (Table 4, Figures 2 and 3). The median OS in the learning sample was 29.8 months (95% CI, 26.4–34.7) in the low-risk group (n = 184, 34%), 19.5 months (95% CI, 16.5–21.1) in the intermediate-risk group (n = 223, 42%), and 13.9 months (95% CI, 11.1–14.8) in the high-risk group (n = 128, 24%). According to low risk, univariate HR was 1.92 (95% CI, 1.52–2.43) for the intermediate-risk group and 3.23 (95% CI, 2.50–4.18) for the high-risk group. The Harrell's C index for this model was 0.64. Optimism-corrected Harrell C index was 0.63. As shown in supplemental online Figure 1, calibration was satisfactory.
Table 4.
Overall survivals according to scoring system
Abbreviations: ALP, alkaline phosphatase; CI, confidence interval; LDH, lactate dehydrogenase; N, number of patients; ND, Not done; no. sites, number of metastatic sites; OS, overall survival; PS, performance status.
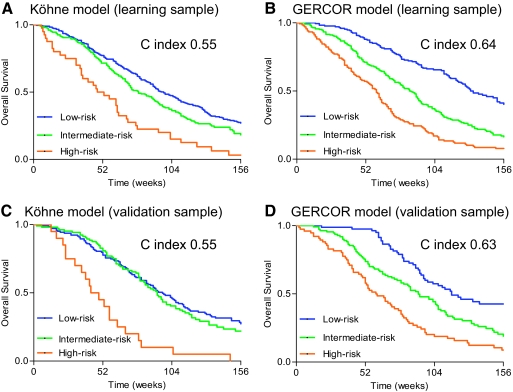
Figure 2.
Survivals in high-, intermediate-, and low-risk groups in Köhne and GERCOR models (learning and validation samples). (A): Köhne model in learning sample, (B): GERCOR model in learning sample, (C): Köhne model in validation sample, and (D): GERCOR model in validation sample.
Figure 3.
GERCOR prognostic model.
Abbreviations: CI, confidence interval; LDH, lactate dehydrogenase; OS, overall survival; PS performance status; ULN, upper limit of normal.
The median OS in the validation sample was 26.9 months (95% CI, 22.0–36.5) in the low-risk group (n = 84, 31%), 21.2 months (95% CI, 18.1–24.3) in the intermediate-risk group (n = 112, 42%), and 14.0 months (95% CI, 11.4–16.5) in the high-risk group (n = 72, 27%). According to low risk, univariate HR was 1.68 (95% CI, 1.20–2.37) for the intermediate-risk group and 2.84 (95% CI, 1.97–4.10) for the high-risk group. The Harrell's C index for this model was 0.63. Optimism-corrected Harrell C index was 0.61. As shown in supplemental online Figure 1, calibration remained satisfactory in the validation sample.
Köhne Model
By applying the Köhne model in the learning and validation samples, median OS was determined to be 22.5 months (95% CI, 20.6–25.1) (n = 294, 55%) and 22.4 months (95% CI, 19.5–25.7) (n = 143, 53%) in the low-risk group, 18.1 months (95% CI, 16.3–21.1) (n = 201, 38%) and 21.4 months (95% CI, 18.4–23.6) (n = 105, 39%) in the intermediate-risk group, and 11.1 months (95% CI, 8.3–14.5) (n = 40, 7%) and 9.9 months (95% CI, 5.5–14.4) (n = 20, 7%) in the high-risk group, respectively.
According to low risk, univariate HR was 1.31 (95% CI, 1.07–1.61) for the intermediate-risk group and 2.64 (95% CI, 1.86–3.74) for the high-risk group in the learning sample. According to low risk, univariate HR was 1.12 (95% CI, 0.84–1.50) for the intermediate-risk group and 3.73 (95% CI, 2.29–6.08) for the high-risk group in the validation sample. The Harrell's C index for this model was 0.55 for both samples (Table 4). Optimism-corrected Harrell C index was 0.52 in the learning sample and 0.50 in the validation sample. As shown in supplemental online Figure 1, calibration was average.
Discussion
We identified three risk groups for death depending on two baseline parameters, WHO PS and LDH level. Patients with good prognosis had WHO PS0 and normal LDH level at baseline, whereas patients with poor prognosis had WHO PS 1–2 with increased LDH level at baseline. An intermediate-risk group was defined as either WHO PS 0 and increased LDH level or WHO PS 1–2 with normal LDH level at baseline. Median OS in patients with good prognosis was twofold better than patients with poor prognosis in both learning and validation sample and 1.5-fold better than patients with an intermediate prognosis for survival. This simplified prognostic model with only two variables was more discriminate and easier to practice than a model with three variables (WHO PS, LDH level, and number of organs involved). Moreover, the distribution of the patients between the three groups (high-, intermediate-, and low-risk groups) was better in the simplified model than in the model with three variables and in the Köhne model. This model with only two variables will be evaluated in trials using targeted therapies [24].
In this study, LDH was the most important prognostic factor, with a twofold risk for mortality in the case of increased level at baseline. LDH has been previously identified as a prognostic factor in colorectal cancer [4, 5, 9, 11, 25] and other tumor localizations [26–30]. Of note, LDH has also been identified as a potential predictive factor for a vascular endothelial growth factor inhibitor and chemotherapy combination in patients with metastatic colorectal cancer [31].
After LDH, performance status was the strongest prognostic factor associated with OS, with a twofold risk for mortality in case of PS2 compared to patients with PS0 (HR = 2.00, p < .001). This result is consistent with a previous study that found that PS2 was prognostic for OS (HR = 2.18; p < .0001) [32].
The number of metastatic sites was the third independent prognostic factor identified in this study. As previously shown, a high alkaline phosphatase level at baseline was associated with shorter OS in the univariate analysis [33] but not in the multivariate analysis, like synchronous disease.
CEA level at baseline (p = .054) and age (p = .101) were not significant in univariate analysis. Sex, liver involvement, primitive tumor localization, and use of prior adjuvant chemotherapy were not prognostic factors in the univariate analysis. An adjuvant therapy without oxaliplatin or irinotecan was given in 20% of the patients. The use of oxaliplatin as adjuvant chemotherapy could influence the therapeutic strategy after relapse, leading to a potential impact on overall survival [34].
There were some drawbacks in the Köhne model, which was useful in the fluoropyrimidine era. Despite a strong association with survival in the univariate analysis (p < .0001), LDH was not introduced in the model, because its value was not available for 72% of the patients. Variations between centers with respect to cutoff values for ALP level were problematic. No information related to discriminant capabilities like Harrell's C index was reported stipulating that recursive partition and amalgamation was the better method to improve discrimination. When this model was applied to our patients treated with more recent chemotherapy regimens, it was not able to separate patients with low- and intermediate-risk for death. Better discrimination was found for our proposed score. Although it could be improved, this model had similar discrimination level (C index 0.64) to validated scores in other settings [35].
Two studies have evaluated the applicability of the Köhne model in patients treated with irinotecan- or oxaliplatin-based first-line chemotherapy. The first was a monocentric and retrospective series of 142 patients [36]. The median survival was 20.0, 15.7, and 6.8 months in the low-risk, intermediate-risk, and high-risk groups, respectively. A larger analysis from a randomized study involving 1,499 patients reported similar results [37]. These two studies have shown that the Köhne model was applicable in this setting. Nevertheless, >50% of the patients were in the low-risk group in the retrospective study, and in the second study, the low-risk and the intermediate-risk groups represented nearly 90% of the whole population.
Better discrimination is needed to improve therapeutic decisions. A combination model of prognostic and predictive factors could lead to a better selection of patients before initiating treatment. A complementary approach could combine different fields in the same model, such as clinico-biological markers, health-related quality of life, [38, 39], molecular markers such as KRAS, BRAF, and PTEN [40–42], gene expression signatures, and detection of circulating tumor cells. However, before identifying new biomarkers, this simple model with only two variables could be used in clinical research for patients' stratification and to adapt the strategy.
Serum LDH level at baseline and WHO PS were the main prognostic factors in predicting survival for patients with previously untreated metastatic colorectal cancer receiving either oxaliplatin-based or irinotecan-based first-line chemotherapy. These two variables should be available in all patients with metastatic colorectal cancer. Three risk groups for death were identified based on these two baseline parameters. This simple prognostic model can be useful for designing future clinical trials to ensure balanced randomization.
Supplementary Material
Acknowledgments
This work was supported by GERCOR (Groupe Coopérateur Multidisciplinaire en Oncologie), Paris, France. Presented in part at 2010 ASCO Annual Meeting.
Author Contributions
Conception/Design: Benoist Chibaudel, Franck Bonnetain, Christophe Tournigand, Pascal Artru, Thierry André, Christophe Louvet, Aimery de Gramont
Provision of study material or patients: Benoist Chibaudel, Christophe Tournigand, Pascal Artru, Thierry André, Christophe Louvet, Aimery de Gramont
Collection and/or assembly of data: Benoist Chibaudel, Franck Bonnetain, Christophe Tournigand, Thierry André, Christophe Louvet, Aimery de Gramont
Data analysis and interpretation: Benoist Chibaudel, Franck Bonnetain, Christophe Tournigand, Leïla Bengrine-Lefevre, Luis Teixeira, Pascal Artru, Jérôme Desramé, Annette K. Larsen, Thierry André, Christophe Louvet, Aimery de Gramont
Manuscript writing: Benoist Chibaudel, Franck Bonnetain, Christophe Tournigand, Leïla Bengrine-Lefevre, Luis Teixeira, Pascal Artru, Jérôme Desramé, Annette K. Larsen, Thierry André, Christophe Louvet, Aimery de Gramont
Final approval of manuscript: Benoist Chibaudel, Franck Bonnetain, Christophe Tournigand, Leïla Bengrine-Lefevre, Luis Teixeira, Pascal Artru, Jérôme Desramé, Annette K. Larsen, Thierry André, Christophe Louvet, Aimery de Gramont
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Rougier P, Milan C, Lazorthes F, et al. Prospective study of prognostic factors in patients with unresected hepatic metastases from colorectal cancer. Fondation Française de Cancérologie Digestive. Br J Surg. 1995;82:1397–1400. doi: 10.1002/bjs.1800821034. [DOI] [PubMed] [Google Scholar]
- 3.Giacchetti S, Perpoint B, Zidani R, et al. Phase III Multicenter Randomized Trial of Oxaliplatin Added to Chronomodulated Fluorouracil-Leucovorin as First-Line Treatment of Metastatic Colorectal Cancer. J Clin Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 4.de Gramont A, Figer A, Seymour M, et al. Leucovorin and Fluorouracil With or Without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 5.Saltz L, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 6.Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282–2292. doi: 10.1200/JCO.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Twelves C, Cassidy J, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19:4097–4106. doi: 10.1200/JCO.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]
- 8.Mitry E, Douillard JY, Van Cutsem E, et al. Predictive factors of survival in patients with advanced colorectal cancer: an individual data analysis of 602 patients included in irinotecan phase III trials. Ann Oncol. 2004;15:1013–1017. doi: 10.1093/annonc/mdh267. [DOI] [PubMed] [Google Scholar]
- 9.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 10.Colucci G, Gebbia V, Paoletti G, et al. Phase III Randomized Trial of FOLFIRI Versus FOLFOX4 in the Treatment of Advanced Colorectal Cancer: A Multicenter Study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23:4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 11.Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—a GERCOR study. J Clin Oncol. 2006;24:394–400. doi: 10.1200/JCO.2005.03.0106. [DOI] [PubMed] [Google Scholar]
- 12.Giacchetti S, Bjarnason G, Garufi C, et al. Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: The European Organisation for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol. 2006;24:3562–3569. doi: 10.1200/JCO.2006.06.1440. [DOI] [PubMed] [Google Scholar]
- 13.Souglakos J, Androulakis N, Syrigos K, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG) Br J Cancer. 2006;94:798–805. doi: 10.1038/sj.bjc.6603011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porschen R, Arkenau HT, Kubicka S, et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol. 2007;25:4217–4223. doi: 10.1200/JCO.2006.09.2684. [DOI] [PubMed] [Google Scholar]
- 15.Falcone A, Ricci S, Brunetti I, et al. Phase III Trial of Infusional Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan (FOLFOXIRI) Compared With Infusional Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) As First-Line Treatment for Metastatic Colorectal Cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 16.Köhne CH, Cunningham D, Di CF, et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13:308–317. doi: 10.1093/annonc/mdf034. [DOI] [PubMed] [Google Scholar]
- 17.Chibaudel B, Maindrault F, Lledo G, et al. Can a planned treatment break be given to patients with unresectable metastatic colorectal cancer? Results of the GERCOR OPTIMOX 2 study. J Clin Oncol. 2009;27:5727–5733. doi: 10.1200/JCO.2009.23.4344. [DOI] [PubMed] [Google Scholar]
- 18.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453–473. doi: 10.1002/(sici)1097-0258(20000229)19:4<453::aid-sim350>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34B:187–220. [Google Scholar]
- 22.Harrell FE, Lee KL, Mark DB, et al. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Gramont A, Haller DG, Sargent DJ, et al. Toward efficient trials in colorectal cancer: The ARCAD Clinical Trials Program. J Clin Oncol. 2010;28:527–530. doi: 10.1200/JCO.2009.25.2544. [DOI] [PubMed] [Google Scholar]
- 25.Kemeny N, Braun DW., Jr Prognostic factors in advanced colorectal carcinoma. Importance of lactic dehydrogenase level, performance status, and white blood cell count. Am J Med. 1983;74:786–794. doi: 10.1016/0002-9343(83)91066-5. [DOI] [PubMed] [Google Scholar]
- 26.Tas F, Aykan F, Alici S, et al. Prognostic factors in pancreatic carcinoma: serum LDH levels predict survival in metastatic disease. Am J Clin Oncol. 2001;24:547–550. doi: 10.1097/00000421-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Tas F, Aydiner A, Demir C, et al. Serum lactate dehydrogenase levels at presentation predict outcome of patients with limited-stage small-cell lung cancer. Am J Clin Oncol. 2001;24:376–378. doi: 10.1097/00000421-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Nisman B, Barak V, Hubert A, et al. Prognostic factors for survival in metastatic breast cancer during first-line paclitaxel chemotherapy. Anticancer Res. 2003;23:1939–1942. [PubMed] [Google Scholar]
- 29.Coiffier B, Gisselbrecht C, Vose JM, et al. Prognostic factors in aggressive malignant lymphomas. Description and validation of a prognostic index that could identify patients requiring a more intensive therapy. J Clin Oncol. 1991;9:211–219. doi: 10.1200/JCO.1991.9.2.211. [DOI] [PubMed] [Google Scholar]
- 30.Dimopoulos MA, Barlogie B, Smith TL, et al. High serum lactate dehydrogenase level as a marker for drug resistance and short survival in multiple myeloma. Ann Intern Med. 1991;115:931–935. doi: 10.7326/0003-4819-115-12-931. [DOI] [PubMed] [Google Scholar]
- 31.Hecht JR, Trarbach T, Jaeger E, et al. A randomized, double-blind, placebo-controlled, phase III study in patients (Pts) with metastatic adenocarcinoma of the colon or rectum receiving first-line chemotherapy with oxaliplatin/5-fluorouracil/leucovorin and PTK787/ZK 222584 or placebo (CONFIRM-1) J Clin Oncol. 2005;23:16S. (abstr LBA3) [Google Scholar]
- 32.Sargent DJ, Köhne CH, Sanoff HK, et al. Pooled Safety and Efficacy Analysis Examining the Effect of Performance Status on Outcomes in Nine First-Line Treatment Trials Using Individual Data From Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2009;27:1948–1955. doi: 10.1200/JCO.2008.20.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chibaudel B, Tournigand C, Artru P, et al. FOLFOX in patients with metastatic colorectal cancer and high alkaline phosphatase level: an exploratory cohort of the GERCOR OPTIMOX1 study. Ann Oncol. 2009;20:1383–1386. doi: 10.1093/annonc/mdp012. [DOI] [PubMed] [Google Scholar]
- 34.Tournigand C, Andre T, Bachet J, et al. FOLFOX4 as adjuvant therapy in elderly patients (pts) with colon cancer (CC): subgroup analysis of the MOSAIC trial. J Clin Oncol. 2010;28:15S. (abstr 3522) [Google Scholar]
- 35.Tournoux-Facon C, Paoletti X, Barbare JC, et al. Development and validation of a new prognostic score of death for patients with hepatocellular carcinoma in palliative setting. J Hepatol. 2011;54:108–114. doi: 10.1016/j.jhep.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Díaz R, Aparicio J, Gironés R, et al. Analysis of prognostic factors and applicability of Köhne's prognostic groups in patients with metastatic colorectal cancer treated with first-line irinotecan or oxaliplatin-based chemotherapy. Clin Colorectal Cancer. 2005;5:197–202. doi: 10.3816/ccc.2005.n.031. [DOI] [PubMed] [Google Scholar]
- 37.Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol. 2008;26:5721–5727. doi: 10.1200/JCO.2008.17.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Efficace F, Innominato PF, Bjarnason G, et al. Validation of patient's self-reported social functioning as an independent prognostic factor for survival in metastatic colorectal cancer patients: results of an international study by the Chronotherapy Group of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2008;26:2020–2026. doi: 10.1200/JCO.2007.12.3117. [DOI] [PubMed] [Google Scholar]
- 39.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10:865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 40.Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 41.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 42.Laurent-Puig P, Cayre A, Manceau G, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924–5930. doi: 10.1200/JCO.2008.21.6796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.