Patients with locally advanced rectal cancer (cUICC stages II/III) are typically treated with preoperative 5-fluorouracil–based (5-FU–based) radiochemotherapy. However, trials are currently being conducted to improve the complete remission rates and the systemic control by combining 5-FU with oxaliplatin in order to identify the subgroups of rectal cancer patients at risk for high-grade toxicity. The results indicate that there are basic clinical parameters, such as gender and body mass index, that may be potential markers for generating individual risk profiles of radiochemotherapy-induced toxicity.
Keywords: Rectal cancer, Gender effect, Radiochemotherapy, Toxicity, Body mass index
Learning Objectives
After completing this course, the reader will be able to:
Describe present strategies of treatment of locally advanced rectal cancer and ongoing clinical trials, including neoadjuvant radiochemotherapy with 50.4 Gy and concomitant 5-FU +/− oxaliplatin.
Define the basic clinical parameters, with special emphasis on gender and BMI, correlating with radiochemotherapy-associated side effects in rectal cancer patients and differences in severity of toxicity.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Patients with locally advanced rectal cancer (cUICC stages II/III) are typically treated with preoperative 5-fluorouracil–based (5-FU–based) radiochemotherapy (RCT). However, trials are currently being conducted to improve the complete remission rates and the systemic control by combining 5-FU with oxaliplatin. The primary objective was to identify the subgroups of rectal cancer patients who were at risk for high-grade toxicity.
All 196 patients who were included in the present study were treated with 50.4 Gy and chemotherapy that included either 5-FU (n = 115) or 5-FU+oxaliplatin (n = 81). The preoperative RCT was followed by a total mesorectal excision and adjuvant chemotherapy. Acute toxicity was monitored weekly and a toxicity grade ≥3 (Common Toxicity Criteria) for a skin reaction, cystitis, proctitis, or enteritis was defined as high-grade acute organ toxicity. After RCT with 5-FU+oxaliplatin, complete tumor remission was achieved in 13.6% of the patients and in 11.3% after RCT with 5-FU alone.
Complete irradiation dosages of 50.4 Gy were given to 99% (5-FU) and 95% (5-FU+oxaliplatin) of the patients. Concomitant chemotherapy was fully administered in 95% of the patients treated with 5-FU compared with the 84% of patients treated with 5-FU+oxaliplatin.
A significantly higher proportion of acute organ toxicity was found in the patients who were treated with 5-FU+oxaliplatin compared with those who were treated with 5-FU. Additionally, women with a low body mass index were at the highest risk for acute organ toxicity.
These results suggest that there are basic clinical parameters, such as gender and body mass index, that may be potential markers for generating individual risk profiles of RCT-induced toxicity.
Introduction
Rectal cancer is a common oncological diagnosis in the Western world [1] and treating rectal cancer is a major socioeconomic and health issue [2]. The German Rectal Cancer Study Group and other groups [3–6] have shown that preoperative radiotherapy (RT) combined with 5-FU chemotherapy (used as a radiosensitizer) improves locoregional tumor control with less acute and chronic toxicity compared with postoperative radiochemotherapy (RCT). Therefore, this preoperative setting became the standard treatment for stage II and III rectal cancer, as defined by the Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) [7]. Although local control rates were improved by this preoperative multimodal strategy, distant metastases still remained the major mode of failure and recurrence rates of 30%–45% have been reported 5 years after treatment [1, 3]. Intensified chemotherapy regimens, including standard RCT with 5-FU monotherapy and additional oxaliplatin, have been tested in different clinical trials to increase the rate of complete pathological tumor regression (pCR) and long-term progression-free survival [6].
After chemotherapy treatment modalities are intensified, the risk of high-grade acute toxicity during therapy increases [8–11]. Gérard et al. [12] observed a nonsignificant positive trend after 5-FU+oxaliplatin treatment, as compared with 5-FU (capecitabine) alone, in preoperative RCT for rectal cancer patients in the ACCORD 12/0405-Prodige 2 trial. This trend was based on the pathological complete response rates (19% versus 14%, p = .11) and the high-grade acute organ toxicity rates significantly increased (15% versus 3%) when the treatment was intensified with additional oxaliplatin. Long-term follow-up results from intensified RCT regimes that included oxaliplatin are not currently available.
The goal of the present study was to identify subgroups with a higher risk of severe toxicity during intensified preoperative RCT. The current analysis also aimed to correlate those groups with the rate of pCR in patients with locally advanced rectal cancer that was treated within prospective randomized trials (CAO/AIO/ARO-94 [13], XelOx [14], and the ongoing CAO/AIO/ARO-04 [6]).
To identify reliable markers for predicting tumor response and treatment-related toxicity, a risk profile that is associated with tailoring of RCT could be a promising step toward the individualization of risk-adapted treatment in rectal cancer patients.
Patients and Methods
Patient Characteristics
Between March 2001 and April 2010, 196 patients with rectal cancer (cUICC stage II or III), with tumors localized in the middle (6–12 cm) or the lower third (≤6 cm) of the rectum, were treated according to the protocols of the prospective clinical phase II or phase III trials (CAO/AIO/ARO-94 [13], XelOx [14], CAO/ARO/AIO-04 [6]) at the University Medical Center of Göttingen.
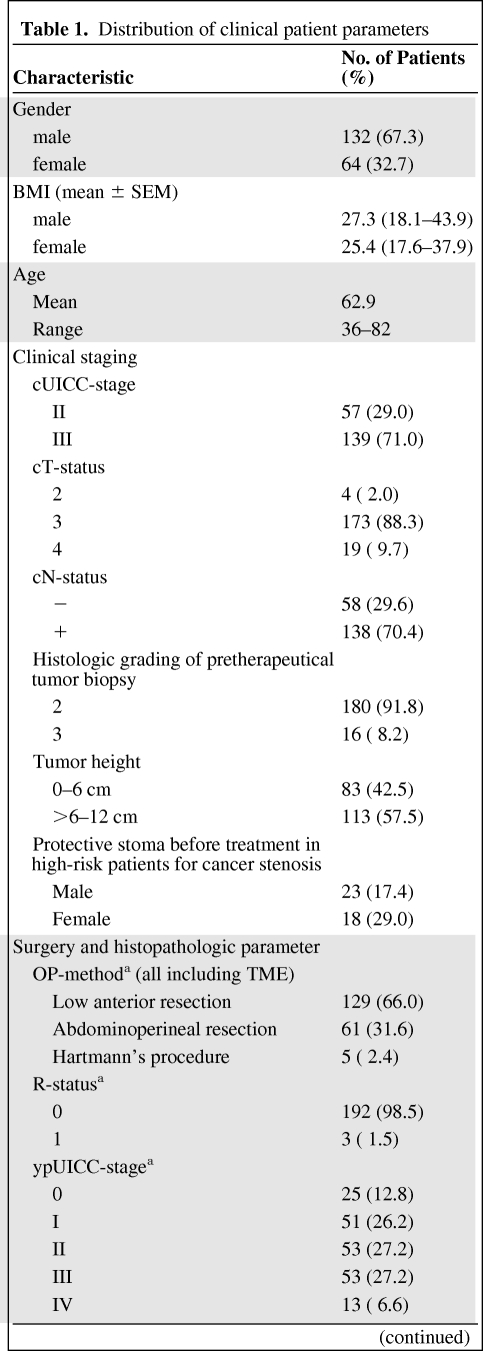
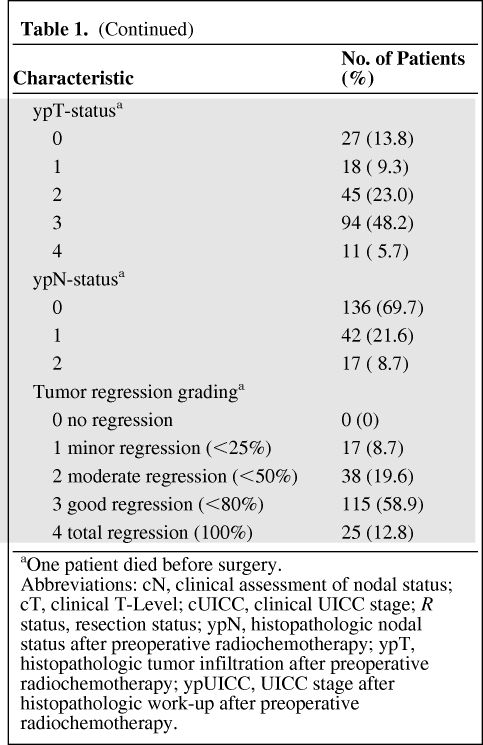
Of the 196 patients, 132 patients were male and 64 were female. The patients' ages at the time of the diagnosis ranged from 36 to 82 years (the median age was 63 years). Tumor staging was performed according to the UICC/AJCC criteria [7]. The initial staging procedures included a medical history, a clinical examination, complete peripheral blood counts, a carcinoembryonic antigen (CEA) test, a chest x-ray, and a tumor biopsy, which was located within 12 cm of the anocutaneous verge, as measured by rigid rectoscopy. According to the study protocols, the pretherapeutic staging was completed using an endorectal ultrasound and contrast-enhanced magnetic resonance imaging (MRI) of the pelvis to confirm the presence of locally advanced, but resectable, rectal cancer. Contrast-enhanced computed tomography (CT) scans of the chest, abdomen, and pelvis were performed to identify patients with evidence of distant metastatic disease. The distribution of the tumor stages is shown in Table 1. Specifically, 57 (29.1%) patients were classified as UICC stage II and 139 (70.9%) of the patients were classified as UICC stage III before multimodal treatment. The lower border of all of the tumors was between 0 and 12 cm when the tumor was measured from the anal verge using rigid endoscopy. Therefore, 90 (46%) tumors were localized ≤6 cm of the rectum and 106 (54%) tumors were localized between >6 and 12 cm of the rectum. All of the tumors were histologically characterized as adenocarcinomas.
Table 1.
Distribution of clinical patient parameters
Table 1a.
(Continued)
aOne patient died before surgery.
Abbreviations: cN, clinical assessment of nodal status; cT, clinical T-Level; cUICC, clinical UICC stage; R status, resection status; ypN, histopathologic nodal status after preoperative radiochemotherapy; ypT, histopathologic tumor infiltration after preoperative radiochemotherapy; ypUICC, UICC stage after histopathologic work-up after preoperative radiochemotherapy.
RCT might induce an initial tumor swelling that can lead to stenosis in patients with substenotic tumor growth. Therefore, all of the patients at high risk for an ileus under preoperative RCT received a protective ileostoma before the start of the RCT (n = 41, 20.9%) because of an interdisciplinary decision by the local tumor board for gastrointestinal cancer.
The pretreatment patient characteristics before RCT are summarized in Table 1.
All of the procedures were conducted according to the ethical standards of the committee on human experimentation and the Helsinki Declaration of 1975, which was revised in 2000. All of the study protocols were approved by the institutional ethics committee. Informed consent was obtained from all of the patients after they were provided with a detailed explanation of the treatment procedures.
Radiochemotherapy
The radiotherapy was delivered using a Varian Clinac 600 C/D accelerator (Varian, Palo Alto, CA). The dose was defined according to the International Commission on Radiation Units and Measurements' Report [15]. All of the patients received 3D-planned irradiation with 20 MV photons. The daily fraction size was 1.8 Gy (5 times per week), with a total dose of 50.4 Gy. As described previously, the target volume definition was determined according to the guidelines from the trials of the German Rectal Cancer Study Group [13, 14].
All of the patients were treated while in a prone position, using a belly board to reduce the doses for the small bowels [16]. Additionally, gold markers were implanted in the tumor region of the patients with tumors that were localized more than 5 cm above the anal sphincter [17]. These markers provided a more precise definition of the target volume while protecting the anal structures. Dose-volume-histogram analyses were performed and monitored to evaluate the impact of hot spots and the differential exposure on organs that were at risk for high-grade acute organ toxicity.
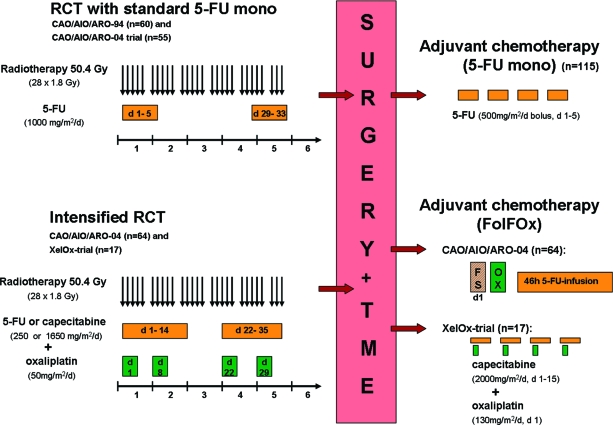
Concomitant chemotherapy was administered according to the study protocols for the CAO/AIO/ARO-94, XelOx, and CAO/AIO/ARO-04 trials. Before therapy, all of the patients were tested for a dihydropyrimidine dehydrogenase deficiency to prevent severe adverse events associated with the administration of 5-FU [18]. The treatment details are summarized in Figure 1.
Figure 1.
Treatment of studied patients. One hundred fifteen patients underwent preoperative RCT with 5-FU monotherapy and concomitant radiotherapy. Five to 6 weeks after completion of RCT, all patients underwent surgery. Adjuvant chemotherapy consisted of either 5-FU monotherapy or a combination of 5-FU with additional oxaliplatin.
Abbreviations: 5-FU, 5-fluorouracil; d, day; RCT, radiochemotherapy; TME, total mesorectal excision.
The level of toxicity was monitored weekly during RCT and every second week until the acute side effects of the preoperative therapy disappeared. The acute side effects were classified using the Common Toxicity Criteria (CTC 3.0) score [19, 20] and the late side effects (which occurred >90 days after the RCT) were categorized according to the LENT (Late Effects of Normal Tissue) scoring system for chronic toxicity [21, 22]. The follow-up examinations included rectoscopy, abdominal ultrasound, contrast-enhanced computed tomography of the abdomen/pelvis, and an x-ray of the chest. Follow-up examinations were performed at 3-month intervals during the first 2 years and at 6-month intervals after 2 years, according to guidelines of the German Cancer Society [23]. For the following analyses, the highest CTC score concerning acute toxicity during treatment was assessed, which included one item or more of the following: skin reaction, enteritis, proctitis, or cystitis.
Surgery and Histopathological Examination
Five to 6 weeks after the preoperative RCT was completed, a quality-controlled rectal resection, including a total mesorectal excision, was performed using a standard technique [24]. Assessment of the intended surgical procedure and of the possibility of sphincter preservation was performed by the surgeon before the oncological resection. The quality of the surgical resection was documented perioperatively with an injection of methylene blue into the inferior mesenteric artery to assess the integrity of the mesorectal fascia [25]. The total mesorectal excision specimen was macroscopically examined by a pathologist using the MERCURY criteria [26].
The residual tumor tissue in the resected specimen was classified according to the TNM staging system of the UICC [7]. The residual tumor mass and any RCT-induced fibrotic changes were semiquantitatively evaluated using an established five-point rectal cancer regression grading system [27, 28]. Briefly, tumor samples without any fibrosis/regression were considered to be TRG 0, whereas complete regression (TRG 4) was defined as the absence of viable tumor cells in the primary tumor and in the lymph nodes (ypT0N0). The tumor samples that were comprised of >75% viable tumor cells (<25% fibrosis) were considered to be TRG 1. A regression of 25% to 50% was classified as TGR 2, and a regression of >50% was classified as TRG 3.
Statistical Methods and Subgroup Analyses
After the data assessment, clinical parameters (such as the body mass index [BMI] or gender), treatment regime, and the presence of pretherapeutically created stomata were analyzed according to their grades of toxicity. Statistical significance was assessed using the Wilcoxon rank test to compare the toxicity levels between the two groups (e.g., 5-FU therapy versus 5-FU+oxaliplatin therapy). For the continuous and multigroup variables, correlation tests were performed using the Spearman rank correlation. Comparisons between the ordinal variable toxicities and the quantitative variable BMIs were performed using the Kruskal-Wallis test. Because the results of the univariate analyses did not have more than one significant result per item, the multivariate analyses were not performed. The analyses were performed using the statistical computing software R. p-values <.05 were considered to be statistically significant.
Results
Surgical Procedures
The surgical procedures consisted of 141 (73%) low anterior resections, 50 (26%) abdominoperineal resections, and 3 (1%) Hartmann's procedures (discontinuous resection). All of the resection procedures included a total mesorectal excision and a perioperatively performed staining of the mesorectal fascia and tissue with methylene blue via the arteria mesenterica [29]. A complete resection (R0), including a negative circumferential resection margin [4], was performed in all of the patients. In one patient, the surgery was not conducted because of death, which was unrelated to the treatment and occurred during the interval between the completion of the intensified RCT and the planned surgery. However, the toxicity data during the RCT were regularly monitored for this patient and were included in the analyses.
Pathological Characteristics and the Influence of the Treatment Arm
The histopathologic tumor regression was analyzed after the RCT and the surgery. TRG 4 was observed in 25 patients (12.8%), TRG 3 was observed in 115 patients (58.9%), TRG 2 was observed in 38 patients (19.6%), and TRG 1 was observed in 17 patients (8.7%). When both treatment arms were analyzed, 11 patients (13.6%) that were treated in the intensified treatment arm displayed TRG 4 compared with the 13 patients (11.3%) from the standard treatment arm. In conclusion, no statistically significant difference was observed among the groups when all of the grades of tumor regression were included in the analysis (p = .37).
Compliance and Toxicity
The irradiation was planned and applied for a time window of <44 days for all of the patients, as commonly recommended [30]. Overall, 190 of 196 patients (97%) received the intended dose of radiotherapy. In the other six patients (five receiving 5-FU+oxaliplatin and one receiving 5-FU), a dose reduction (mean 5.4 Gy) that led to a minimum irradiated dose of 43 Gy at the end of planned irradiation was necessary because of acute side effects with a CTC score grade ≥3 (proctitis was observed in three patients, a skin reaction was observed in two patients, and one patient developed enteritis).
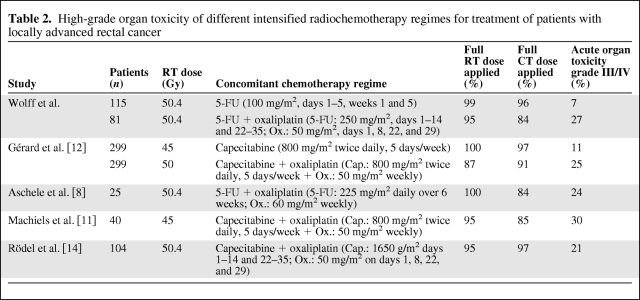
The discontinuation of chemotherapy occurred in 5 patients that were in the group that received standard chemotherapy after the first cycle (1 patient exhibited hand-foot syndrome, 2 patients had coronary spasms, and 2 patients developed distinctive treatment-associated exanthema) and 13 patients in the intensified regime stopped receiving chemotherapy (7 patients exhibited pronounced diarrhea, 4 patients exhibited pronounced interleukin-releasing syndrome, and 2 patients exhibited an electrolyte imbalance). Overall, 90.8% of the patients received concomitant chemotherapy, according to the study protocols [13, 14]. The RCT dosages are presented in Table 2.
Table 2.
High-grade organ toxicity of different intensified radiochemotherapy regimes for treatment of patients with locally advanced rectal cancer
The distribution of the acute toxicity symptoms during RCT was as follows: a skin reaction was observed in 170 (86.7%) patients (85 patients with a grade 1 reaction, 73 patients with a grade 2 reaction, and 12 patients with a grade 3 reaction). Overall, 68 (34.6%) of the 196 patients developed cystitis during RCT (58 patients exhibited grade 1, 7 patients exhibited grade 2, and 3 patients exhibited grade 3). Proctitis was observed in 175 patients (110 patients exhibited grade 1, 51 patients exhibited grade 2, 11 patients exhibited grade 3, and 3 patients exhibited grade 4). Additionally, 66 patients developed enteritis (47 patients classified as grade 1, 10 patients classified as grade 2, 4 patients classified as grade 3, and 5 patients classified as grade 4).
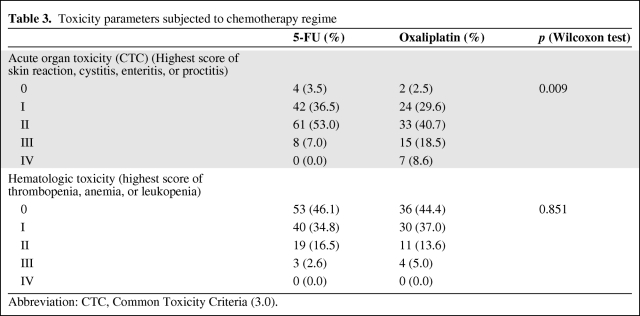
Overall, the prevalence of acute organ toxicity for grades 3 and 4 was 7.0% in the standard treatment regime and 27.1% in the group that received RCT with 5-FU+oxaliplatin (p = .009) (Table 3).
Table 3.
Toxicity parameters subjected to chemotherapy regime
Abbreviation: CTC, Common Toxicity Criteria (3.0).
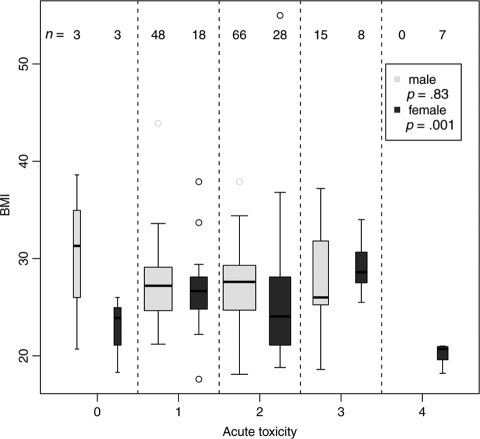
Gender and BMI correlated significantly with the acute toxicity classes (Kruskal-Wallis test p = .001) (Figure 2): All seven grade 4 toxicities occurred exclusively in women who received intensified RCT (5-FU+oxaliplatin) and had a low BMI (<22 kg/m2). Furthermore, a gender-specific evaluation revealed that, for male patients, BMI had no significant effect on observed toxicity.
Figure 2.
Acute toxicity for all patients subjected to gender and body mass indices. p-values for the two groups male and female were computed using the Kruskal-Wallis test.
To preclude irradiation technique-related influences on the individual incidence of high-grade toxicity, such as a failed bowel exclusion during the use of the belly board (especially for patients with low body weights) or different exposures for organs at risk due to individual anatomy or tumor localization, detailed dose-volume-histogram analyses were performed. These analyses indicated that there were no noticeable differences among the tested parameters for the patients with or without high-grade toxicity. Specifically, the results for the seven women with low BMIs and grade 4 toxicity in the intensified chemotherapy arm were unremarkable without hot spots in sensitive organs.
The patients with pretherapeutically created stomata (n = 41) did not exhibit any significant differences when compared with the patients without stomata and these two groups of patients exhibited similar levels of high-grade acute organ toxicity during therapy (12% versus 14%).
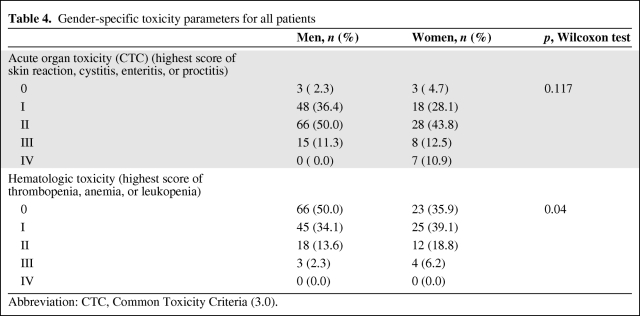
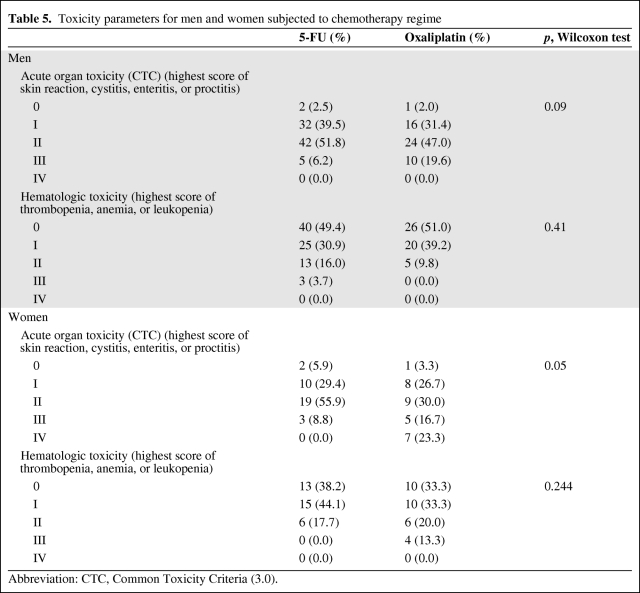
Acute hematological toxicity during RCT appeared very infrequently and no grade 4 toxicity was observed in the present study. Specifically, 27 patients exhibited grade 1 anemia, 20 patients exhibited grade 2 anemia, and 2 patients exhibited grade 3 anemia. Thus, substitution therapy with two erythrocyte concentrates was necessary in 2 patients. Grade 1 leukopenia was observed in 68 patients, grade 2 leukopenia was observed in 17 patients, and grade 3 leukopenia was observed in 5 patients. Grade 1 thrombocytopenia was observed in 5 patients. After analyzing the subgroups for hematological toxicity, we found that there were no significant differences in treatment arm, gender, or BMI (Tables 3, 4, and 5).
Table 4.
Gender-specific toxicity parameters for all patients
Abbreviation: CTC, Common Toxicity Criteria (3.0).
Table 5.
Toxicity parameters for men and women subjected to chemotherapy regime
Abbreviation: CTC, Common Toxicity Criteria (3.0).
Discussion
Currently, patients with locally advanced rectal cancer (cUICC II/III) are being treated in clinical prospective trials with intensified preoperative RCT regimes to achieve higher levels of complete histopathologically confirmed remission rates and to improve systemic tumor control. In the patient cohorts of the present study that were treated according to standard (5-FU) or intensified (additional oxaliplatin) RCT protocols, we found a significantly higher proportion of high-grade acute organ toxicity in the treatment group that received 5-FU+oxaliplatin. In addition, a gender-specific distribution of the data revealed that women had a significantly higher risk of developing high-grade acute toxicity during therapy. Furthermore, all seven incidences of grade 4 toxicity occurred in female patients with comparatively low BMIs who were treated with additional oxaliplatin. Notably, a gender-specific risk of developing this toxicity was not reported in the reviewed literature.
The findings of the present study might be useful to conduct patient-adapted risk stratification in future clinical trials.
Concerning the influence of BMI on toxicity, our results were similar to those in the literature. For example, Meyerhardt et al. [31] found that individuals with a normal weight and advanced rectal cancer developed a higher rate of adjuvant chemotherapy-related toxicity (grades 3 and 4) compared with obese patients. Furthermore, other studies that analyzed the BMI of patients with colonic, breast, or lung cancer also reported this trend [32–34]. This finding could be explained by a different level of absorption of the chemotherapy agent due to different body volumes and muscle-to-fat ratios.
The patients in our study who needed a protective stoma attachment before RCT to adjust for conditions, such as constricted tumor growth or tumor localization near the anal sphincter, were assumed to be at high risk for tumor-related ileus or perforation. However, these patients (n = 41) may have been prevented from achieving complete stenosis because of tumor swelling that was caused by the initial RT. These patients were able to receive the prescribed dose of the cumulative preoperative RCT without interruptions. Therefore, these patients benefited from this procedure, when considering the comparable toxicity rates between the patients with and without protective stomata.
Previous studies on prostate and rectal cancer reported a correlation between acute organ toxicity and the incidence of late side effects. For example, Schultheiss et al. [35] described a significantly higher rate of late gastrointestinal toxicity after the occurrence of acute gastrointestinal organ toxicity. In another study by Denham et al. [36], the presence of acute proctitis was a significant factor for predicting the occurrence of three late symptoms (urgency, frequency, and diarrhea) and for predicting the late EORTC/RTOG score (p < .05). Because the follow-up results for the late toxicity are not yet available for the entire patient cohort, it remains to be seen whether the occurrence of acute toxicity also predicts late side effects in these patients.
The results of the current monocentric study demonstrated that additional oxaliplatin application with 5-FU during preoperative RT did not result in a significantly higher rate of histopathologically confirmed regression in patients with UICC stage II or III rectal cancer (13.6% versus 11.3% [5-FU alone], p = 0.37). We analyzed a cohort of patients from three prospective clinical trials. At the moment, a final conclusion cannot be drawn until the definitive results of the ongoing CAO/AIO/ARO-04 trial are available. However, results similar to those in the present study were reported by Gérard et al. [12] after analyzing the results of the ACCORD 12/0405-Prodige 2 trial. In this study, additional oxaliplatin was not found to be more effective than 5-FU alone for preoperative RCT in rectal cancer patients. However, a larger trend was observed in the tumor response rate, which was 19.2% (for 5-FU + oxaliplatin) versus 13.9% (for 5-FU alone) (p = .09). In conclusion, the authors recommended omitting the use of oxaliplatin with concurrent irradiation because of the occurrence of increased early toxicity. Analogous results with higher rates of acute toxicity have also been reported in several other studies (Table 2). Therefore, clinical characteristics and additional biomarkers, for example, survivin, are required for risk stratification in rectal cancer patients [37, 38].
Proven indications exist for the use of intensified chemotherapy as a standard regimen for advanced stage local tumors and expanding tumor growth [12, 14, 39, 40]. The use of an induction chemotherapy followed by standard RCT with 5-FU monotherapy might be more effective concerning tumor response without increasing acute toxicity during RCT [41, 42]. Another possible benefit of this therapy sequence would be the ability to administer the scheduled chemotherapy dosage over time, which often needs to be reduced in an adjuvant setting [43, 44].
Several ongoing and future trials have been designed to investigate treatment with an induction chemotherapy regimen before preoperative RCT because the first phase I and phase II studies have already shown the feasibility of these induction regimens [40, 45].
It remains to be seen whether toxicity rates and overall outcome will change as a result of the use of these new concepts. However, the long-term results for overall- and disease-free survival are not available for the ongoing studies and the risks of high-grade acute organ toxicity must be taken into account. These risks should be considered especially for women with low BMIs to avoid serious complications. The interruption of therapy or a dose reduction because of high-grade acute organ toxicity should be avoided whenever possible [30, 46]. Thus, for patients at a high risk of organ toxicity, the best standard of care should include a stationary survey or a frequently monitored, ambulant, interdisciplinary treatment to complete the planned therapy schedule, especially for patients receiving intensified regimens. To ensure compliance, we implemented close monitoring for high-risk patients, which included full inpatient treatment during the combined chemotherapy regimen and a daily physical examination during ambulant irradiation at the University Medical Center Göttingen. Furthermore, the development of new irradiation techniques, such as intensity-modulated volumetric arcs or protons, might reduce the exposure of organs at risk and normal tissues without compromising tumor control rates.
Conclusion
The current study identified basic clinical parameters, such as gender and BMI, as potential markers for generating individual risk profiles of RCT-induced toxicity.
The results of the present study need to be validated in future clinical trials. If the current findings are replicated, the gender and BMI of a patient will be useful parameters in choosing a treatment modality. The final goal is to provide data that will result in the development of the best therapy for individual patients and will lead to maximized response rates and minimized therapy-associated toxicity.
Acknowledgments
This work was supported by the German Research Foundation (DFG, KFO 179). All authors are participants of the KFO 179 (clinical research unit 179) of the University Medical Center Göttingen, Germany.
H.A.W. and L.-C.C. share first authorship. H.C. and T.L. share senior authorship.
This manuscript was edited by the American Journal Experts editorial service.
Author Contributions
Conception/Design: Hendrik Andreas Wolff, Lena-Christin Conradi, Margret Rave-Fränk, Clemens Friedrich Hess, Heinz Becker, Hans Christiansen, Torsten Liersch
Provision of study material or patients: Hendrik Andreas Wolff, Lena- Christin Conradi, Thilo Sprenger, Steffen Hennies
Collection and/or assembly of data: Hendrik Andreas Wolff, Lena-Christin Conradi, Markus Schirmer, Tim Beissbarth, Thilo Sprenger, Steffen Hennies
Data analysis and interpretation: Hendrik Andreas Wolff, Lena-Christin Conradi, Markus Schirmer, Tim Beissbarth, Clemens Friedrich Hess, Heinz Becker, Hans Christiansen, Torsten Liersch
Manuscript writing: Hendrik Andreas Wolff, Lena-Christin Conradi, Margret Rave-Fränk, Hans Christiansen, Torsten Liersch
Final approval of manuscript: Hendrik Andreas Wolff, Lena-Christin Conradi, Markus Schirmer, Tim Beissbarth, Thilo Sprenger, Margret Rave-Fränk, Steffen Hennies, Clemens Friedrich Hess, Heinz Becker, Hans Christiansen, Torsten Liersch
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Midgley R, Kerr D. Colorectal cancer. Lancet. 1999;353:391–399. doi: 10.1016/S0140-6736(98)07127-X. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 4.Nagtegaal ID, Gosens MJ, Marijnen CA, et al. Combinations of tumor and treatment parameters are more discriminative for prognosis than the present TNM system in rectal cancer. J Clin Oncol. 2007;25:1647–1650. doi: 10.1200/JCO.2005.05.4825. [DOI] [PubMed] [Google Scholar]
- 5.Crane CH, Janjan NA, Mason K, et al. Preoperative chemoradiation for locally advanced rectal cancer: emerging treatment strategies. Oncology (Williston Park) 2002;16:39–44. [PubMed] [Google Scholar]
- 6.Rödel C, Sauer R. Integration of novel agents into combined-modality treatment for rectal cancer patients. Strahlenther Onkol. 2007;183:227–235. doi: 10.1007/s00066-007-9000-9. [DOI] [PubMed] [Google Scholar]
- 7.Sobin LH, Wittekind C, editors. TNM classification of malignant tumours. 6th ed. New York: Wiley-Liss; 2002. pp. 66–70. [Google Scholar]
- 8.Aschele C, Friso ML, Pucciarelli S, et al. A phase I-II study of weekly oxaliplatin, 5-fluorouracil continuous infusion and preoperative radiotherapy in locally advanced rectal cancer. Ann Oncol. 2005;16:1140–1146. doi: 10.1093/annonc/mdi212. [DOI] [PubMed] [Google Scholar]
- 9.de Castro G, Snitcovsky IM, Gebrim EM, et al. High-dose cisplatin concurrent to conventionally delivered radiotherapy is associated with unacceptable toxicity in unresectable, non-metastatic stage IV head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2007;264:1475–1482. doi: 10.1007/s00405-007-0395-9. [DOI] [PubMed] [Google Scholar]
- 10.Franzmann EJ, Lundy DS, Abitbol AA, et al. Complete hypopharyngeal obstruction by mucosal adhesions: a complication of intensive chemoradiation for advanced head and neck cancer. Head Neck. 2006;28:663–670. doi: 10.1002/hed.20392. [DOI] [PubMed] [Google Scholar]
- 11.Machiels JP, Duck L, Honhon B, et al. Phase II study of preoperative oxaliplatin, capecitabine and external beam radiotherapy in patients with rectal cancer: the RadiOxCape study. Ann Oncol. 2005;16:1898–1905. doi: 10.1093/annonc/mdi406. [DOI] [PubMed] [Google Scholar]
- 12.Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–1644. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 13.Sauer R, Fietkau R, Wittekind C, et al. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis. 2003;5:406–415. doi: 10.1046/j.1463-1318.2003.00509.x. [DOI] [PubMed] [Google Scholar]
- 14.Rödel C, Liersch T, Hermann RM, et al. Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol. 2007;25:110–117. doi: 10.1200/JCO.2006.08.3675. [DOI] [PubMed] [Google Scholar]
- 15.Chavaudra J. [Last ICRU recommendations for the prescription, recording and reporting of external bean therapy] Cancer Radiother. 1998;2:607–614. doi: 10.1016/s1278-3218(98)80104-2. [DOI] [PubMed] [Google Scholar]
- 16.Vorwerk H, Hermann RM, Christiansen H, et al. A special device (double-hole belly board) and optimal radiation technique to reduce testicular radiation exposure in radiotherapy of rectal cancer. Radiother Oncol. 2007;84:320–327. doi: 10.1016/j.radonc.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Vorwerk H, Liersch T, Rothe H, et al. Gold markers for tumor localization and target volume delineation in radiotherapy for rectal cancer. Strahlenther Onkol. 2009;185:127–133. doi: 10.1007/s00066-009-1928-5. [DOI] [PubMed] [Google Scholar]
- 18.van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer. 2004;40:939–950. doi: 10.1016/j.ejca.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Trotti A, Byhardt R, Stetz J, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:13–47. doi: 10.1016/s0360-3016(99)00559-3. [DOI] [PubMed] [Google Scholar]
- 20.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 21.Hendry JH, Jeremic B, Zubizarreta EH. Normal tissue complications after radiation therapy. Rev Panam Salud Publica. 2006;20:151–160. doi: 10.1590/s1020-49892006000800012. [DOI] [PubMed] [Google Scholar]
- 22.Rubin P, Constine LS, 3rd, Fajardo LF, et al. EORTC Late Effects Working Group. Overview of late effects normal tissues (LENT) scoring system. Radiother Oncol. 1995;35:9–10. doi: 10.1016/0167-8140(95)97447-l. [DOI] [PubMed] [Google Scholar]
- 23.Schmiegel W, Reinacher-Schick A, Arnold D, et al. [Update S3-guideline “colorectal cancer” 2008] Z Gastroenterol. 2008;46:799–840. doi: 10.1055/s-2008-1027726. [DOI] [PubMed] [Google Scholar]
- 24.Sprenger T, Rothe H, Homayounfar K, et al. Preoperative chemoradiotherapy does not necessarily reduce lymph node retrieval in rectal cancer specimens-results from a prospective evaluation with extensive pathological work-up. J Gastrointest Surg. 2010;14:96–103. doi: 10.1007/s11605-009-1057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markl B, Kerwel TG, Jahnig HG, et al. Methylene blue-assisted lymph node dissection in colon specimens: a prospective, randomized study. Am J Clin Pathol. 2008;130:913–919. doi: 10.1309/AJCPVAPB5APABJNX. [DOI] [PubMed] [Google Scholar]
- 26.Nagtegaal ID, van de Velde CJ, van der Worp E, et al. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729–1734. doi: 10.1200/JCO.2002.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Gavioli M, Bagni A, Piccagli I, et al. Usefulness of endorectal ultrasound after preoperative radiotherapy in rectal cancer: comparison between sonographic and histopathologic changes. Dis Colon Rectum. 2000;43:1075–1083. doi: 10.1007/BF02236553. [DOI] [PubMed] [Google Scholar]
- 28.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 29.Brown G, Daniels IR. Preoperative staging of rectal cancer: the MERCURY research project. Recent Results Cancer Res. 2005;165:58–74. doi: 10.1007/3-540-27449-9_8. [DOI] [PubMed] [Google Scholar]
- 30.Fietkau R, Rödel C, Hohenberger W, et al. Rectal cancer delivery of radiotherapy in adequate time and with adequate dose is influenced by treatment center, treatment schedule, and gender and is prognostic parameter for local control: results of study CAO/ARO/AIO-94. Int J Radiat Oncol Biol Phys. 2007;67:1008–1019. doi: 10.1016/j.ijrobp.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004;22:648–657. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 32.Georgiadis MS, Steinberg SM, Hankins LA, et al. Obesity and therapy-related toxicity in patients treated for small-cell lung cancer. J Natl Cancer Inst. 1995;87:361–366. doi: 10.1093/jnci/87.5.361. [DOI] [PubMed] [Google Scholar]
- 33.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 34.Poikonen P, Blomqvist C, Joensuu H. Effect of obesity on the leukocyte nadir in women treated with adjuvant cyclophosphamide, methotrexate, and fluorouracil dosed according to body surface area. Acta Oncol. 2001;40:67–71. doi: 10.1080/028418601750071082. [DOI] [PubMed] [Google Scholar]
- 35.Schultheiss TE, Lee WR, Hunt MA, et al. Late GI and GU complications in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 1997;37:3–11. doi: 10.1016/s0360-3016(96)00468-3. [DOI] [PubMed] [Google Scholar]
- 36.Denham JW, O'Brien PC, Dunstan RH, et al. Is there more than one late radiation proctitis syndrome? Radiother Oncol. 1999;51:43–53. doi: 10.1016/s0167-8140(99)00027-4. [DOI] [PubMed] [Google Scholar]
- 37.Sprenger T, Rödel F, Beissbarth T, et al. Failure of down-regulation of Survivin following neoadjuvant radiochemotherapy in rectal cancer is associated with distant metastases and shortened survival. Clin Cancer Res. 2011;17:1623–1631. doi: 10.1158/1078-0432.CCR-10-2592. [DOI] [PubMed] [Google Scholar]
- 38.Rödel F, Reichert S, Sprenger T, et al. The role of survivin for radiation oncology: moving beyond apoptosis inhibition. Curr Med Chem. 2011;18:191–199. doi: 10.2174/092986711794088362. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859–865. doi: 10.1200/JCO.2009.25.8541. [DOI] [PubMed] [Google Scholar]
- 40.Rödel C. Radiotherapy: preoperative chemoradiotherapy for rectal cancer. Nat Rev Clin Oncol. 2010;7:129–130. doi: 10.1038/nrclinonc.2010.10. [DOI] [PubMed] [Google Scholar]
- 41.Glynne-Jones R, Harrison M. Locally advanced rectal cancer: what is the evidence for induction chemoradiation? The Oncologist. 2007;12:1309–1318. doi: 10.1634/theoncologist.12-11-1309. [DOI] [PubMed] [Google Scholar]
- 42.Rödel C, Arnold D, Becker H, et al. Induction chemotherapy before chemoradiotherapy and surgery for locally advanced rectal cancer: is it time for a randomized phase III trial? Strahlenther Onkol. 2010;186:658–664. doi: 10.1007/s00066-010-2194-2. [DOI] [PubMed] [Google Scholar]
- 43.Rödel C, Sauer R. Neoadjuvant radiotherapy and radiochemotherapy for rectal cancer. Recent Results Cancer Res. 2005;165:221–230. doi: 10.1007/3-540-27449-9_24. [DOI] [PubMed] [Google Scholar]
- 44.Bujko K, Glynne-Jones R, Bujko M. Adjuvant chemotherapy for rectal cancer. Ann Oncol. 2010;21:2443. doi: 10.1093/annonc/mdq616. [DOI] [PubMed] [Google Scholar]
- 45.Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241–248. doi: 10.1016/S1470-2045(09)70381-X. [DOI] [PubMed] [Google Scholar]
- 46.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of hospital procedure volume on surgical operation and long-term outcomes in high-risk curatively resected rectal cancer: findings from the Intergroup 0114 Study. J Clin Oncol. 2004;22:166–174. doi: 10.1200/JCO.2004.04.172. [DOI] [PubMed] [Google Scholar]