Abstract
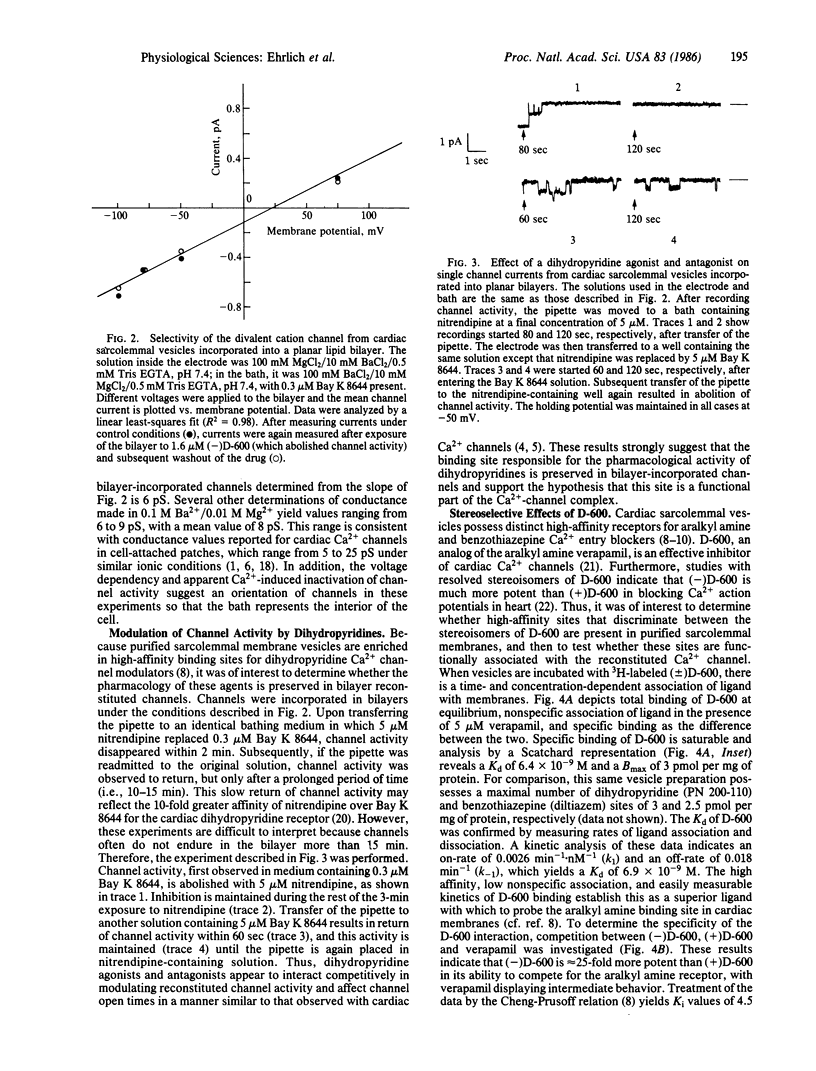
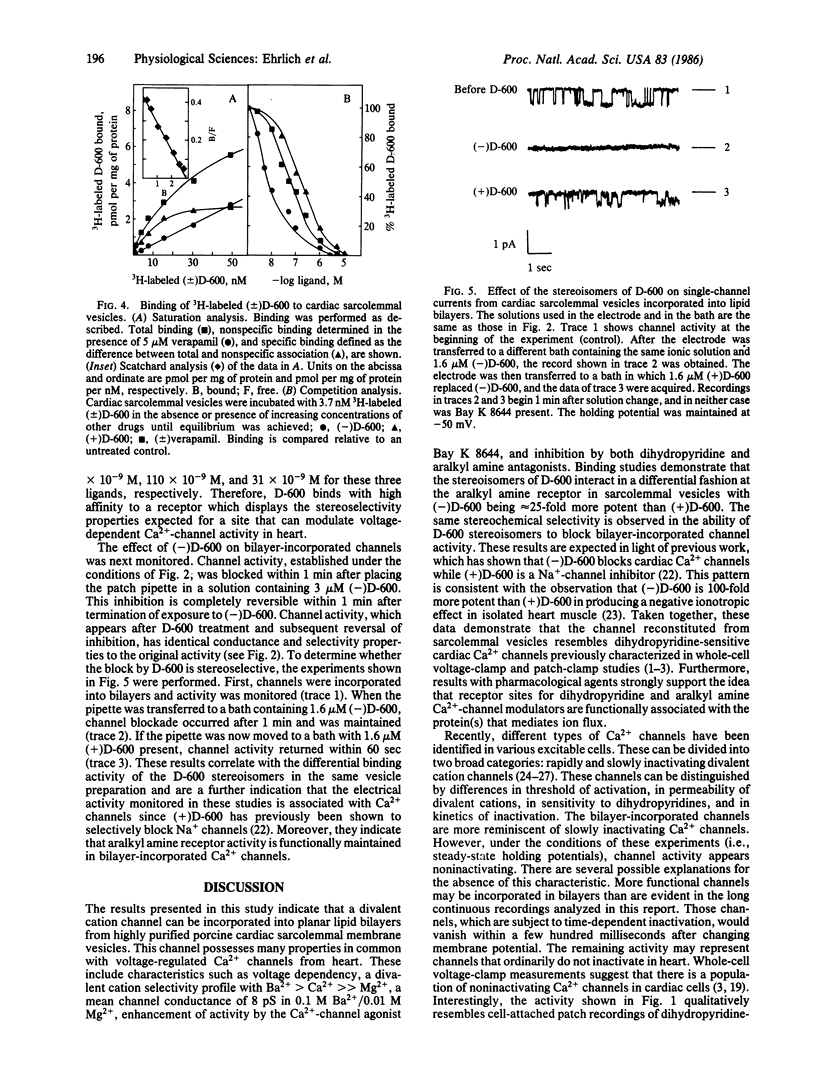
When purified porcine cardiac sarcolemmal membrane vesicles are incorporated into planar lipid bilayers formed at the tip of patch electrode pipettes, individual divalent cation channels can be monitored. Channel activity is increased in the presence of the Ca2+ channel agonist Bay K 8644, is voltage dependent, and selects for divalent cations over anions. The activity does not inactivate because it is maintained during prolonged depolarizations. Determination of divalent cation selectivity from the reversal potential of single-channel currents indicates a relative permeability ratio for Ba/Ca/Mg of 1:0.45:0.08. Mean channel conductance in 0.1 M Ba2+/0.01 M Mg2+ is 8 pS. Channels are reversibly blocked by the Ca2+ channel inhibitor nitrendipine, and inhibition can be competitively antagonized by Bay K 8644. Binding studies with 3H-labeled D-600 demonstrate the presence of high-affinity receptors for D-600 in sarcolemmal membranes (Kd = 6.4 X 10(-9) M; Bmax = 3 pmol per mg of protein). In addition, experiments with resolved D-600 stereoisomers indicate that (-)D-600 is at least 25-fold more potent than (+)D-600 in competing for this aralkyl amine receptor. Consistent with this, (-)D-600 is much more effective than the (+) isomer in inhibiting bilayer-incorporated channels. These results demonstrate that the divalent cation channel that has been reconstituted in planar lipid bilayers possesses many of the characteristics of voltage-regulated Ca2+ channels in heart and suggest that receptors for Ca2+ entry blockers are functionally associated with this channel.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter H., Coronado R. Agonists Bay-K8644 and CGP-28392 open calcium channels reconstituted from skeletal muscle transverse tubules. Biophys J. 1985 Aug;48(2):341–347. doi: 10.1016/S0006-3495(85)83789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Matteson D. R. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science. 1985 Jan 4;227(4682):65–67. doi: 10.1126/science.2578071. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Swenson R. P., Jr, Taylor S. R. Block of squid axon K channels by internally and externally applied barium ions. J Gen Physiol. 1982 Nov;80(5):663–682. doi: 10.1085/jgp.80.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer R., Kalusche D., Kaufmann R., Mannhold R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. III. Effects of the optical isomers on transmembrane action potentials. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(1):81–97. doi: 10.1007/BF00499991. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Ochi R., Pelzer D., Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflugers Arch. 1983 Sep;398(4):284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Coronado R., Latorre R. Phospholipid bilayers made from monolayers on patch-clamp pipettes. Biophys J. 1983 Aug;43(2):231–236. doi: 10.1016/S0006-3495(83)84343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich B. E., Finkelstein A., Forte M., Kung C. Voltage-dependent calcium channels from Paramecium cilia incorporated into planar lipid bilayers. Science. 1984 Jul 27;225(4660):427–428. doi: 10.1126/science.6330895. [DOI] [PubMed] [Google Scholar]
- Garcia M. L., Trumble M. J., Reuben J. P., Kaczorowski G. J. Characterization of verapamil binding sites in cardiac membrane vesicles. J Biol Chem. 1984 Dec 25;259(24):15013–15016. [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Holck M., Thorens S., Haeusler G. Characterization of [3H]nifedipine binding sites in rabbit myocardium. Eur J Pharmacol. 1982 Dec 3;85(3-4):305–315. doi: 10.1016/0014-2999(82)90217-5. [DOI] [PubMed] [Google Scholar]
- Janis R. A., Rampe D., Sarmiento J. G., Triggle D. J. Specific binding of a calcium channel activator, [3H]BAY k 8644, to membranes from cardiac muscle and brain. Biochem Biophys Res Commun. 1984 May 31;121(1):317–323. doi: 10.1016/0006-291x(84)90725-3. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Reuter H. Dihydropyridine derivatives prolong the open state of Ca channels in cultured cardiac cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4824–4827. doi: 10.1073/pnas.81.15.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Alvarez O., Cecchi X., Vergara C. Properties of reconstituted ion channels. Annu Rev Biophys Biophys Chem. 1985;14:79–111. doi: 10.1146/annurev.bb.14.060185.000455. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., French R. J., Krueger B. K. Voltage-dependent calcium channels from brain incorporated into planar lipid bilayers. Nature. 1984 Mar 1;308(5954):77–80. doi: 10.1038/308077a0. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Long-opening mode of gating of neuronal calcium channels and its promotion by the dihydropyridine calcium agonist Bay K 8644. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2178–2182. doi: 10.1073/pnas.82.7.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F., Tsien R. W., Yellen G. Properties of single calcium channels in cardiac cell culture. Nature. 1982 Jun 10;297(5866):501–504. doi: 10.1038/297501a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. L., Tomiko S. A., Agnew W. S. Single-channel properties of the reconstituted voltage-regulated Na channel isolated from the electroplax of Electrophorus electricus. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5594–5598. doi: 10.1073/pnas.81.17.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Uehara A., Hume J. R. Interactions of organic calcium channel antagonists with calcium channels in single frog atrial cells. J Gen Physiol. 1985 May;85(5):621–647. doi: 10.1085/jgp.85.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]



